Abstract
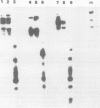
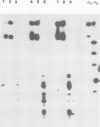
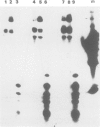
We have developed an assay for transient gene expression using a dominant-selectable marker previously employed to transform Drosophila cultured cells. Drosophila hydei cells transfected with a functional Escherichia coli xanthine guanine phosphoribosyl transferase gene (gpt), under the control of the long terminal repeats (LTRs) of the copia transposable element, rapidly incorporate guanine into acid-precipitable counts. Autoradiographic analysis in situ shows that approximately 20% of cells take up, and express, the gpt gene. This transient gpt expression depends on the Drosophila promoter sequences since vectors with the gpt gene in reverse orientation to the copia LTRs fail to incorporate guanine. Deletion analysis confirms that the LTRs are essential for gpt gene expression. Similarly, cells transfected with gpt controlled by the Drosophila 70 000 mol. wt. heat-shock (hsp 70) promoter show regulated guanine incorporation when heat shocked. The efficiency of the copia LTRs varies considerably between the cell lines we tested, whereas that of the hsp 70 promoter does not. The heterologous promoters of the Rous sarcoma virus (RSV) and simian virus 40 (SV40) function poorly in these cells.
Full text
PDF
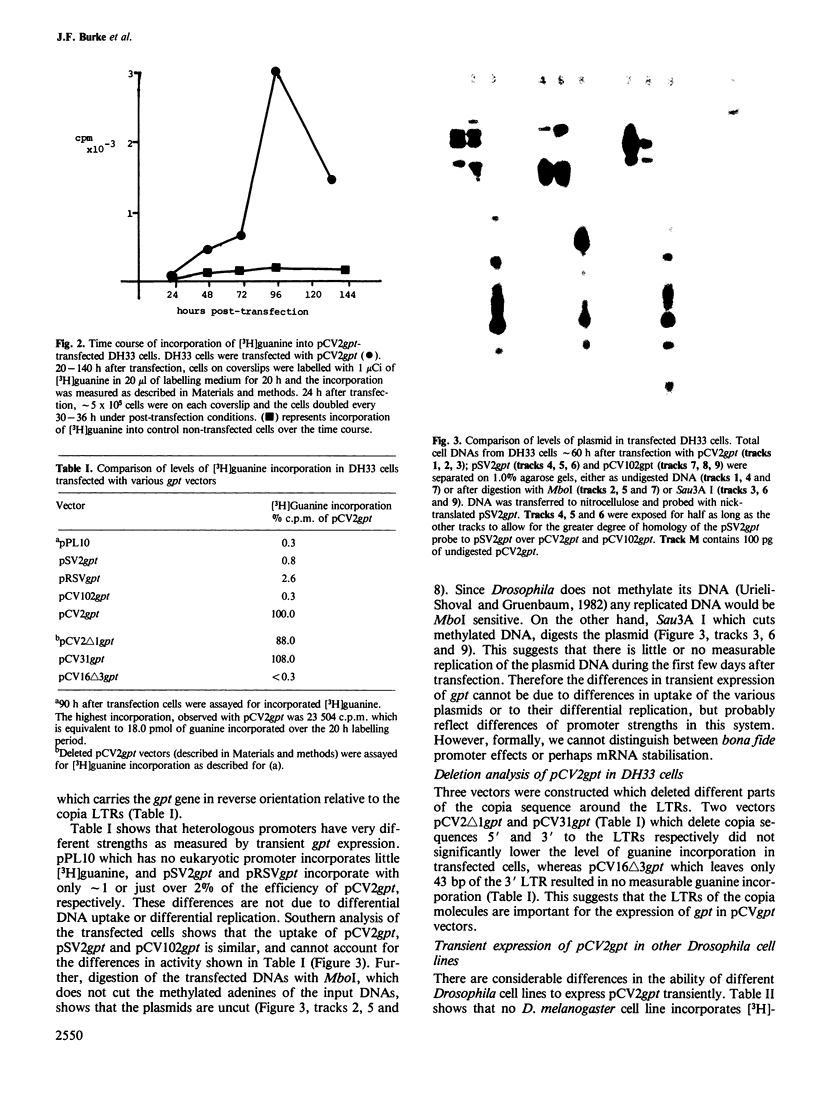
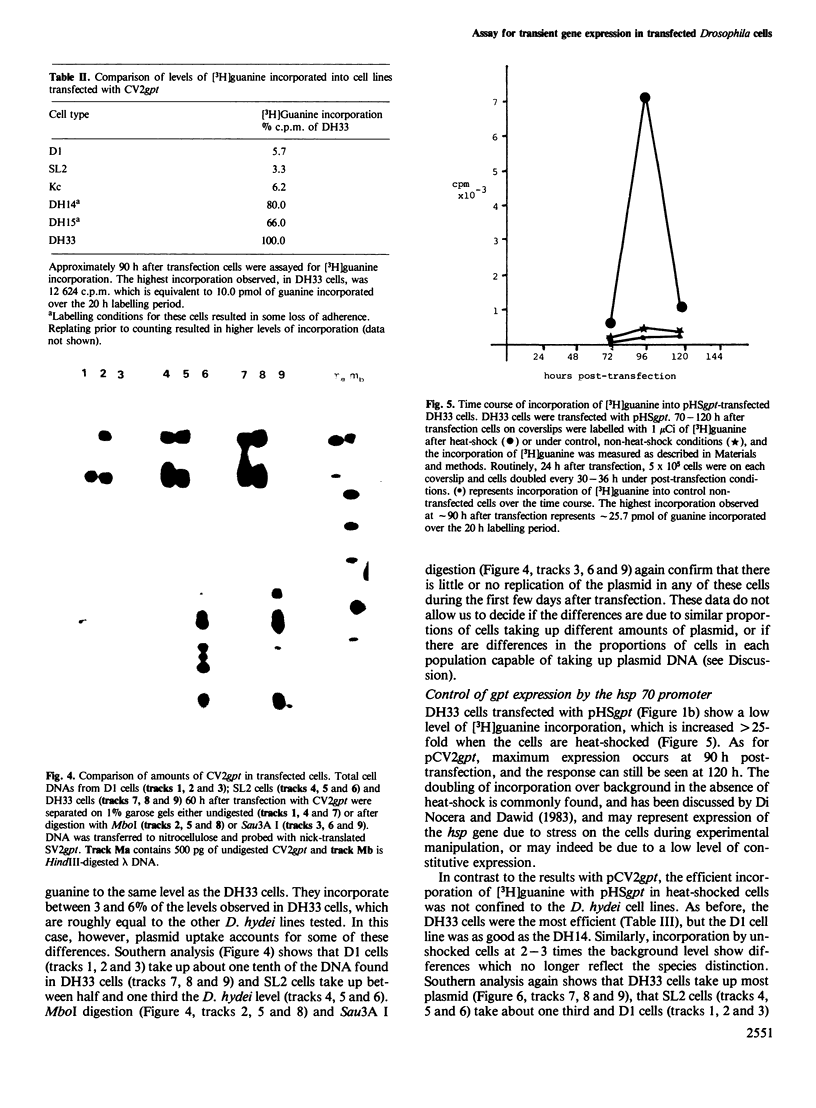
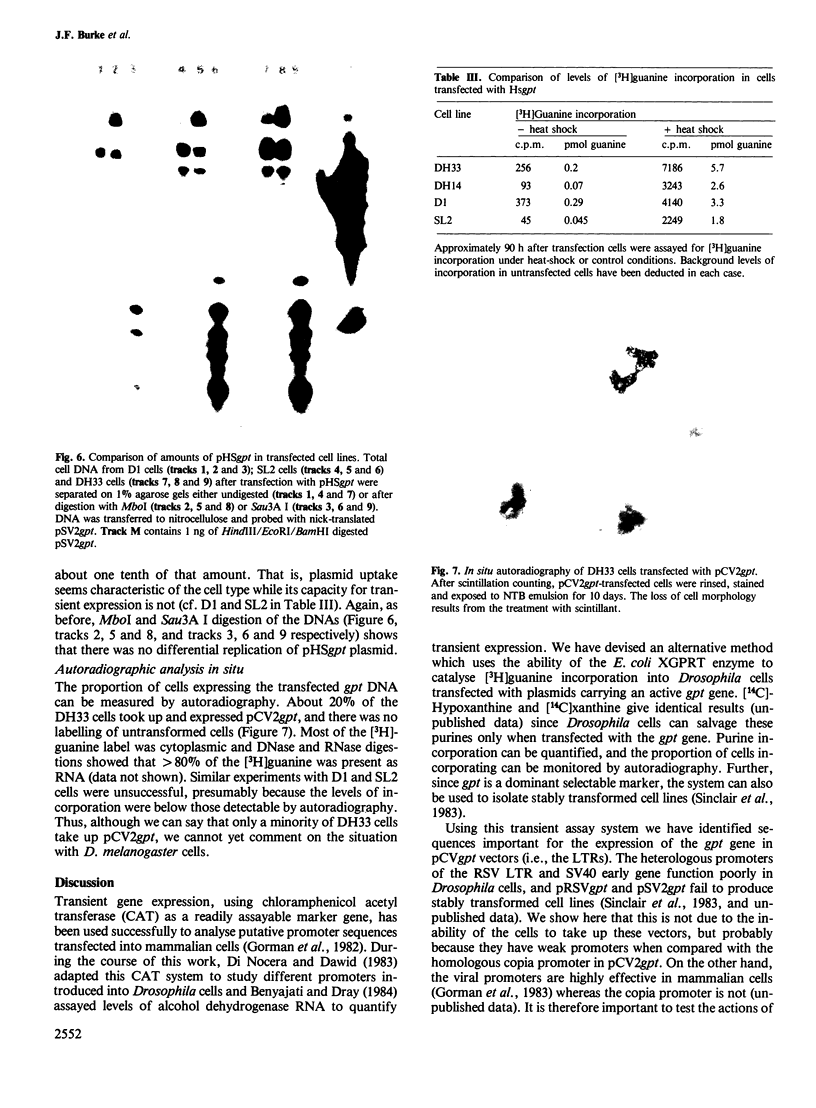


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashburner M., Bonner J. J. The induction of gene activity in drosophilia by heat shock. Cell. 1979 Jun;17(2):241–254. doi: 10.1016/0092-8674(79)90150-8. [DOI] [PubMed] [Google Scholar]
- Becker J. L. Métabolisme des purines dans des cellules de Drosophila melanogaster en culture in vitro: Interconversion des purines. Biochimie. 1974;56(9):1249–1253. doi: 10.1016/s0300-9084(74)80018-0. [DOI] [PubMed] [Google Scholar]
- Benyajati C., Dray J. F. Cloned Drosophila alcohol dehydrogenase genes are correctly expressed after transfection into Drosophila cells in culture. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1701–1705. doi: 10.1073/pnas.81.6.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourouis M., Jarry B. Vectors containing a prokaryotic dihydrofolate reductase gene transform Drosophila cells to methotrexate-resistance. EMBO J. 1983;2(7):1099–1104. doi: 10.1002/j.1460-2075.1983.tb01552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke J. F., Ish-Horowicz D. Expression of Drosophila heat shock genes is regulated in Rat-cells. Nucleic Acids Res. 1982 Jul 10;10(13):3821–3830. doi: 10.1093/nar/10.13.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Nocera P. P., Dawid I. B. Transient expression of genes introduced into cultured cells of Drosophila. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7095–7098. doi: 10.1073/pnas.80.23.7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell A. J., Ish-Horowicz D. Extrachromosomal circular copies of the eukaryotic transposable element copia in cultured Drosophila cells. Nature. 1981 Aug 13;292(5824):591–595. doi: 10.1038/292591a0. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Merlino G. T., Willingham M. C., Pastan I., Howard B. H. The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6777–6781. doi: 10.1073/pnas.79.22.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C., Padmanabhan R., Howard B. H. High efficiency DNA-mediated transformation of primate cells. Science. 1983 Aug 5;221(4610):551–553. doi: 10.1126/science.6306768. [DOI] [PubMed] [Google Scholar]
- Huttner K. M., Barbosa J. A., Scangos G. A., Pratcheva D. D., Ruddle F. H. DNA-mediated gene transfer without carrier DNA. J Cell Biol. 1981 Oct;91(1):153–156. doi: 10.1083/jcb.91.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirault M. E., Southgate R., Delwart E. Regulation of heat-shock genes: a DNA sequence upstream of Drosophila hsp70 genes is essential for their induction in monkey cells. EMBO J. 1982;1(10):1279–1285. doi: 10.1002/j.1460-2075.1982.tb00025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Selection for animal cells that express the Escherichia coli gene coding for xanthine-guanine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2072–2076. doi: 10.1073/pnas.78.4.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R. A regulatory upstream promoter element in the Drosophila hsp 70 heat-shock gene. Cell. 1982 Sep;30(2):517–528. doi: 10.1016/0092-8674(82)90249-5. [DOI] [PubMed] [Google Scholar]
- Schedl P., Artavanis-Tsakonas S., Steward R., Gehring W. J., Mirault M. E., Goldschmidt-Clermont M., Moran L., Tissières A. Two hybrid plasmids with D. melanogaster DNA sequences complementary to mRNA coding for the major heat shock protein. Cell. 1978 Aug;14(4):921–929. doi: 10.1016/0092-8674(78)90346-x. [DOI] [PubMed] [Google Scholar]
- Sondermeijer P. J., Derksen J. W., Lubsen N. H. New cell line: established cell lines of Drosophila hydei. In Vitro. 1980 Nov;16(11):913–914. doi: 10.1007/BF02619327. [DOI] [PubMed] [Google Scholar]
- Urieli-Shoval S., Gruenbaum Y., Sedat J., Razin A. The absence of detectable methylated bases in Drosophila melanogaster DNA. FEBS Lett. 1982 Sep 6;146(1):148–152. doi: 10.1016/0014-5793(82)80723-0. [DOI] [PubMed] [Google Scholar]
- Wyss C. Purine and pyrimidine salvage in a clonal Drosophila cell line. J Insect Physiol. 1977;23(6):739–747. doi: 10.1016/0022-1910(77)90092-0. [DOI] [PubMed] [Google Scholar]