Abstract
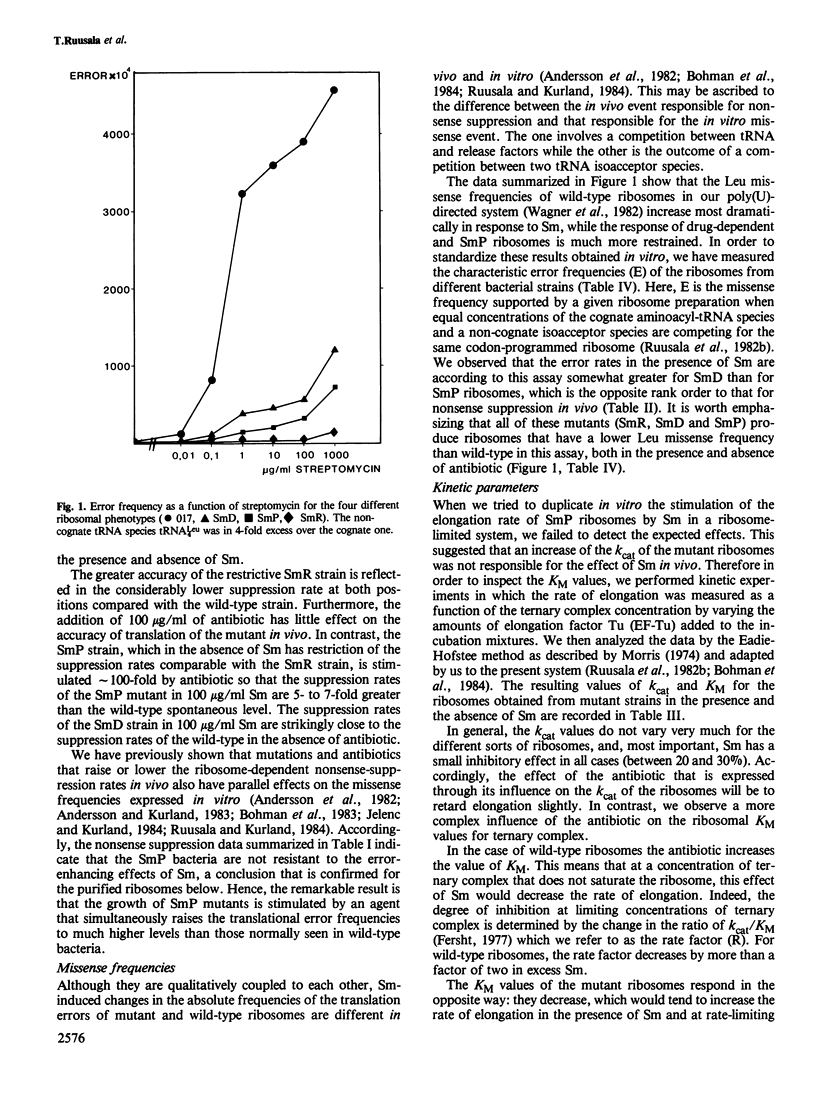
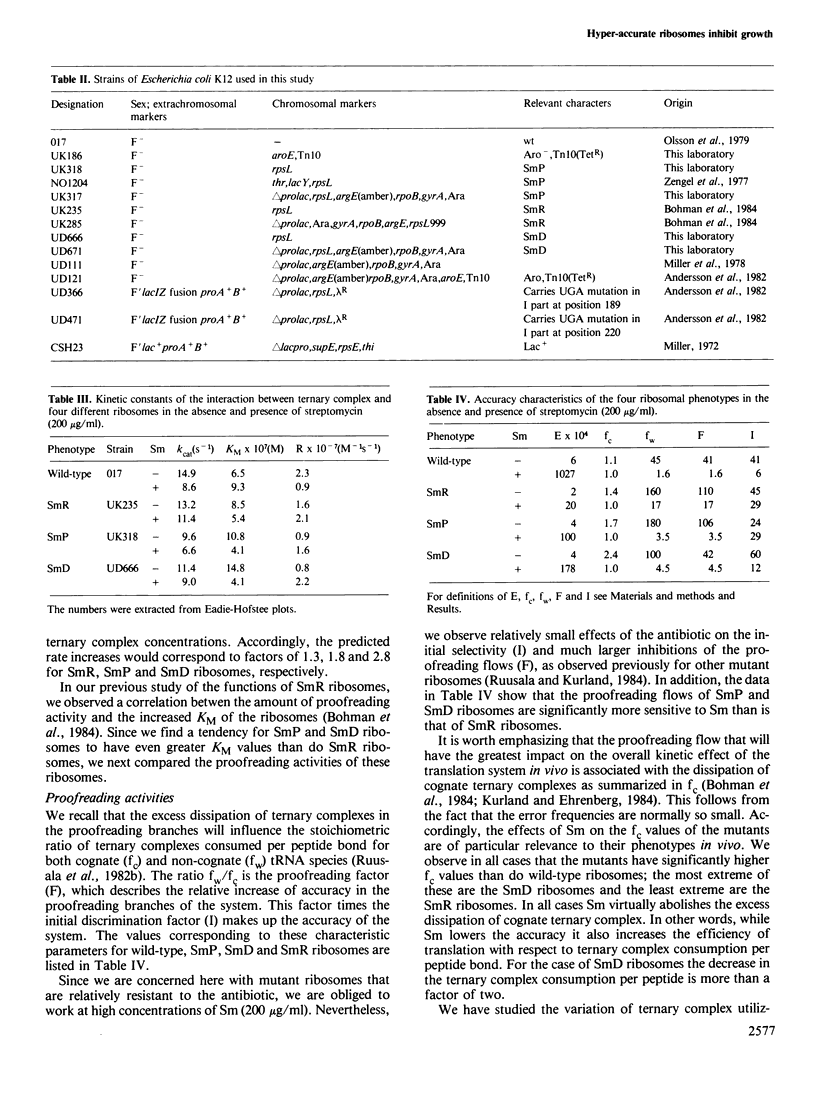
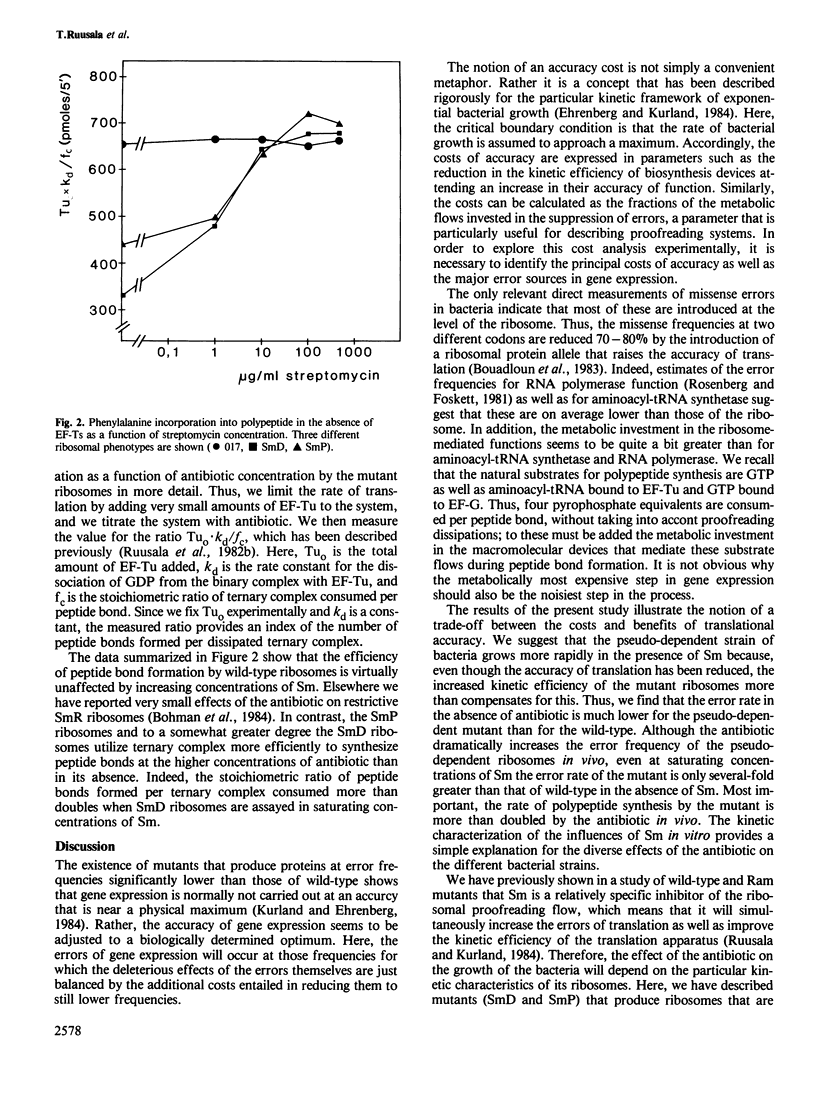
We have compared both in vivo and in vitro translation by ribosomes from wild-type bacteria with those from streptomycin-resistant (SmR), streptomycin-dependent (SmD) and streptomycin-pseudo-dependent (SmP) mutants. The three mutant bacteria translate more accurately and more slowly in the absence of streptomycin (Sm) than do wild-type bacteria. In particular, the SmP bacteria grow at roughly half the rate of the wild-type in the absence of Sm. The antibiotic stimulates both the growth rate and the translation rate of SmP bacteria by approximately 2-fold, but it simultaneously increases the nonsense suppression rate quite dramatically. Kinetic experiments in vitro show that the greater accuracy and slower translation rates of mutant ribosomes compared with wild-type ribosomes are associated with much more rigorous proofreading activities of SmR, SmD and SmP ribosomes. Sm reduces the proofreading flows of the mutant ribosomes and stimulates their elongation rates. The data suggest that these excessively accurate ribosomes are kinetically less efficient than wild-type ribosomes, and that this inhibits mutant growth rates. The stimulation of the growth of the mutants by Sm results from the enhanced translational efficiency due to the loss of proofreading, which more than offsets the loss of accuracy caused by the antibiotic.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson D. I., Bohman K., Isaksson L. A., Kurland C. G. Translation rates and misreading characteristics of rpsD mutants in Escherichia coli. Mol Gen Genet. 1982;187(3):467–472. doi: 10.1007/BF00332630. [DOI] [PubMed] [Google Scholar]
- Andersson D. I., Kurland C. G. Ram ribosomes are defective proofreaders. Mol Gen Genet. 1983;191(3):378–381. doi: 10.1007/BF00425749. [DOI] [PubMed] [Google Scholar]
- Arai K. I., Kawakita M., Kaziro Y. Studies on polypeptide elongation factors from Escherichia coli. II. Purification of factors Tu-guanosine diphosphate, Ts, and Tu-Ts, and crystallization of Tu-guanosine diphosphate and Tu-Ts. J Biol Chem. 1972 Nov 10;247(21):7029–7037. [PubMed] [Google Scholar]
- Birge E. A., Kurland C. G. Reversion of a streptomycin-dependent strain of Escherichia coli. Mol Gen Genet. 1970;109(4):356–369. doi: 10.1007/BF00267704. [DOI] [PubMed] [Google Scholar]
- Bouadloun F., Donner D., Kurland C. G. Codon-specific missense errors in vivo. EMBO J. 1983;2(8):1351–1356. doi: 10.1002/j.1460-2075.1983.tb01591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenberg M., Kurland C. G. Costs of accuracy determined by a maximal growth rate constraint. Q Rev Biophys. 1984 Feb;17(1):45–82. doi: 10.1017/s0033583500005254. [DOI] [PubMed] [Google Scholar]
- Hummel H., Böck A. On the basis of aminoglycoside-dependent growth of mutants from E. coli: physiological studies. Mol Gen Genet. 1983;191(2):167–175. doi: 10.1007/BF00334809. [DOI] [PubMed] [Google Scholar]
- Jelenc P. C., Kurland C. G. Multiple effects of kanamycin on translational accuracy. Mol Gen Genet. 1984;194(1-2):195–199. doi: 10.1007/BF00383516. [DOI] [PubMed] [Google Scholar]
- Jelenc P. C., Kurland C. G. Nucleoside triphosphate regeneration decreases the frequency of translation errors. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3174–3178. doi: 10.1073/pnas.76.7.3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelenc P. C. Rapid purification of highly active ribosomes from Escherichia coli. Anal Biochem. 1980 Jul 1;105(2):369–374. doi: 10.1016/0003-2697(80)90472-8. [DOI] [PubMed] [Google Scholar]
- Leberman R., Antonsson B., Giovanelli R., Guariguata R., Schumann R., Wittinghofer A. A simplified procedure for the isolation of bacterial polypeptide elongation factor EF-Tu. Anal Biochem. 1980 May 1;104(1):29–36. doi: 10.1016/0003-2697(80)90272-9. [DOI] [PubMed] [Google Scholar]
- Momose H., Gorini L. Genetic analysis of streptomycin dependence in Escherichia coli. Genetics. 1971 Jan;67(1):19–38. doi: 10.1093/genetics/67.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki M., Mizushima S., Nomura M. Identification and functional characterization of the protein controlled by the streptomycin-resistant locus in E. coli. Nature. 1969 Apr 26;222(5191):333–339. doi: 10.1038/222333a0. [DOI] [PubMed] [Google Scholar]
- Rosenberger R. F., Foskett G. An estimate of the frequency of in vivo transcriptional errors at a nonsense codon in Escherichia coli. Mol Gen Genet. 1981;183(3):561–563. doi: 10.1007/BF00268784. [DOI] [PubMed] [Google Scholar]
- Ruusala T., Ehrenberg M., Kurland C. G. Catalytic effects of elongation factor Ts on polypeptide synthesis. EMBO J. 1982;1(1):75–78. doi: 10.1002/j.1460-2075.1982.tb01127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruusala T., Ehrenberg M., Kurland C. G. Is there proofreading during polypeptide synthesis? EMBO J. 1982;1(6):741–745. doi: 10.1002/j.1460-2075.1982.tb01240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleif R., Hess W., Finkelstein S., Ellis D. Induction kinetics of the L-arabinose operon of Escherichia coli. J Bacteriol. 1973 Jul;115(1):9–14. doi: 10.1128/jb.115.1.9-14.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E. G., Jelenc P. C., Ehrenberg M., Kurland C. G. Rate of elongation of polyphenylalanine in vitro. Eur J Biochem. 1982 Feb;122(1):193–197. doi: 10.1111/j.1432-1033.1982.tb05866.x. [DOI] [PubMed] [Google Scholar]
- Wagner E. G., Kurland C. G. Escherichia coli elongation factor G blocks stringent factor. Biochemistry. 1980 Mar 18;19(6):1234–1240. doi: 10.1021/bi00547a030. [DOI] [PubMed] [Google Scholar]
- Zengel J. M., Young R., Dennis P. P., Nomura M. Role of ribosomal protein S12 in peptide chain elongation: analysis of pleiotropic, streptomycin-resistant mutants of Escherichia coli. J Bacteriol. 1977 Mar;129(3):1320–1329. doi: 10.1128/jb.129.3.1320-1329.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]



