Abstract
Tumor necrosis factor-α (TNF-α) induces serum amyloid A (SAA) 3 among acute-phase proteins in mouse granulosa cells by activating NF-κB signaling via p55 TNF-α receptor type 1. However, the localization of SAA3 within the ovary is unknown. Here we investigated ovarian localization of SAA3 in a mouse ovulation model and in response to IL-1β, a proinflammatory mediator. For the ovulation model, equine chorionic gonadotropin (eCG; 2.5 IU) was administered to mice subcutaneously (sc) to stimulate follicular development on day 25 of age and then 50 h after eCG, human chorionic gonadotropin (hCG; 2.5 IU) was administered sc to induce ovulation. The mouse ovulation model was characterized by the localization of CYP19 mRNA expression to granulosa layers of larger follicles. SAA3 mRNA, determined by in situ hybridization, was broadly expressed throughout the whole ovary. Granulosa layers and small follicles expressed higher SAA3 mRNA compared to thecal-interstitial layers and large follicles, respectively. Interestingly, atretic follicles contained cells expressing intense SAA3 mRNA. After ovulation, SAA3 mRNA expression was intensely evident in ruptured follicles and corpora lutea (CL). The intraperitoneal administration of IL-1β revealed the intense and extensive appearance of specific cells expressing SAA3 mRNA around follicles and in CL. In addition, Gene Expression Omnibus (GEO) database analysis supported expression pattern of SAA3 mRNA observed in mouse ovulation model. Taken together, SAA3 was broadly distributed through the whole ovary, but intensely expressed in atretic follicles and CL. Furthermore, proinflammatory mediators could trigger the intense appearance of SAA3 around follicles and in CL.
Keywords: Serum amyloid A, Ovulation, Ovary
INTRODUCTION
Acute-phase serum amyloid A (SAA) is primarily expressed in the liver in response to inflammation or infections (1,2). Recently, extrahepatic expression of SAA has been elucidated in many tumor (3), inflammatory (4), obese (5,6), and normal tissues (2). Human and mouse have 4 SAA isoforms (SAA1, 2, 3, and 4) (7). In human, SAA1 and SAA2 are mainly synthesized during acute-phase inflammatory reactions (8) and share approximately 95% of overall sequence identity in their promoter regions, exons, and introns (9). On the other hand, human SAA3 is pseudogene and SAA4 is constitutively expressed. Human SAA1/2 is comparable to mouse SAA3 which is expressed abundantly and extrahepatically (10). SAA is released in response to proinflammatory cytokines such as IL-6, IL-1, and tumor necrosis factor-α (TNF-α). The concentration levels of SAA during inflammation reach up to 1,000-fold greater than those in the non-inflammatory state.
Reproductive processes such as ovulation, menstruation, implantation, and labor are comparable to inflammatory processes (11,12,13). Inflammation is an active process which involves the release of inflammatory cytokines, chemokines, and peptide growth factors. All of reproductive events involve upregulation of inflammatory mediators such as cytokines, and growth factors (11). In particular, cytokines expressed locally in the granulosa cells and endometrial tissue cause ovulation and implantation, respectively. Moreover, ovarian hormones are likely to be mediators of inflammatory process (11). Furthermore, nonsteroidal anti-inflammatory agents such as indomethacin inhibit ovulation in mammalian species (14).
Our previous study revealed that TNF-α specifically and abundantly increased SAA3 among acute-phase proteins in mouse granulosa cells (15). TNF-α, a multi-functional hormone-like polypeptide, is well known to modulate many genes involved in inflammation, infection, and malignancy. In particular, ovarian TNF-α locally regulates steroidogenesis, folliculogenesis, ovulation, and fertility (16,17,18,19). Although SAA3 is the most abundant gene induced by TNF-α in granulosa cells (7,15), the ovarian localization of SAA3 is unknown. Thus, the present study was designed to investigate the localization of SAA3 in the mouse ovary using both a mouse ovulation model and model of inflammatory condition.
MATERIALS AND METHODS
Animal handling
C57BL6 female mice from Harlan, Inc. (Indianapolis, IN, USA) were used from breeding colonies in our laboratory. All handling of animals and procedures were approved by the Institutional Animal Care and Use Committee at the University of Kansas Medical Center.
Mouse ovulation model and ovarian inflammatory condition
For mouse ovulation model, mice were subcutaneously (sc) injected with 2.5 IU equine chorionic gonadotropin (eCG; Sigma-Adrich, St. Louis, MO, USA) on day 25 to initiate follicle development for 48 h. Fifty hours after eCG, 2.5 IU human chorionic gonadotropin (hCG; Sigma-Adrich) was administered sc to induce ovulation and luteinization by 72 h (Fig. 1). Mice were euthanized at the indicated time beginning on day 25 through day 28 and ovaries were collected for 10 μm frozen sectioning and in situ hybridization. For ovarian inflammatory condition, 2 months old mice were treated intraperitoneally with IL-1β (1 μg/mouse), were euthanized at 2 h post-injection and ovaries were collected for frozen sectioning and in situ hybridization.
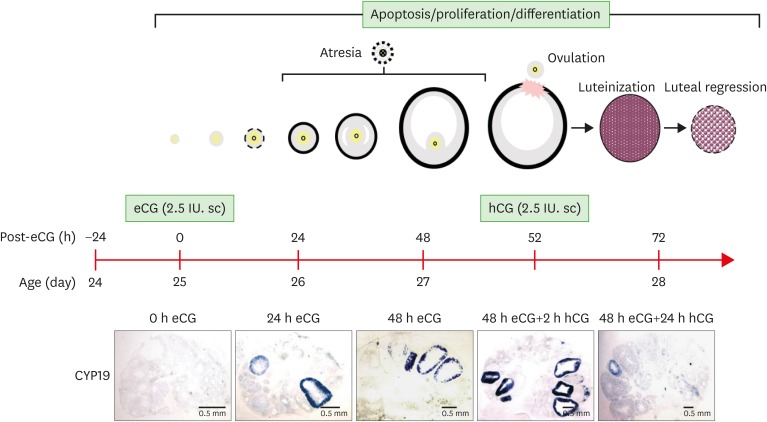
Figure 1.
Schematic of mouse ovulation model and ovarian localization of CYP19 mRNA. Ovaries were collected at 0, 24, 48 h after eCG (2.5 IU) to initiate follicular development, and 2, 24 h after hCG (2.5 IU) to induce ovulation and luteinization. The mouse ovulation model is characterized by the expected time- and cell-specific expression of CYP19 mRNA using in situ hybridization with digoxigenin CYP19 RNA probe.
Granulosa cell culture and collection
Primary culture of granulosa cells was performed as described previously (7,15,20). Mouse granulosa cells were collected immediately from ovaries of mice during the ovulation model and after 2 h intraperitoneal lipopolysaccharide (LPS; 100 μg/mouse) challenge as indicated in Results and Discussion.
Digoxigenin CYP19 and SAA3 RNA probes
Total RNA was isolated using TRIzol® reagent (Invitrogen, Grand Island, NY, USA). The reverse transcription (RT) reaction conditions, using random primers with M-MLV (Invitrogen), were at 42°C for 60 min, followed by 94°C for 10 min. Specific primers for mouse CYP19 aromatase and SAA3 were designed as follows: 5′-TCA ATA CCA GGT CCT GGC TA-3′ (forward) and 5′-TGC TTG ATG GAC TCC ACA CA-3′ (reversed) for CYP19; and 5′-AGC CTT CCA TTG CCA TCA TTC TT-3′ (forward) and 5′-AGT ATC TTT TAG GCA GGC CAG CA-3′ (reversed) for SAA3. PCR was performed in 30-cycle increments under the following conditions: denaturation at 94°C for 1 min; annealing at 58°C for 1 min; and extension at 74°C for 1 min. Amplified PCR product (374 bp) was analyzed by electrophoresis in 2% agarose gels containing 1 µg ethidium bromide/ml and was purified by a spin-column (Gel Extraction System; Qiagen, Valencia, CA, USA) to absorb the DNA. The PCR products were inserted into the pGEM®-T vector (Promega, Madison, WI, USA). Antisense and sense linear template DNAs were made by digestion with NcoI and SpeI enzymes, respectively, according to inserted orientation. Linear template DNAs were labeled with digoxigenin-UTP (Roche, Indianapolis, IN, USA) by in vitro transcription with SP6 (antisense) and T7 (sense) RNA polymerase as described previously (20).
In situ hybridization
Nonradioactive methods for in situ hybridization on frozen sections were performed using digoxigenin-labeled CYP19 and SAA3 RNA probes (antisense and sense) as described previously (20).
RT-PCR
Total RNA was isolated using TRIzol® reagents. RT-PCR was performed as described previously (7,15,20), using specific primers for mouse SAA3 as follows: 5′-AGC CTT CCA TTG CCA TCA TTC TT-3′ (sense) and 5′-AGT ATC TTT TAG GCA GGC CAG CA-3′ (antisense). L19 was used as a loading control (21).
Data analysis from Gene Expression Omnibus (GEO) dataset
Data analysis was performed using microarray data sets deposited in the National Center for Biotechnology Information (NCBI) GEO (https://www.ncbi.nlm.nih.gov/geo/) database under accession number GSE23084 and GDS1677. We employed Gitools 2.2.3 (Biomedical Genomics Group, Barcelona, Spain; http://www.gitools.org) based on Oracle Java 7, an open-source tool to perform Genomic Data Analysis and Visualization as interactive heat-maps (22) as described previously (23).
RESULTS AND DISCUSSION
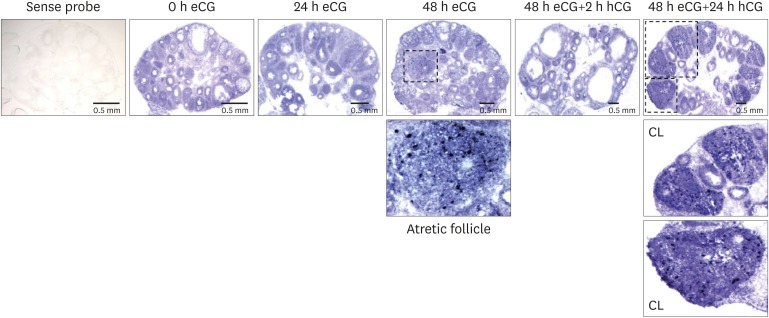
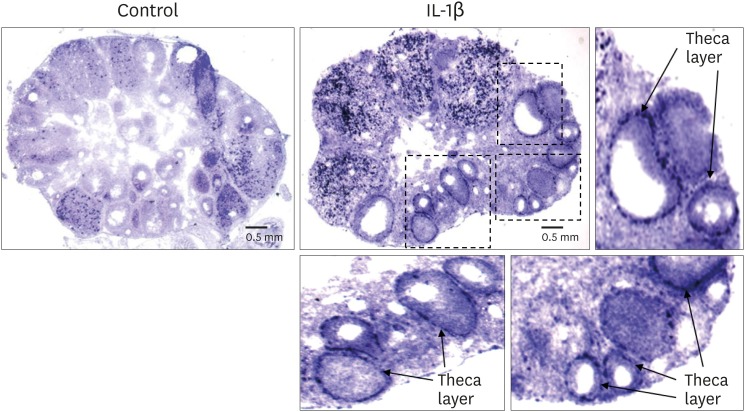
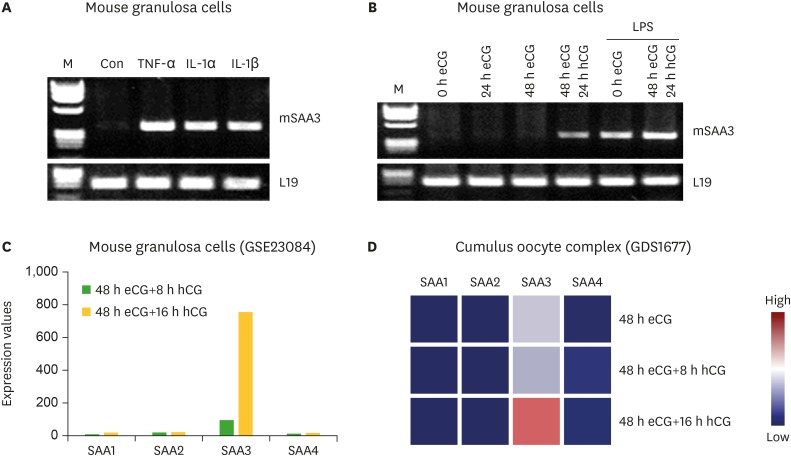
The localization of SAA3 mRNA in the whole ovary was performed in mouse ovulation model using in situ hybridization as described previously (7,20). Ovaries were collected at post-eCG injection (0, 24, and 48 h) and post-hCG (2 and 24 h) (Fig. 1). First, ovarian CYP19 (aromatase) mRNA was localized to characterize and validate the mouse ovulation model (Fig. 1). CYP19 mRNAs are expressed in the granulosa layer of large follicles during follicular development, with maximal expression occurring immediately after ovulation and loss of expression with luteinization (Fig. 1) as anticipated (24,25). The expression pattern of ovarian CYP19 mRNA indicates that the mouse ovulation model reflects the physiological process of follicular development and ovulation. Next, we determined the localization of SAA3 mRNA expression in the whole ovaries using the mouse ovulation model. We confirmed no signal with sense SAA3 probe (Fig. 2). SAA3 mRNA is lightly and broadly expressed throughout the whole ovary even prior to eCG injection (Fig. 2). During follicle development, granulosa cells express higher SAA3 mRNA than thecal-interstitial cells, and small follicles express higher SAA3 mRNA than large follicles (Fig. 2). Interestingly, SAA3 mRNA is intensely expressed in ovarian follicle atresia (Fig. 2) in which immature ovarian follicles are degenerated by granulosa cell apoptosis. Because atresia in ovarian follicles involves atretogenic factors such as TNF-α and Fas ligands (26), these factors are likely to induce SAA3 mRNA which is strictly regulated by NF-κB signaling (7,15). At 72 h ovary (48 h post-eCG and 24 h post-hCG injections), corpora lutea (CL) contain cells with intense SAA3 mRNA hydridization (Fig. 2). Because TNF-α is a product of luteal macrophages that infiltrate into the CL (27), immune cell-derived proinflammatory cytokines are likely to induce SAA3 during CL formation and luteal regression. NF-κB mediated signaling is primary pathway inducing SAA3 expression in mouse granulosa cells (7,15). Therefore, we employed treatment with IL-1β a proinflammatory cytokine activating NF-κB signaling, and assessed localization of SAA3 mRNA by in situ hybridization. Intense SAA3 mRNA hybridization was localized to CL (Fig. 3) similar to the pattern observed in newly formed CL using the ovulation model (Fig. 2). IL-1β-treated ovary has more intense and extensive SAA3 mRNA expression compared to non-treated ovary (Fig. 3). Interestingly, following IL-1b treatment SAA3 mRNA was intensely localized to the thecal-interstitial cell layers in both small and large follicles (Fig. 3). These findings indicate that ovarian follicles likely protect their oocytes by blocking harmful effects due to inflammatory stimulation. Direct effects of proinflammatory cytokines on SAA3 expression were then assessed using cultured primary granulosa cells. In vitro treatment with TNF-α, IL-1α, or IL-1β strongly induces SAA3 mRNA expression in mouse granulosa cells compared to non-treated granulosa cells (Fig. 4A). Granulosa cell-specific expression of SAA3 was further assessed by collection of granulosa throughout the induction of follicle development and ovulation in the mouse ovulation model. Granulosa cells collected at 48 h post-eCG plus 24 h post-hCG injection highly express SAA3 mRNA (Fig. 4B), reflecting CL derived SAA3 expression as indicated in Fig. 2. On the other hand, granulosa cells collected prior to this time point expressed modest SAA3 documented by only a faint band following RT-PCR. SAA3 expression in immature granulosa cells could be stimulated by LPS, a strong inflammatory mediator. In another set of experiments mice were injected intraperitoneally with LPS and then treated with gonadotropins as the ovulation model with granulosa cells collected at various time points. Granulosa cells collected following injection with LPS and prior to eCG injection express increase SAA3 mRNA compared to mice not receiving LPS (Fig. 4B). In addition, SAA3 mRNA expression was further increased in cells collected at the 48 h post-eCG plus 24 h post-hCG time point in LPS treated mice compared to mice not receiving LPS (Fig. 4B). SAA expression in mouse ovaries was further assessed by examination of 2 different GEO data sets. Each data set indicates that SAA3 is the primary SAA isoform in the ovary (Fig. 4C and 4D). Furthermore, SAA3 expression was increased following eCG plus 24 h post hCG (Fig. 4C) similar to the findings of the current study (Fig. 4B). Additional data indicate that SAA3 expression in granulosa cells comprising the cumulus-oocyte-complex is increased following eCG plus 16 h post hCG (Fig. 4D). Together these 2 GEO datasets support the findings of the current study on high expression of SAA3 in CL as shown in Fig. 2.
Figure 2.
Localization of SAA3 mRNA in the mouse ovary during gonadotropin-induced follicle development and ovulation. Hybridization of antisense SAA3-labeled riboprobes was visualized as purple-blue precipitate in the whole ovary at respective time points (0, 24, 48 h post-eCG injection, and 2, 24 h post-hCG injection). Incubation of tissue sections with labeled sense probe revealed no hybridization.
Figure 3.
IL-1β treatment in vivo induced SAA3 mRNA in the mouse ovary. Adult cycle mice (2 months old) were treated intraperitoneally with IL-1β (1 μg/mouse). Mice were euthanized at 2 h post-injection and ovaries were collected for 10 μm frozen sectioning and in situ hybridization.
Figure 4.
Expression of SAA3 mRNA in mouse granulosa cells. (A) Effect of proinflammatory cytokines on SAA3 mRNA in mouse granulosa cells. Mouse granulosa cells were incubated with vehicle, TNF-α (10 ng/mL), IL-1α (10 ng/mL), and IL-1β (10 ng/mL) for 2 h. RT-PCR was performed using SAA3 primers. L19 was used as a loading control. (B) Expression of SAA3 mRNA in mouse granulosa cells collected from ovaries of mice during the ovulation model and after 2 h post-LPS (100 μg/mouse) challenge. (C) Expression levels of SAA isoforms in mouse granulosa cells based on the NCBI GEO dataset (GSE23084). (D) Expression levels of SAA isoforms in cumulus oocyte complex based on the NCBI GEO dataset (GDS1677).
NCBI, National Center for Biotechnology Information; M, molecular marker in base pairs; Con, control.
In conclusion, SAA3 mRNA is broadly expressed by in situ hybridization throughout the whole ovary. In particular, atretic follicles and CL express intensely elevated levels of SAA3 mRNA. In addition, proinflammatory conditions such as treatment with LPS or IL-1β potentiate SAA3 mRNA expression in CL and in thecal-interstitial layers around follicles. Further study will be required to identify the specific cell type expressing SAA3, however, these results indicate that SAA3 is likely to play a role in ovarian follicular atresia and luteal formation and regression.
ACKNOWLEDGEMENTS
This research was supported, in whole or in part, by National Institutes of Health (NIH) as the following grants: NIGMS SC1 089630 and R01ES024756 (Eun-Sook Lee), NCI CA50616 (Paul F. Terranova), and NIAID SC1AI089073, NCI SC1CA200519 and U54CA163069 (Deok-Soo Son). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIH.
Abbreviations
- CL
corpora lutea
- eCG
equine chorionic gonadotropin
- GEO
Gene Expression Omnibus
- hCG
human chorionic gonadotropin
- LPS
lipopolysaccharide
- SAA
serum amyloid A
- sc
subcutaneously
- TNF-α
tumor necrosis factor-α
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest.
Author Contributions: Conceptualization: Terranova PF, Son DS. Data curation: Choi H, Son DS. Formal analysis: Choi H, Roby KF, Terranova PF, Son DS. Funding acquisition: Lee ES, Terranova PF, Son DS. Investigation: Choi H, Ignacio RMC, Roby KF, Son DS. Methodology: Choi H, Ignacio RMC, Son DS. Project administration: Lee ES, Roby KF, Terranova PF, Son DS. Resources: Lee ES, Roby KF, Terranova PF, Son DS. Software: Choi H, Ignacio RMC, Son DS. Supervision: Terranova PF, Son DS. Validation: Choi H, RMI, Lee ES, Roby KF, Terranova PF, Son DS. Visualization: Choi H, Ignacio RMC, Lee ES, Roby KF, Terranova PF, Son DS. Writing - original draft: Choi H, Son DS. Writing - review & editing: Choi H, Ignacio RMC, Lee ES, Roby KF, Terranova PF, Son DS.
References
- 1.Targońska-Stępniak B, Majdan M. Serum amyloid A as a marker of persistent inflammation and an indicator of cardiovascular and renal involvement in patients with rheumatoid arthritis. Mediators Inflamm. 2014;2014:793628. doi: 10.1155/2014/793628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Urieli-Shoval S, Cohen P, Eisenberg S, Matzner Y. Widespread expression of serum amyloid A in histologically normal human tissues. Predominant localization to the epithelium. J Histochem Cytochem. 1998;46:1377–1384. doi: 10.1177/002215549804601206. [DOI] [PubMed] [Google Scholar]
- 3.Urieli-Shoval S, Finci-Yeheskel Z, Dishon S, Galinsky D, Linke RP, Ariel I, Levin M, Ben-Shachar I, Prus D. Expression of serum amyloid a in human ovarian epithelial tumors: implication for a role in ovarian tumorigenesis. J Histochem Cytochem. 2010;58:1015–1023. doi: 10.1369/jhc.2010.956821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meek RL, Urieli-Shoval S, Benditt EP. Expression of apolipoprotein serum amyloid A mRNA in human atherosclerotic lesions and cultured vascular cells: implications for serum amyloid A function. Proc Natl Acad Sci USA. 1994;91:3186–3190. doi: 10.1073/pnas.91.8.3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Z, Nakayama T. Inflammation, a link between obesity and cardiovascular disease. Mediators Inflamm. 2010;2010:535918. doi: 10.1155/2010/535918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang RZ, Lee MJ, Hu H, Pollin TI, Ryan AS, Nicklas BJ, Snitker S, Horenstein RB, Hull K, Goldberg NH, et al. Acute-phase serum amyloid A: an inflammatory adipokine and potential link between obesity and its metabolic complications. PLoS Med. 2006;3:e287. doi: 10.1371/journal.pmed.0030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Son DS, Terranova PF, Roby KF. Interaction of adenosine 3′,5′-cyclic monophosphate and tumor necrosis factor-alpha on serum amyloid A3 expression in mouse granulosa cells: dependence on CCAAT-enhancing binding protein-beta isoform. Endocrinology. 2010;151:3407–3419. doi: 10.1210/en.2009-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takase H, Tanaka M, Miyagawa S, Yamada T, Mukai T. Effect of amino acid variations in the central region of human serum amyloid A on the amyloidogenic properties. Biochem Biophys Res Commun. 2014;444:92–97. doi: 10.1016/j.bbrc.2014.01.029. [DOI] [PubMed] [Google Scholar]
- 9.Uhlar CM, Burgess CJ, Sharp PM, Whitehead AS. Evolution of the serum amyloid A (SAA) protein superfamily. Genomics. 1994;19:228–235. doi: 10.1006/geno.1994.1052. [DOI] [PubMed] [Google Scholar]
- 10.Uhlar CM, Whitehead AS. Serum amyloid A, the major vertebrate acute-phase reactant. Eur J Biochem. 1999;265:501–523. doi: 10.1046/j.1432-1327.1999.00657.x. [DOI] [PubMed] [Google Scholar]
- 11.Jabbour HN, Sales KJ, Catalano RD, Norman JE. Inflammatory pathways in female reproductive health and disease. Reproduction. 2009;138:903–919. doi: 10.1530/REP-09-0247. [DOI] [PubMed] [Google Scholar]
- 12.Espey LL. Ovulation as an inflammatory reaction--a hypothesis. Biol Reprod. 1980;22:73–106. doi: 10.1095/biolreprod22.1.73. [DOI] [PubMed] [Google Scholar]
- 13.Espey LL. Current status of the hypothesis that mammalian ovulation is comparable to an inflammatory reaction. Biol Reprod. 1994;50:233–238. doi: 10.1095/biolreprod50.2.233. [DOI] [PubMed] [Google Scholar]
- 14.Gaytán M, Morales C, Bellido C, Sánchez-Criado JE, Gaytán F. Non-steroidal anti-inflammatory drugs (NSAIDs) and ovulation: lessons from morphology. Histol Histopathol. 2006;21:541–556. doi: 10.14670/HH-21.541. [DOI] [PubMed] [Google Scholar]
- 15.Son DS, Roby KF, Terranova PF. Tumor necrosis factor-alpha induces serum amyloid A3 in mouse granulosa cells. Endocrinology. 2004;145:2245–2252. doi: 10.1210/en.2003-1261. [DOI] [PubMed] [Google Scholar]
- 16.Ghersevich S, Isomaa V, Vihko P. Cytokine regulation of the expression of estrogenic biosynthetic enzymes in cultured rat granulosa cells. Mol Cell Endocrinol. 2001;172:21–30. doi: 10.1016/s0303-7207(00)00396-8. [DOI] [PubMed] [Google Scholar]
- 17.Nakayama M, Manabe N, Inoue N, Matsui T, Miyamoto H. Changes in the expression of tumor necrosis factor (TNF) alpha, TNFalpha receptor (TNFR) 2, and TNFR-associated factor 2 in granulosa cells during atresia in pig ovaries. Biol Reprod. 2003;68:530–535. doi: 10.1095/biolreprod.102.004820. [DOI] [PubMed] [Google Scholar]
- 18.Brännström M, Bonello N, Wang LJ, Norman RJ. Effects of tumour necrosis factor alpha (TNF alpha) on ovulation in the rat ovary. Reprod Fertil Dev. 1995;7:67–73. doi: 10.1071/rd9950067. [DOI] [PubMed] [Google Scholar]
- 19.Roby KF, Son DS, Terranova PF. Alterations of events related to ovarian function in tumor necrosis factor receptor type I knockout mice. Biol Reprod. 1999;61:1616–1621. doi: 10.1095/biolreprod61.6.1616. [DOI] [PubMed] [Google Scholar]
- 20.Son DS, Roby KF. Interleukin-1alpha-induced chemokines in mouse granulosa cells: impact on keratinocyte chemoattractant chemokine, a CXC subfamily. Mol Endocrinol. 2006;20:2999–3013. doi: 10.1210/me.2006-0001. [DOI] [PubMed] [Google Scholar]
- 21.Park-Sarge OK, Mayo KE. Regulation of the progesterone receptor gene by gonadotropins and cyclic adenosine 3′,5′-monophosphate in rat granulosa cells. Endocrinology. 1994;134:709–718. doi: 10.1210/endo.134.2.8299566. [DOI] [PubMed] [Google Scholar]
- 22.Perez-Llamas C, Lopez-Bigas N. Gitools: analysis and visualisation of genomic data using interactive heat-maps. PLoS One. 2011;6:e19541. doi: 10.1371/journal.pone.0019541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ignacio RM, Kabir SM, Lee ES, Adunyah SE, Son DS. NF-κB-mediated CCL20 reigns dominantly in CXCR2-driven ovarian cancer progression. PLoS One. 2016;11:e0164189. doi: 10.1371/journal.pone.0164189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fitzpatrick SL, Carlone DL, Robker RL, Richards JS. Expression of aromatase in the ovary: down-regulation of mRNA by the ovulatory luteinizing hormone surge. Steroids. 1997;62:197–206. doi: 10.1016/s0039-128x(96)00181-x. [DOI] [PubMed] [Google Scholar]
- 25.Inaoka Y, Yazawa T, Mizutani T, Kokame K, Kangawa K, Uesaka M, Umezawa A, Miyamoto K. Regulation of P450 oxidoreductase by gonadotropins in rat ovary and its effect on estrogen production. Reprod Biol Endocrinol. 2008;6:62. doi: 10.1186/1477-7827-6-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaipia A, Hsueh AJ. Regulation of ovarian follicle atresia. Annu Rev Physiol. 1997;59:349–363. doi: 10.1146/annurev.physiol.59.1.349. [DOI] [PubMed] [Google Scholar]
- 27.Gadsby J, Rose L, Sriperumbudur R, Ge Z. The role of intra-luteal factors in the control of the porcine corpus luteum. Soc Reprod Fertil Suppl. 2006;62:69–83. [PubMed] [Google Scholar]