Abstract
Cytokines are molecules that play critical roles in the regulation of a wide range of normal functions leading to cellular proliferation, differentiation and survival, as well as in specialized cellular functions enabling host resistance to pathogens. Cytokines released in response to infection, inflammation or immunity can also inhibit cancer development and progression. The predominant intracellular signaling pathway triggered by cytokines is the JAK-signal transducer and activator of transcription (STAT) pathway. Knockout mice and clinical human studies have provided evidence that JAK-STAT proteins regulate the immune system, and maintain immune tolerance and tumor surveillance. Moreover, aberrant activation of the JAK-STAT pathways plays an undeniable pathogenic role in several types of human cancers. Thus, in combination, these observations indicate that the JAK-STAT proteins are promising targets for cancer therapy in humans. The data supporting this view are reviewed herein.
Keywords: Cytokine, JAK-STAT, Cancer, Kinase inhibitor
INTRODUCTION
The importance of inflammation for tumorigenesis and malignant progression has become the considerable interests for good reasons. Inflammatory conditions can initiate or promote various accessary responses such as oncogenic transformation, and genetic and epigenetic changes in malignant cells that further enhance tumor progression. The presence of specific inflammatory cells and mediators, including cytokines and chemokines provoke cancer-related inflammation and cytokines apply broad immunoregulatory roles to human disease (1,2,3).
Over the last 25 years, it has been shown that the majority of cytokines transfer their signals via the JAKs and signal transducer and activator of transcriptions (STATs). The different JAKs associate constitutively with different cytokine receptors. Binding of specific ligands to such receptors induces conformational changes in the receptors, resulting in activation of JAKs. The activated JAKs subsequently induce phosphorylation of specific tyrosine-based motifs in the cytokine receptors, which provide docking sites for Src homology 2 (SH2)-containing STATs, as well as for other proteins with SH2 domains (4,5,6). Inactivating JAK3 mutations in humans are seen is a severe combined immunodeficiency syndrome, whereas mutations of tyrosine kinase 2 (Tyk2) result in another primary immunodeficiency such as autosomal recessive hyperimmunoglobulin E syndrome (7). These findings imply a critical role of JAK-STAT pathways in promoting normal immunity (2).
Conversely, activating mutations of JAKs are found in the connection with malignant transformation in humans as gain-of-function mutation of JAK2 in myeloproliferative disorders (8). Moreover, JAK3 and Tyk2 are also related with clinical disorders in humans and mouse models (9,10). In addition, recent evidences provide important roles for STAT family that potentiate candidates to induce a pro-carcinogenic inflammatory microenvironment as well as the initiation of malignant transformation and cancer progression (10). STAT3 is linked to inflammation-associated tumorigenesis initiated by genetic alterations in malignant cells and induced by various environmental factors such as chemical carcinogens, sunlight, infection, cigarette smoking, and stress (11). Thus, JAK-STATs have complex roles, either direct or indirect, in promoting cancer progression.
In this review, we will discuss the role of JAK-STAT pathways in promoting cancer development and progression. We will show that the involvement of JAK-STAT pathways in these processes can be either direct or indirect. We will also summarize the data regarding the creation and testing of pharmacological inhibitors of the JAK-STAT pathways for the treatment of various types of human cancers.
THE JAK-STAT PATHWAYS
As referred to above, cytokine receptors are non-covalently associated with the JAK family member of cytoplasmic protein tyrosine kinases (PTKs) (2,4,5). JAK kinases are so called, because they have 2 tandem kinase-like domains, one true kinase domain and one pseudo-kinase domain, and thus reminiscent of the 2-headed mythical Roman god Janus (2,12). There are 4 common members of the JAK family, which are JAK1, JAK2, JAK3, and Tyk2 (10). Most JAK family members are ubiquitously expressed except JAK3 predominantly expressed in hematopoietic cells.
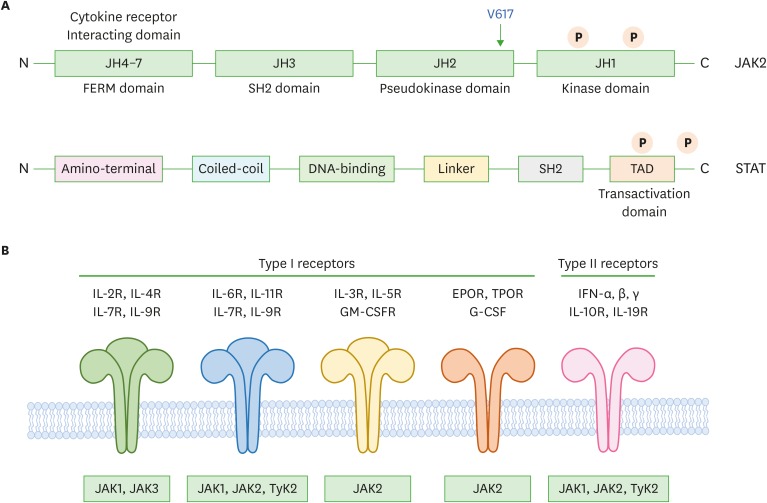
As mice deficient for individual JAK family members show different phenotypes, it is presumed that each kinase has a distinct function (4). JAK family members show very similar structures and functions and are more than 1,000 amino acids, which consist of unique 7 JAK homology (JH) regions (JH1 to JH7), and these form the alleged domains of JAK family members (Fig. 1A and 1B) (2).
Figure 1.
Schematic representation of JAK-STAT structure. (A) The domain structure of JAKs and STATs. Four JAKs consist of the domains JH1 to JH7 based on their sequence similarity including 2 tyrosines after cytokine stimulation. JH1 is kinase domain and JH2 is the pseudo-kinase domain. The JH6 and JH7 domains introduce the binding of JAKs to main receptors. STATs consist of 7 specific domains, which are involved with various responses resulting in the regulation of protein modification by tyrosine and serine phosphorylation, methylation, sumoylation, and acetylation. (B) Classification of cytokine receptors which are type I and II according to their ligands and the association with JAKs to deliver their signals to the downstream.
FERM, 4.1, ezrin, radixin, moesin; TAD, transactivation domain; GM-CSFR, granulocyte-macrophage colony-stimulating factor receptor; EPOR, erythropoietin receptor; TPOR, thrombopoietin receptor; G-CSF, granulocyte-colony stimulating factor; IFN, interferon.
It is likely that the usage of different combinations of JAKs by different cytokine receptors enables a diversity of signaling responses. The dimerization or clustering of the signaling chains allows the JAKs to cross-phosphorylate each other, thus stimulating their kinase activity. The activated JAKs then phosphorylate the cytokine receptors on specific tyrosine residues, to generate binding sites for proteins with SH2 domains (Fig. 1A). Some of the tyrosine phosphorylated sites recruit SH2 domain-containing latent transcription factors known as STATs (5,12).
There are 7 known STATs (1–4, 5a, 5b, and 6) (4,12). The specificity of particular STATs for a particular receptor is determined by the recognition of distinctive phosphotyrosine sequences on the activated receptors by the SH2 domain of the STATs. Recruitment of a STAT to an activated receptor brings the STAT close to the activated JAK, which can then phosphorylate the STAT (4). This leads to a conformational change in the STAT that allows it to bind to another STAT and form a STAT dimer. STATs can form homodimers or heterodimers. The phosphorylated STAT dimer then dissociates and enters the nucleus to initiate the transcription of particular genes (4). These STAT-regulated genes contribute to the growth and differentiation of specific subsets of lymphocytes.
As cytokines have so many powerful effects, the activation of cytokine signaling pathways must be tightly controlled; breakdown in control can lead to significant pathological effects. A variety of cytokine-specific inhibitory mechanisms ensure that cytokine signaling pathways can be efficiently terminated (3,13). As cytokine receptor signaling depends on tyrosine phosphorylation, dephosphorylation of receptor complexes by protein tyrosine phosphatases is one important means of signal termination. A variety of protein tyrosine phosphatases have been implicated in the dephosphorylation of cytokine receptors, JAKs or STATs; these include CD45, SH2 domain-containing phosphatase-1 (SHP-1) or SHP-2, and the T-cell protein tyrosine phosphatase (TCPTP) (14,15).
Cytokine signaling can also be terminated by a negative feedback process involving specific inhibitors induced by cytokine activation. One class of inhibitors contains the suppressors of cytokine signaling (SOCS) proteins, which terminate signaling in variety of ways, including promoting the ubiquitination and subsequent degradation of receptors, JAKs and STATs (16). Another class of inhibitory proteins consists of the protein inhibitors of activated STAT (PIAS) proteins, which also seem to be involved in promoting the degradation of receptors and pathway components (17).
Among the genes known to be transcriptionally upregulated by mammalian STAT proteins are some encoding cell survival factors, such as the B-cell lymphoma 2 (Bcl-2) family of proteins, others involved in cell proliferation, such as cyclin D1 and Myc, and some implicated in angiogenesis or metastasis, such as vascular endothelial growth factor (16,18). Since it is conceivable that upregulation of genes promotes cancer formation, it has been presumed that upregulation of these genes mediates the physiological effects of STAT activation on cell behavior and, also, may promote cancer formation.
INVOLVEMENT OF JAK FAMILY KINASES IN CANCER
Several lines of evidence have directly implicated the JAK-STAT pathways in the pathogenesis of cancers. Abnormal translocations or mutations involving certain genes coding for JAKs have been observed in leukemias and other hematologic malignancies in humans. Moreover, JAK-STATs are hyperactivated in a variety of hematological malignancies and solid tumors, and such abnormal activations are likely involved in the pathogenesis of these diseases (34,35).
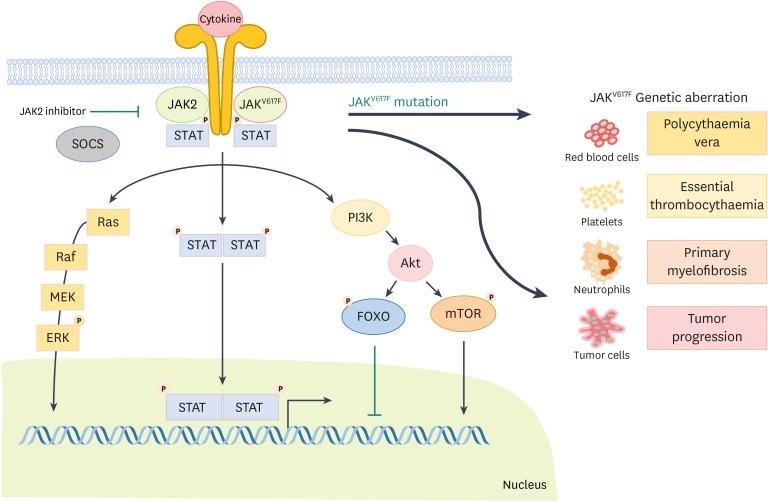
Discovery of mutations in JAK2, in particular JAK2 valine 617 to phenylalanine 617 (V617F) mutation in myeloproliferative neoplasms (MPNs) such as polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF) has caused remarkable interest in examining the direct involvement of JAK-STAT pathways in cancer (19). These mutations result in a constitutively active kinase domain, leading to gain-of-function and tumor development. The V617F mutation happens in the pseudokinase domain of JAK2 and results in an impaired ability of the pseudokinase domain to regulate negatively the kinase domain (the active part of an enzyme) (20). JAK2 V617F mutation exists in most PV patients and about half of PMF patient as well as ET have a JAK2 V617F mutation, even though different levels of allele burden (21). Although JAK2 V617F is mostly related with MPNs, abnormally activated JAK2 mutation have been recognized in a few patients with MPN without a JAK2 V617F mutation. These mutations include JAK2 mutations residing in exon 12 and mutations in the myeloproliferative leukemia virus oncogene (MPL) receptor (MPL W515L) (Fig. 2) (21). All these mutations result in abnormal proliferation and survival of stem cells and hypersensitivity or independence from hematopoietic cytokines (6,22). Enforced expression of these mutant proteins in mice, either by transgenesis or by retroviral transfer in bone marrow stem cells, cause PV, ET, and post-PV/ET myelofibrosis (MF) phenotypes implying a direct causal role for these mutations in MPNs (23,24).
Figure 2.
Schematic representation of the JAK-STAT pathway. The cytokine receptor induces activation of JAKs after cytokine stimulation following the phosphorylation of STATs. Furthermore, phosphorylated STATs undergo dimerization and translocate to the nucleus to activate target gene transcription. Genetic aberration in JAKs provoke serious diseases such as PV, ET, PMF as well as cancer.
SOCS, suppressors of cytokine signaling; PI3K, phosphatidyl inositol 3 kinase; Akt, protein kinase B; FOXO, Forkhead box protein O; mTOR, mammalian target of rapamycin.
Recently, several published studies identified a genetic haplotype that affect the development of JAK2 V617F mutation and MPN through somatic mutation (25,26). These reports suggested that the JAK2 V617F mutation is not causing MPN, but rather is a contributing factor for disease existence (25,27). Identification of the abnormalities that lead to the existence of MPN and the occurrence of JAK2 V617F mutations, and to the distinct clinical entities of PV, ET, and PMF in humans, is a subject of intense investigation (28,29).
It has been reported that acute lymphoblastic leukemia (ALL), acute myelogenous leukemia (AML), and acute megakaryoblastic leukemia (AMKL) are also affected by JAK2 mutations (30). Point mutations of V617F enhance cell proliferation and survival via tyrosine phosphorylation of JAK2. Rare cases of point mutations in JAK3/STAT5 phosphorylation causing AMKL have been reported contrast to high prevalence of patients with JAK2 alterations. Interestingly, JAK3 mutations have been reported in solid cancers such as breast cancer or gastric cancer (31).
Another abnormal translocation, which results in production of the 2146 transformation-specific leukemia (TEL)-JAK2 fusion protein, has been identified in myeloid and lymphoid malignancies in humans (32). Direct evidence that this abnormal JAK protein with constitutive activation of its kinase domain can induce leukemia-like syndromes was obtained by the creation of TEL-JAK2 transgenic mice, which exhibited uncontrolled expansion of CD8+ T cells (32,33). Studies to understand how the TEL-JAK2 fusion protein promotes leukemogenesis have shown that the constitutive activity of JAK2 in the fusion protein results in phosphorylation/activation of STAT proteins (STAT1, STAT3, and STAT5) (32,34). The ability to induce myelo- and lymphoproliferative diseases in STAT5a/b-deficient mice, using a bicistronic retrovirus encoding both TEL-JAK2 and STAT5a, established the critical role of STAT5 in the pathogenesis of TEL-JAK2-induced syndromes (35). The TEL-JAK2 fusion protein induces the transformation of a hematopoietic pro-B cell line (Ba/F3), seemingly through constitutive STAT3 and STAT5 activation (36,37).
Besides TEL-JAK2, different JAK2 fusion proteins are involved with atypical chronic myelogenous leukemias (CMLs), including pericentriolar material 1 (PCM1)-JAK2 or B-cell receptor (BCR)-JAK2 fusions (38). Similarly, in the setting of acute leukemias, JAK2 fusion proteins have been reported (38). The oncogenic potential of such PTK fusion proteins was featured by translocations leading to production of TEL-platelet-derived growth factor receptor (PDGFR) or TEL-Abelson kinase (Abl) with chronic myelomonocytic leukemia (CMML) or ALL, respectively (39,40,41).
Another important hematologic malignancy in which JAK-STAT pathways appear to play roles in pathogenesis is multiple myeloma. Studies have shown that constitutive activation of STAT3 occurs in bone marrow mononuclear cells from patients with multiple myeloma (42,43). Similarly, STAT3 was found to be activated in IL-6-independent multiple myeloma cell lines and such activation was associated with activation of JAK1, JAK2, or JAK3 (43). STAT3 inhibitors were found to downregulate B-cell lymphoma-extra large (Bcl-xL) expression and increase Fas-mediated apoptosis (44). In addition, they were shown to suppress cell proliferation and inhibit JAK2 kinase activity, as well as extracellular signal-regulated kinase (ERK2) and STAT3 phosphorylation, in IL-6-dependent multiple myeloma cell lines.
STAT3 was also shown to be constitutively activated in solid tumors. These include primary breast carcinoma cells, breast cancer cell lines, and primary melanoma cells (45,46). STAT3 is also constitutively activated in prostate carcinomas (46). Blockade of activated STAT3 in prostate cancer cells expressively suppressed their growth and tumorigenicity. Constitutive activation of STAT3 has also been described in squamous cell carcinomas of the head and neck, and such an activation decreases apoptosis through increased Bcl-xL expression (47,48,49). In addition, correlation between increased levels of the activated form of phosphorylated STAT3 and cyclin D1 levels was found in one study in which 51 primary tumor samples (50).
Thus, in combination, these results clearly established that constitutive and aberrant activation of JAK-STAT pathways can have a direct pathogenic role in various hematological malignancies, as well as in certain types of solid tumors, in humans.
INDIRECT PARTICIPATION OF JAK-STAT PATHWAYS IN HUMAN MALIGNANCIES
There is also indication that the JAK-STAT pathways can participate indirectly in the pathogenesis of human malignancies. Indeed, JAKs regulate signaling pathways activated by a variety of cytokines and growth factors such as various interleukins; granulocyte-macrophage colony-stimulating factor receptor (GM-CSFR), granulocyte-colony stimulating factor (G-CSF), and erythropoietin (10). As a result, members of the JAK family activate normal mitogenic pathways and this feature may contribute to creation of the oncogenic state. For instance, JAK kinases activate the Ras-Raf-mitogen activated protein kinase (MAPK) cascade that is implicated in malignant transformation and neoplastic cell growth (51). JAK kinases have also been linked to malignant transformation by other oncogene proteins such as v-Abl (52). The capacity of v-Abl to transform cells is directly linked to its ability to induce constitutive activation of STAT5 and STAT6, and v-Abl-dependent STAT activation correlates with its ability to activate JAK1, JAK2, and JAK3, depending on the cellular context and cell type involved (52).
Another mechanism by which JAK kinases appear to promote malignant cell survival and growth is modulation of apoptosis via regulation of the activities of anti-apoptotic the Bcl-2 family proteins. JAK kinases regulate the levels of Bcl-xL, Bcl-2, and Bcl-2-associated X protein (Bax), independently of their effects on STATs and other associated pathways such as the phosphatidyl inositol 3 kinase (PI3K) and the Ras-MAPK pathways (53,54). JAKs also regulate apoptosis and p53 dependent cell cycle arrest.
Lastly, the JAK-STAT pathways can indirectly favor malignant transformation by promoting immune cell-mediated inflammation at sites of tumor development. While immunity can help prevent or terminate the oncogenic process, it is now becoming abundantly clear that inflammation at tumor sites can also have pro-oncogenic effects. This activity is mediated through multiple mechanisms by way of the ability of some immune cells, in particular macrophages, to secrete growth factors that promote tumor cell growth, to stimulate blood vessel development that enhances blood perfusion and arrival of nutrients at tumor sites and to suppress anti-tumor-specific immune reactions (55).
JAKS AS CLINICAL DRUG TARGETS
Considering the evidence that activation of JAK-STAT pathways plays a role, direct or, at times, indirect in malignant transformation of hematopoietic and non-hematopoietic cells, there has been significant interest in developing and using pharmacological inhibitors of the JAK-STAT pathways to treat these disorders (Table 1). In support of this, JAK-specific kinase inhibitors have been shown to inhibit cell proliferation in several systems (56).
Table 1. Type of JAK-STAT inhibitors.
| Type | Name | Efficacy | Clinical stage |
|---|---|---|---|
| JAK2 inhibitor | SB1518 (pacritinib) | Hematological malignancies, CIMF, MF, MDS | Phase III |
| XL019 | MPD, MF | Phase I/II | |
| TG101348 | MF, renal impairment | Phase I/II | |
| INCB018424 (ruxolitinib) | Prostate cancer, multiple myeloma, AML, CML, IBC, advanced hematologic malignancies, MF | Phase II/III | |
| CEP701 (lestaurtinib) | AML, pancreatic cancer, prostate cancers, Neuroblastoma | Phase II | |
| Dasatinib | JAK2 mutant dependent PV, CML, prostate cancer | Phase IV | |
| JAK3 inhibitor | WHI-P131 | Glioblastoma | N/A |
| WHI-P154 | Glioblastoma | N/A | |
| Tyrphostin AG 490 | Pre-B acute leukemia (ALL) | N/A | |
| PNU156804 | Block allograft rejection | N/A | |
| CP-690,550 (tofacitinib) | RA, psoriasis, inflammatory bowel disease, organ transplant rejection, ulcerative colitis, ankylosing spondylitis | Phase III | |
| NC1153 | Block allograft rejection | N/A | |
| STAT3 inhibitor | Sorafenib | HCC, RCC, breast cancer, thyroid cancer | Phase II/III |
| Sunitinib | GIST, esophageal cancer, RCC, pNET | Phase II/III | |
| Bendamustine | CLL, multiple myeloma, non-Hodgkin's lymphoma | Phase II/III | |
| Napabucasin | Colon cancer, rectal cancer, colorectal cancer | Phase II |
CIMF, chronic idiopathic myelofibrosis; MDS, myelodysplastic syndrome; MPD, myeloproliferative disorder; IBC, inflammatory breast cancer; N/A, not applicable; RA, rheumatoid arthritis; HCC, advanced hepatocellular carcinoma; RCC, advanced renal cell carcinoma; GIST, gastrointestinal stromal tumor; pNET, pancreatic neuroendocrine tumor; CLL, chronic lymphocytic leukemia.
At first, the quinazoline derivatives such as WHI-P131 and WHI-P154, which were therapeutic agent for glioblastoma, are also considered to potential activity against JAK3 (57,58,59). However, in retrospect, these compounds were neither selective nor potent JAK3 inhibitors (60). Other inhibitors such as tyrphostin AG 490 (Sigma-Aldrich, St. Louis, MO, USA) or PNU156804 (Pfizer Inc., New York, NY, USA) were also observed to inhibit JAK3 and show significant effects in vitro, but, again, their selectivity was not confirmed in vivo (61,62). Another study showed that CP-690,550 (Tofacitinib; Pfizer Inc.) had high affinity for JAK3, with little effect on unrelated kinases (63,64). But the clinical efficiency of this compound remains unproven.
More significantly, the discovery of an activating mutation (JAK2 V617F) in hematological malignancies like MPNs, PV, ET, and PMF led to the accelerated development of JAK2 inhibitors, of which are in clinical studies. Various JAK2 inhibitors such as pacritinib (SB1518; S*BIO Pte Ltd., Singapore, Singapore), XL019 (Exelixis, Inc., South San Francisco, CA, USA), ruxolitinib (INCB018424; Incyte Corporation, Wilmington, DE, USA), TG101348 (Sanofi, Paris, France), and lestaurtinib (CEP701; Abcam Biochemicals, Cambridge, UK) are under being examined to develop for hematological malignancies (65,66,67,68,69,70). Clinical studies of ruxolitinib are being conducted for prostate cancer, multiple myeloma, AML, and CML (71,72). Lestaurtinib is a U.S. Food and Drug Administration (FDA)-designated orphan drug for AML, which was considered by targetting Fms-like tyrosine kinase 3 (FLT3) and tropomyosin-related kinase A (TrkA) (73,74,75,76). However, lestaurtinib was also reported to inhibit JAK2. Consequently, phase II clinical trials are testing this drug in AML patients with JAK2 mutations (70,77). Furthermore, although imatinib (Gleevec®, STI571; Novartis Oncology, East Hanover, NJ, USA) show activity towards JAK2, efficacy of imatinib was tested in clinical trials for PV (78). Finally, dasatinib (Sprycel®, BMS-354825; Bristol-Myers Squibb, Princeton, NJ, USA), a PTK inhibitor approved for CML after imatinib, is a potential inhibitor of Src family PTKs and BCR-Abl (79). Dasatinib is less efficacious in vivo model of JAK2 mutant dependent PV model though in vitro dasatinib inhibit myeloid and erythroid colony growth in peripheral blood cells (80). Dasatinib can inhibit JAK2 activity in vitro at least at high-doses and more clinical trials are underway to determine how it inhibits JAK2 mutation-driven proliferation. Moreover, several FDA-approved tyrosine kinase inhibitors already in the clinic, including sorafenib (Nexavar®, BAY43-9006; Bayer HealthCare Pharmaceuticals Inc., Wayne, NJ, USA) and sunitinib (Sutent®, SU11248; Pfizer Inc.), were found to inhibit STAT3 signaling indirectly, resulting in tumor cell cycle arrest and apoptosis (81,82). Sorafenib inhibits of phosphotyrosine site of STAT3 and decreases expression of anti-apoptotic protein myeloid cell leukemia 1 (Mcl-1), a member of Bcl-2 family (82,83). Sunitinib has further been found to inhibit STAT3 activity in tumor-associated immune cells, modulating the tumor immunological microenvironment in favor of cancer therapy (84). It inhibits immunosuppressive myeloid lineage-derived suppressor cells (MDSCs) and regulatory T cells both in mouse models and in human clinical trials (85,86). Although the inhibition of STAT3 signaling contributes to the anti-tumor activities of sorafenib and sunitinib, their precise molecular mechanisms of action in terms of STAT3 inhibition remain to be determined. In sum, targeting of JAKs-STATs — a central regulatory node on which many oncogenic and inflammatory pathways converge — hold great promise for cancer therapy. In addition to the inhibitory functions for the tumor cell proliferation and survival, such JAK2 or STAT3 inhibitors may convert inflammation in the tumor microenvironment from tumor-promoting to tumor-suppressing. This possibility deserves consideration.
CONCLUSION
Significant progresses have occurred over the recent years in the field of JAK-STAT signaling. The original identification of the components of the JAK-STAT pathways led to the development of important basic research studies that have provided valuable information for mechanisms by which different combinations of JAK kinases and their substrates participate in the regulation of malignant cell growth, survival and death. Depending on the specific JAK kinase involved and the downstream effectors activated, different biological outcomes can occur. While it is clear that JAK-STAT pathways negatively regulate neoplastic cell proliferation under certain circumstances, activation of JAKs or STATs promotes malignant transformation and neoplastic cell growth in most of cases. The tumor-promoting activity of JAKs and STATs is highly relevant both to hematologic malignancies and to solid tumors, and has provided potential targets for the development of specific anti-tumor therapies. The several reports of JAK2 inhibitors in the treatment of various cancer models were a remarkable scientific and clinical advance in the field of leukemia. The studies of these drugs have provided a model for the development of other small molecules that target kinases involved in the pathogenesis of malignancies. It is likely that continuation and expansion of current research efforts will provide additional important information that could be applied towards the future development of novel anticancer therapies.
ACKNOWLEDGEMENTS
This research was supported by Basic Science Research program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2015R1C1A1A02037462).
Abbreviations
- Abl
Abelson kinase
- ALL
acute lymphoblastic leukemia
- AML
acute myelogenous leukemia
- Bcl-2
B-cell lymphoma 2
- Bcl-xL
B-cell lymphoma-extra large
- CML
chronic myelogenous leukemia
- ET
essential thrombocythemia
- JH
JAK homology
- MF
myelofibrosis
- MPN
myeloproliferative neoplasm
- PMF
primary myelofibrosis
- PTK
protein tyrosine kinase
- PV
polycythemia vera
- SH2
Src homology 2
- STAT
signal transducer and activator of transcription
- TEL
transformation-specific leukemia
- Tyk2
tyrosine kinase 2
- V617F
valine 617 to phenylalanine 617
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest.
Author Contributions: Conceptualization: Lee M, Rhee I. Funding acquisition: Rhee I. Investigation: Lee M, Rhee I. Methodology: Lee M, Rhee I. Project administration: Rhee I. Supervision: Rhee I. Validation: Rhee I. Visualization: Rhee I. Writing - original draft: Lee M, Rhee I. Writing - review & editing: Lee M, Rhee I.
References
- 1.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 2.O'Shea JJ, Holland SM, Staudt LM. JAKs and STATs in immunity, immunodeficiency, and cancer. N Engl J Med. 2013;368:161–170. doi: 10.1056/NEJMra1202117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spangler JB, Moraga I, Mendoza JL, Garcia KC. Insights into cytokine-receptor interactions from cytokine engineering. Annu Rev Immunol. 2015;33:139–167. doi: 10.1146/annurev-immunol-032713-120211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kisseleva T, Bhattacharya S, Braunstein J, Schindler CW. Signaling through the JAK/STAT pathway, recent advances and future challenges. Gene. 2002;285:1–24. doi: 10.1016/s0378-1119(02)00398-0. [DOI] [PubMed] [Google Scholar]
- 5.Rane SG, Reddy EP. Janus kinases: components of multiple signaling pathways. Oncogene. 2000;19:5662–5679. doi: 10.1038/sj.onc.1203925. [DOI] [PubMed] [Google Scholar]
- 6.Vainchenker W, Constantinescu SN. JAK/STAT signaling in hematological malignancies. Oncogene. 2013;32:2601–2613. doi: 10.1038/onc.2012.347. [DOI] [PubMed] [Google Scholar]
- 7.Woellner C, Schäffer AA, Puck JM, Renner ED, Knebel C, Holland SM, Plebani A, Grimbacher B. The hyper IgE syndrome and mutations in TYK2. Immunity. 2007;26:535–536. doi: 10.1016/j.immuni.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 8.Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, Tichelli A, Cazzola M, Skoda RC. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352:1779–1790. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- 9.Casanova JL, Holland SM, Notarangelo LD. Inborn errors of human JAKs and STATs. Immunity. 2012;36:515–528. doi: 10.1016/j.immuni.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Shea JJ, Schwartz DM, Villarino AV, Gadina M, McInnes IB, Laurence A. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu Rev Med. 2015;66:311–328. doi: 10.1146/annurev-med-051113-024537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Durant L, Watford WT, Ramos HL, Laurence A, Vahedi G, Wei L, Takahashi H, Sun HW, Kanno Y, Powrie F, et al. Diverse targets of the transcription factor STAT3 contribute to T cell pathogenicity and homeostasis. Immunity. 2010;32:605–615. doi: 10.1016/j.immuni.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leonard WJ, O'Shea JJ. Jaks and STATs: biological implications. Annu Rev Immunol. 1998;16:293–322. doi: 10.1146/annurev.immunol.16.1.293. [DOI] [PubMed] [Google Scholar]
- 13.Kim JS, Kim YG, Park EJ, Kim B, Lee HK, Hong JT, Kim Y, Han SB. Cell-based immunotherapy for colorectal cancer with cytokine-induced killer cells. Immune Netw. 2016;16:99–108. doi: 10.4110/in.2016.16.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Irie-Sasaki J, Sasaki T, Matsumoto W, Opavsky A, Cheng M, Welstead G, Griffiths E, Krawczyk C, Richardson CD, Aitken K, et al. CD45 is a JAK phosphatase and negatively regulates cytokine receptor signalling. Nature. 2001;409:349–354. doi: 10.1038/35053086. [DOI] [PubMed] [Google Scholar]
- 15.David M, Chen HE, Goelz S, Larner AC, Neel BG. Differential regulation of the alpha/beta interferon-stimulated Jak/Stat pathway by the SH2 domain-containing tyrosine phosphatase SHPTP1. Mol Cell Biol. 1995;15:7050–7058. doi: 10.1128/mcb.15.12.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Constantinescu SN, Girardot M, Pecquet C. Mining for JAK-STAT mutations in cancer. Trends Biochem Sci. 2008;33:122–131. doi: 10.1016/j.tibs.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Clark JD, Flanagan ME, Telliez JB. Discovery and development of Janus kinase (JAK) inhibitors for inflammatory diseases. J Med Chem. 2014;57:5023–5038. doi: 10.1021/jm401490p. [DOI] [PubMed] [Google Scholar]
- 18.Yu H, Jove R. The STATs of cancer--new molecular targets come of age. Nat Rev Cancer. 2004;4:97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- 19.Bandaranayake RM, Ungureanu D, Shan Y, Shaw DE, Silvennoinen O, Hubbard SR. Crystal structures of the JAK2 pseudokinase domain and the pathogenic mutant V617F. Nat Struct Mol Biol. 2012;19:754–759. doi: 10.1038/nsmb.2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geron I, Abrahamsson AE, Barroga CF, Kavalerchik E, Gotlib J, Hood JD, Durocher J, Mak CC, Noronha G, Soll RM, et al. Selective inhibition of JAK2-driven erythroid differentiation of polycythemia vera progenitors. Cancer Cell. 2008;13:321–330. doi: 10.1016/j.ccr.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 21.Tefferi A. JAK and MPL mutations in myeloid malignancies. Leuk Lymphoma. 2008;49:388–397. doi: 10.1080/10428190801895360. [DOI] [PubMed] [Google Scholar]
- 22.Staerk J, Constantinescu SN. The JAK-STAT pathway and hematopoietic stem cells from the JAK2 V617F perspective. JAK-STAT. 2012;1:184–190. doi: 10.4161/jkst.22071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stein BL, Williams DM, Rogers O, Isaacs MA, Spivak JL, Moliterno AR. Disease burden at the progenitor level is a feature of primary myelofibrosis: a multivariable analysis of 164 JAK2 V617F-positive myeloproliferative neoplasm patients. Exp Hematol. 2011;39:95–101. doi: 10.1016/j.exphem.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steensma DP, Dewald GW, Lasho TL, Powell HL, McClure RF, Levine RL, Gilliland DG, Tefferi A. The JAK2 V617F activating tyrosine kinase mutation is an infrequent event in both “atypical” myeloproliferative disorders and myelodysplastic syndromes. Blood. 2005;106:1207–1209. doi: 10.1182/blood-2005-03-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kralovics R, Teo SS, Li S, Theocharides A, Buser AS, Tichelli A, Skoda RC. Acquisition of the V617F mutation of JAK2 is a late genetic event in a subset of patients with myeloproliferative disorders. Blood. 2006;108:1377–1380. doi: 10.1182/blood-2005-11-009605. [DOI] [PubMed] [Google Scholar]
- 26.Pietra D, Li S, Brisci A, Passamonti F, Rumi E, Theocharides A, Ferrari M, Gisslinger H, Kralovics R, Cremonesi L, et al. Somatic mutations of JAK2 exon 12 in patients with JAK2 (V617F)-negative myeloproliferative disorders. Blood. 2008;111:1686–1689. doi: 10.1182/blood-2007-07-101576. [DOI] [PubMed] [Google Scholar]
- 27.Lippert E, Boissinot M, Kralovics R, Girodon F, Dobo I, Praloran V, Boiret-Dupré N, Skoda RC, Hermouet S. The JAK2-V617F mutation is frequently present at diagnosis in patients with essential thrombocythemia and polycythemia vera. Blood. 2006;108:1865–1867. doi: 10.1182/blood-2006-01-013540. [DOI] [PubMed] [Google Scholar]
- 28.Kilpivaara O, Mukherjee S, Schram AM, Wadleigh M, Mullally A, Ebert BL, Bass A, Marubayashi S, Heguy A, Garcia-Manero G, et al. A germline JAK2 SNP is associated with predisposition to the development of JAK2(V617F)-positive myeloproliferative neoplasms. Nat Genet. 2009;41:455–459. doi: 10.1038/ng.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Villeval JL, James C, Pisani DF, Casadevall N, Vainchenker W. New insights into the pathogenesis of JAK2 V617F-positive myeloproliferative disorders and consequences for the management of patients. Semin Thromb Hemost. 2006;32:341–351. doi: 10.1055/s-2006-942755. [DOI] [PubMed] [Google Scholar]
- 30.Jeong EG, Kim SH, Kim MS, Lee SH, Yoo NJ. Absence of JAK2 exon 12 mutation in acute leukemias. Acta Haematol. 2008;119:38–39. doi: 10.1159/000114205. [DOI] [PubMed] [Google Scholar]
- 31.Jeong EG, Kim MS, Nam HK, Min CK, Lee S, Chung YJ, Yoo NJ, Lee SH. Somatic mutations of JAK1 and JAK3 in acute leukemias and solid cancers. Clin Cancer Res. 2008;14:3716–3721. [Google Scholar]
- 32.dos Santos NR, Rickman DS, de Reynies A, Cormier F, Williame M, Blanchard C, Stern MH, Ghysdael J. Pre-TCR expression cooperates with TEL-JAK2 to transform immature thymocytes and induce T-cell leukemia. Blood. 2007;109:3972–3981. doi: 10.1182/blood-2006-09-048801. [DOI] [PubMed] [Google Scholar]
- 33.Kennedy JA, Barabé F, Patterson BJ, Bayani J, Squire JA, Barber DL, Dick JE. Expression of TEL-JAK2 in primary human hematopoietic cells drives erythropoietin-independent erythropoiesis and induces myelofibrosis in vivo . Proc Natl Acad Sci USA. 2006;103:16930–16935. doi: 10.1073/pnas.0604902103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roncero AM, López-Nieva P, Cobos-Fernández MA, Villa-Morales M, González-Sánchez L, López-Lorenzo JL, Llamas P, Ayuso C, Rodríguez-Pinilla SM, Arriba MC, et al. Contribution of JAK2 mutations to T-cell lymphoblastic lymphoma development. Leukemia. 2016;30:94–103. doi: 10.1038/leu.2015.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwaller J, Parganas E, Wang D, Cain D, Aster JC, Williams IR, Lee CK, Gerthner R, Kitamura T, Frantsve J, et al. Stat5 is essential for the myelo- and lymphoproliferative disease induced by TEL/JAK2. Mol Cell. 2000;6:693–704. doi: 10.1016/s1097-2765(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 36.Monni R, Santos SC, Mauchauffe M, Berger R, Ghysdael J, Gouilleux F, Gisselbrecht S, Bernard O, Penard-Lacronique V. The TEL-Jak2 oncoprotein induces Socs1 expression and altered cytokine response in Ba/F3 cells. Oncogene. 2001;20:849–858. doi: 10.1038/sj.onc.1204201. [DOI] [PubMed] [Google Scholar]
- 37.dos Santos NR, Ghysdael J. A transgenic mouse model for TEL-JAK2-induced B-cell lymphoma/leukemia. Leukemia. 2006;20:182–185. doi: 10.1038/sj.leu.2404026. [DOI] [PubMed] [Google Scholar]
- 38.Onnebo SM, Rasighaemi P, Kumar J, Liongue C, Ward AC. Alternative TEL-JAK2 fusions associated with T-cell acute lymphoblastic leukemia and atypical chronic myelogenous leukemia dissected in zebrafish. Haematologica. 2012;97:1895–1903. doi: 10.3324/haematol.2012.064659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cramer K, Nieborowska-Skorska M, Koptyra M, Slupianek A, Penserga ET, Eaves CJ, Aulitzky W, Skorski T. BCR/ABL and other kinases from chronic myeloproliferative disorders stimulate single-strand annealing, an unfaithful DNA double-strand break repair. Cancer Res. 2008;68:6884–6888. doi: 10.1158/0008-5472.CAN-08-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dierov J, Xu Q, Dierova R, Carroll M. TEL/platelet-derived growth factor receptor beta activates phosphatidylinositol 3 (PI3) kinase and requires PI3 kinase to regulate the cell cycle. Blood. 2002;99:1758–1765. doi: 10.1182/blood.v99.5.1758. [DOI] [PubMed] [Google Scholar]
- 41.Yokota A, Hirai H, Shoji T, Maekawa T, Okuda K. Constitutively active ABL family kinases, TEL/ABL and TEL/ARG, harbor distinct leukemogenic activities in vivo. Leukemia. 2017 doi: 10.1038/leu.2017.114. [DOI] [PubMed] [Google Scholar]
- 42.Shain KH, Yarde DN, Meads MB, Huang M, Jove R, Hazlehurst LA, Dalton WS. Beta1 integrin adhesion enhances IL-6-mediated STAT3 signaling in myeloma cells: implications for microenvironment influence on tumor survival and proliferation. Cancer Res. 2009;69:1009–1015. doi: 10.1158/0008-5472.CAN-08-2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chatterjee M, Stühmer T, Herrmann P, Bommert K, Dörken B, Bargou RC. Combined disruption of both the MEK/ERK and the IL-6R/STAT3 pathways is required to induce apoptosis of multiple myeloma cells in the presence of bone marrow stromal cells. Blood. 2004;104:3712–3721. doi: 10.1182/blood-2004-04-1670. [DOI] [PubMed] [Google Scholar]
- 44.Ranger JJ, Levy DE, Shahalizadeh S, Hallett M, Muller WJ. Identification of a Stat3-dependent transcription regulatory network involved in metastatic progression. Cancer Res. 2009;69:6823–6830. doi: 10.1158/0008-5472.CAN-09-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu F, Zhang H, Song H. Upregulation of MEK5 by Stat3 promotes breast cancer cell invasion and metastasis. Oncol Rep. 2017;37:83–90. doi: 10.3892/or.2016.5256. [DOI] [PubMed] [Google Scholar]
- 46.Dhir R, Ni Z, Lou W, DeMiguel F, Grandis JR, Gao AC. Stat3 activation in prostatic carcinomas. Prostate. 2002;51:241–246. doi: 10.1002/pros.10079. [DOI] [PubMed] [Google Scholar]
- 47.Grandis JR, Drenning SD, Zeng Q, Watkins SC, Melhem MF, Endo S, Johnson DE, Huang L, He Y, Kim JD. Constitutive activation of Stat3 signaling abrogates apoptosis in squamous cell carcinogenesis in vivo . Proc Natl Acad Sci USA. 2000;97:4227–4232. doi: 10.1073/pnas.97.8.4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hang D, Yin Y, Wang L, Yuan H, Du J, Zhu M, Dai J, Chen N, Hu Z, Shen H, et al. Effects of potentially functional polymorphisms in suppressor of cytokine signaling 3 (SOCS3) on the risk of head and neck squamous cancer. J Oral Pathol Med. 2016 doi: 10.1111/jop.12539. [DOI] [PubMed] [Google Scholar]
- 49.Concha-Benavente F, Srivastava RM, Trivedi S, Lei Y, Chandran U, Seethala RR, Freeman GJ, Ferris RL. Identification of the cell-intrinsic and -extrinsic pathways downstream of EGFR and IFNγ that induce pd-l1 expression in head and neck cancer. Cancer Res. 2016;76:1031–1043. doi: 10.1158/0008-5472.CAN-15-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Masuda M, Suzui M, Yasumatu R, Nakashima T, Kuratomi Y, Azuma K, Tomita K, Komiyama S, Weinstein IB. Constitutive activation of signal transducers and activators of transcription 3 correlates with cyclin D1 overexpression and may provide a novel prognostic marker in head and neck squamous cell carcinoma. Cancer Res. 2002;62:3351–3355. [PubMed] [Google Scholar]
- 51.Steelman LS, Abrams SL, Whelan J, Bertrand FE, Ludwig DE, Bäsecke J, Libra M, Stivala F, Milella M, Tafuri A, et al. Contributions of the Raf/MEK/ERK, PI3K/PTEN/Akt/mTOR and Jak/STAT pathways to leukemia. Leukemia. 2008;22:686–707. doi: 10.1038/leu.2008.26. [DOI] [PubMed] [Google Scholar]
- 52.Limnander A, Rothman PB. Abl oncogene bypasses normal regulation of Jak/STAT activation. Cell Cycle. 2004;3:1486–1488. doi: 10.4161/cc.3.12.1297. [DOI] [PubMed] [Google Scholar]
- 53.Zhang M, Mathews Griner LA, Ju W, Duveau DY, Guha R, Petrus MN, Wen B, Maeda M, Shinn P, Ferrer M, et al. Selective targeting of JAK/STAT signaling is potentiated by Bcl-xL blockade in IL-2-dependent adult T-cell leukemia. Proc Natl Acad Sci USA. 2015;112:12480–12485. doi: 10.1073/pnas.1516208112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wen R, Wang D, McKay C, Bunting KD, Marine JC, Vanin EF, Zambetti GP, Korsmeyer SJ, Ihle JN, Cleveland JL. Jak3 selectively regulates Bax and Bcl-2 expression to promote T-cell development. Mol Cell Biol. 2001;21:678–689. doi: 10.1128/MCB.21.2.678-689.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, Coussens LM. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16:91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Telliez JB, Dowty ME, Wang L, Jussif J, Lin T, Li L, Moy E, Balbo P, Li W, Zhao Y, et al. Discovery of a JAK3-selective inhibitor: functional differentiation of jak3-selective inhibition over pan-JAK or JAK1-selective inhibition. ACS Chem Biol. 2016;11:3442–3451. doi: 10.1021/acschembio.6b00677. [DOI] [PubMed] [Google Scholar]
- 57.Narla RK, Liu XP, Myers DE, Uckun FM. 4-(3′-Bromo-4'hydroxylphenyl)-amino-6,7-dimethoxyquinazoline: a novel quinazoline derivative with potent cytotoxic activity against human glioblastoma cells. Clin Cancer Res. 1998;4:1405–1414. [PubMed] [Google Scholar]
- 58.Zhang H, Zhang YK, Wang YJ, Kathawala RJ, Patel A, Zhu H, Sodani K, Talele TT, Ambudkar SV, Chen ZS, et al. WHI-P154 enhances the chemotherapeutic effect of anticancer agents in ABCG2-overexpressing cells. Cancer Sci. 2014;105:1071–1078. doi: 10.1111/cas.12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Linwong W, Hirasawa N, Aoyama S, Hamada H, Saito T, Ohuchi K. Inhibition of the antigen-induced activation of rodent mast cells by putative Janus kinase 3 inhibitors WHI-P131 and WHI-P154 in a Janus kinase 3-independent manner. Br J Pharmacol. 2005;145:818–828. doi: 10.1038/sj.bjp.0706240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Changelian PS, Moshinsky D, Kuhn CF, Flanagan ME, Munchhof MJ, Harris TM, Whipple DA, Doty JL, Sun J, Kent CR, et al. The specificity of JAK3 kinase inhibitors. Blood. 2008;111:2155–2157. doi: 10.1182/blood-2007-09-115030. [DOI] [PubMed] [Google Scholar]
- 61.Stepkowski SM, Erwin-Cohen RA, Behbod F, Wang ME, Qu X, Tejpal N, Nagy ZS, Kahan BD, Kirken RA. Selective inhibitor of Janus tyrosine kinase 3, PNU156804, prolongs allograft survival and acts synergistically with cyclosporine but additively with rapamycin. Blood. 2002;99:680–689. doi: 10.1182/blood.v99.2.680. [DOI] [PubMed] [Google Scholar]
- 62.Rashid S, Bibi N, Parveen Z, Shafique S. Inhibition of Janus kinases by tyrosine phosphorylation inhibitor, Tyrphostin AG-490. J Biomol Struct Dyn. 2015;33:2368–2379. doi: 10.1080/07391102.2015.1050696. [DOI] [PubMed] [Google Scholar]
- 63.Sewgobind VD, Quaedackers ME, van der Laan LJ, Kraaijeveld R, Korevaar SS, Chan G, Weimar W, Baan CC. The Jak inhibitor CP-690,550 preserves the function of CD4CD25FoxP3 regulatory T cells and inhibits effector T cells. Am J Transplant. 2010;10:1785–1795. doi: 10.1111/j.1600-6143.2010.03200.x. [DOI] [PubMed] [Google Scholar]
- 64.Ju W, Zhang M, Jiang JK, Thomas CJ, Oh U, Bryant BR, Chen J, Sato N, Tagaya Y, Morris JC, et al. CP-690,550, a therapeutic agent, inhibits cytokine-mediated Jak3 activation and proliferation of T cells from patients with ATL and HAM/TSP. Blood. 2011;117:1938–1946. doi: 10.1182/blood-2010-09-305425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hart S, Goh KC, Novotny-Diermayr V, Tan YC, Madan B, Amalini C, Ong LC, Kheng B, Cheong A, Zhou J, et al. Pacritinib (SB1518), a JAK2/FLT3 inhibitor for the treatment of acute myeloid leukemia. Blood Cancer J. 2011;1:e44. doi: 10.1038/bcj.2011.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Verstovsek S, Tam CS, Wadleigh M, Sokol L, Smith CC, Bui LA, Song C, Clary DO, Olszynski P, Cortes J, et al. Phase I evaluation of XL019, an oral, potent, and selective JAK2 inhibitor. Leuk Res. 2014;38:316–322. doi: 10.1016/j.leukres.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McCarthy AA. Exelixis: integrated drug-discovery and development platform for human therapeutics. Chem Biol. 2005;12:407–408. doi: 10.1016/j.chembiol.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 68.Moran N. Incyte comes of age with JAK inhibitor approval. Nat Biotechnol. 2012;30:3–5. doi: 10.1038/nbt0112-3. [DOI] [PubMed] [Google Scholar]
- 69.Pardanani A, Gotlib JR, Jamieson C, Cortes JE, Talpaz M, Stone RM, Silverman MH, Gilliland DG, Shorr J, Tefferi A. Safety and efficacy of TG101348, a selective JAK2 inhibitor, in myelofibrosis. J Clin Oncol. 2011;29:789–796. doi: 10.1200/JCO.2010.32.8021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hexner EO, Serdikoff C, Jan M, Swider CR, Robinson C, Yang S, Angeles T, Emerson SG, Carroll M, Ruggeri B, et al. Lestaurtinib (CEP701) is a JAK2 inhibitor that suppresses JAK2/STAT5 signaling and the proliferation of primary erythroid cells from patients with myeloproliferative disorders. Blood. 2008;111:5663–5671. doi: 10.1182/blood-2007-04-083402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Verstovsek S, Kantarjian H, Mesa RA, Pardanani AD, Cortes-Franco J, Thomas DA, Estrov Z, Fridman JS, Bradley EC, Erickson-Viitanen S, et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N Engl J Med. 2010;363:1117–1127. doi: 10.1056/NEJMoa1002028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shi JG, Chen X, Emm T, Scherle PA, McGee RF, Lo Y, Landman RR, McKeever EG, Jr, Punwani NG, Williams WV, et al. The effect of CYP3A4 inhibition or induction on the pharmacokinetics and pharmacodynamics of orally administered ruxolitinib (INCB018424 phosphate) in healthy volunteers. J Clin Pharmacol. 2012;52:809–818. doi: 10.1177/0091270011405663. [DOI] [PubMed] [Google Scholar]
- 73.Aubert L, Guilbert M, Corbet C, Génot E, Adriaenssens E, Chassat T, Bertucci F, Daubon T, Magné N, Le Bourhis X, et al. NGF-induced TrkA/CD44 association is involved in tumor aggressiveness and resistance to lestaurtinib. Oncotarget. 2015;6:9807–9819. doi: 10.18632/oncotarget.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Knapper S, Burnett AK, Littlewood T, Kell WJ, Agrawal S, Chopra R, Clark R, Levis MJ, Small D. A phase 2 trial of the FLT3 inhibitor lestaurtinib (CEP701) as first-line treatment for older patients with acute myeloid leukemia not considered fit for intensive chemotherapy. Blood. 2006;108:3262–3270. doi: 10.1182/blood-2006-04-015560. [DOI] [PubMed] [Google Scholar]
- 75.Hexner EO, Mascarenhas J, Prchal J, Roboz GJ, Baer MR, Ritchie EK, Leibowitz D, Demakos EP, Miller C, Siuty J, et al. Phase I dose escalation study of lestaurtinib in patients with myelofibrosis. Leuk Lymphoma. 2015;56:2543–2551. doi: 10.3109/10428194.2014.1001986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Minturn JE, Evans AE, Villablanca JG, Yanik GA, Park JR, Shusterman S, Groshen S, Hellriegel ET, Bensen-Kennedy D, Matthay KK, et al. Phase I trial of lestaurtinib for children with refractory neuroblastoma: a new approaches to neuroblastoma therapy consortium study. Cancer Chemother Pharmacol. 2011;68:1057–1065. doi: 10.1007/s00280-011-1581-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Diaz T, Navarro A, Ferrer G, Gel B, Gaya A, Artells R, Bellosillo B, Garcia-Garcia M, Serrano S, Martínez A, et al. Lestaurtinib inhibition of the Jak/STAT signaling pathway in hodgkin lymphoma inhibits proliferation and induces apoptosis. PLoS One. 2011;6:e18856. doi: 10.1371/journal.pone.0018856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Soderquist C, Bagg A. Coexistent BCR-ABL1 and JAK2 V617F: converting CML dwarves to ET staghorns with imatinib therapy. Blood. 2014;124:2463. doi: 10.1182/blood-2014-06-585141. [DOI] [PubMed] [Google Scholar]
- 79.Qian XL, Zhang J, Li PZ, Lang RG, Li WD, Sun H, Liu FF, Guo XJ, Gu F, Fu L. Dasatinib inhibits c-src phosphorylation and prevents the proliferation of Triple-Negative Breast Cancer (TNBC) cells which overexpress Syndecan-Binding Protein (SDCBP) PLoS One. 2017;12:e0171169. doi: 10.1371/journal.pone.0171169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhou A, Knoche EM, Engle EK, Fisher DA, Oh ST. Concomitant JAK2 V617F-positive polycythemia vera and BCR-ABL-positive chronic myelogenous leukemia treated with ruxolitinib and dasatinib. Blood Cancer J. 2015;5:e351. doi: 10.1038/bcj.2015.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lang L. FDA approves sorafenib for patients with inoperable liver cancer. Gastroenterology. 2008;134:379. doi: 10.1053/j.gastro.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 82.Rahmani M, Aust MM, Attkisson E, Williams DC, Jr, Ferreira-Gonzalez A, Grant S. Inhibition of Bcl-2 antiapoptotic members by obatoclax potently enhances sorafenib-induced apoptosis in human myeloid leukemia cells through a Bim-dependent process. Blood. 2012;119:6089–6098. doi: 10.1182/blood-2011-09-378141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huynh H, Ngo VC, Choo SP, Poon D, Koong HN, Thng CH, Toh HC, Zheng L, Ong LC, Jin Y, et al. Sunitinib (SUTENT, SU11248) suppresses tumor growth and induces apoptosis in xenograft models of human hepatocellular carcinoma. Curr Cancer Drug Targets. 2009;9:738–747. doi: 10.2174/156800909789271530. [DOI] [PubMed] [Google Scholar]
- 84.Yang F, Jove V, Xin H, Hedvat M, Van Meter TE, Yu H. Sunitinib induces apoptosis and growth arrest of medulloblastoma tumor cells by inhibiting STAT3 and AKT signaling pathways. Mol Cancer Res. 2010;8:35–45. doi: 10.1158/1541-7786.MCR-09-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Finke J, Ko J, Rini B, Rayman P, Ireland J, Cohen P. MDSC as a mechanism of tumor escape from sunitinib mediated anti-angiogenic therapy. Int Immunopharmacol. 2011;11:856–861. doi: 10.1016/j.intimp.2011.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Adotevi O, Pere H, Ravel P, Haicheur N, Badoual C, Merillon N, Medioni J, Peyrard S, Roncelin S, Verkarre V, et al. A decrease of regulatory T cells correlates with overall survival after sunitinib-based antiangiogenic therapy in metastatic renal cancer patients. J Immunother. 2010;33:991–998. doi: 10.1097/CJI.0b013e3181f4c208. [DOI] [PubMed] [Google Scholar]