Abstract
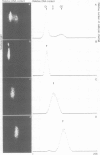
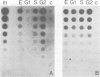
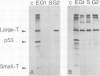
SV40-transformed FR 3T3 rat cells were previously shown to exhibit different patterns of accumulation of the virus-coded T-antigen. One group of transformants accumulates T-antigen throughout the cell cycle, whereas in another group, only the cells in the G2 phase of the cell cycle are stained by immunofluorescence with anti-T antigen antibodies. We investigated the mechanism involved by determining the amounts of early SV40 RNA during the cell cycle. Cells in the various phases of the cell cycle were sorted from an asynchronously growing population using a flow cytofluorimeter. Determination of the amounts of viral RNA in the different nuclear and cytoplasmic RNA fractions showed that in transformants with a G2-restricted accumulation of T-antigen, viral RNA was present in G2, to some extent in S, but could not be detected in cells in G1. In contrast, equivalent amounts of viral RNA were detected in all the phases of the cell cycle in the other group of transformants. Cell sorting, performed after pulse-labeling the cells for 2 h with [35S]methionine, confirmed that translation of the viral mRNAs occurred only in G2 in the first group of transformants, and throughout the cell cycle in the second group.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Chabanas A., Lawrence J. J., Humbert J., Eisen H. Cell cycle regulation of histone H1O in CHO cells: a flow cytofluorimetric study after double staining of the cells. EMBO J. 1983;2(6):833–837. doi: 10.1002/j.1460-2075.1983.tb01510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S. A unifying model for the G1 period in prokaryotes and eukaryotes. Nature. 1979 Jul 5;280(5717):17–19. doi: 10.1038/280017a0. [DOI] [PubMed] [Google Scholar]
- Edwards C. A., Khoury G., Martin R. G. Phosphorylation of T-antigen and control T-antigen expression in cells transformed by wild-type and tsA mutants of simian virus 40. J Virol. 1979 Feb;29(2):753–762. doi: 10.1128/jvi.29.2.753-762.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Felsenfeld G., McGhee J. Methylation and gene control. Nature. 1982 Apr 15;296(5858):602–603. doi: 10.1038/296602a0. [DOI] [PubMed] [Google Scholar]
- Fluck M. M., Benjamin T. L. Comparisons of two early gene functions essential for transformation in polyoma virus and SV-40. Virology. 1979 Jul 15;96(1):205–228. doi: 10.1016/0042-6822(79)90185-5. [DOI] [PubMed] [Google Scholar]
- Gaudray P., Rassoulzadegan M., Cuzin F. Expression of simian virus 40 early genes in transformed rat cells is correlated with maintenance of the transformed phenotype. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4987–4991. doi: 10.1073/pnas.75.10.4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E., Crawford L. V., Pim D. C., Williamson N. M. Monoclonal antibodies specific for simian virus 40 tumor antigens. J Virol. 1981 Sep;39(3):861–869. doi: 10.1128/jvi.39.3.861-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbert J., Clertant P., de Bovis B., Planche J., Birg F. Stabilization of the large T protein in temperature-independent (type A) FR 3T3 rat cells transformed with the simian virus 40 tsA30 mutant. J Virol. 1983 Sep;47(3):442–451. doi: 10.1128/jvi.47.3.442-451.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbert J., Lawrence J. J., Birg F. Simian virus 40 T antigen is detected only in cells in the G2 phase of the cell cycle in one group of rat transformants. Virology. 1983 Apr 30;126(2):711–716. doi: 10.1016/s0042-6822(83)80028-2. [DOI] [PubMed] [Google Scholar]
- Ito Y. Polyoma virus-specific 55K protein isolated from plasma membrane of productively infected cells is virus-coded and important for cell transformation. Virology. 1979 Oct 15;98(1):261–266. doi: 10.1016/0042-6822(79)90545-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levine A. J. Transformation-associated tumor antigens. Adv Cancer Res. 1982;37:75–109. doi: 10.1016/s0065-230x(08)60882-9. [DOI] [PubMed] [Google Scholar]
- Lydon M. J., Keeler K. D., Thomas D. B. Vital DNA staining and cell sorting by flow microfluorometry. J Cell Physiol. 1980 Feb;102(2):175–181. doi: 10.1002/jcp.1041020208. [DOI] [PubMed] [Google Scholar]
- Martin R. G. The transformation of cell growth and transmogrification of DNA synthesis by simian virus 40. Adv Cancer Res. 1981;34:1–68. doi: 10.1016/s0065-230x(08)60238-9. [DOI] [PubMed] [Google Scholar]
- Mercer W. E., Nelson D., DeLeo A. B., Old L. J., Baserga R. Microinjection of monoclonal antibody to protein p53 inhibits serum-induced DNA synthesis in 3T3 cells. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6309–6312. doi: 10.1073/pnas.79.20.6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner J., Milner S. SV40-53K antigen: a possible role for 53K in normal cells. Virology. 1981 Jul 30;112(2):785–788. doi: 10.1016/0042-6822(81)90327-5. [DOI] [PubMed] [Google Scholar]
- Mougneau E., Birg F., Rassoulzadegan M., Cuzin F. Integration sites and sequence arrangement of SV40 DNA in a homogeneous series of transformed rat fibroblast lines. Cell. 1980 Dec;22(3):917–927. doi: 10.1016/0092-8674(80)90569-3. [DOI] [PubMed] [Google Scholar]
- Pardee A. B. A restriction point for control of normal animal cell proliferation. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1286–1290. doi: 10.1073/pnas.71.4.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardee A. B., Dubrow R., Hamlin J. L., Kletzien R. F. Animal cell cycle. Annu Rev Biochem. 1978;47:715–750. doi: 10.1146/annurev.bi.47.070178.003435. [DOI] [PubMed] [Google Scholar]
- Perbal B., Rassoulzadegan M. Distinct transformation phenotypes induced by polyoma virus and simian virus 40 in rat fibroblasts and their control by an early viral gene function. J Virol. 1980 Feb;33(2):697–707. doi: 10.1128/jvi.33.2.697-707.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassoulzadegan M., Mougneau E., Perbal B., Gaudray P., Birg F., Cuzin F. Host-virus interactions critical for cellular transformation by polyoma virus and SV40. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):333–342. doi: 10.1101/sqb.1980.044.01.038. [DOI] [PubMed] [Google Scholar]
- Rassoulzadegan M., Perbal B., Cuzin F. Growth control in simian virus 40-transformed rat cells: temperature-independent expression of the transformed phenotype in tsA transformants derived by agar selection. J Virol. 1978 Oct;28(1):1–5. doi: 10.1128/jvi.28.1.1-5.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich N. C., Levine A. J. Growth regulation of a cellular tumour antigen, p53, in nontransformed cells. Nature. 1984 Mar 8;308(5955):199–201. doi: 10.1038/308199a0. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rossow P. W., Riddle V. G., Pardee A. B. Synthesis of labile, serum-dependent protein in early G1 controls animal cell growth. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4446–4450. doi: 10.1073/pnas.76.9.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif R., Cuzin F. Temperature-sensitive growth regulation in one type of transformed rat cells induced by the tsa mutant of polyoma virus. J Virol. 1977 Dec;24(3):721–728. doi: 10.1128/jvi.24.3.721-728.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif R., Martin R. G. Growth state of the cell early after infection with simian virus 40 determines whether the maintenance of transformation will be A-gene dependent or independent. J Virol. 1979 Aug;31(2):350–359. doi: 10.1128/jvi.31.2.350-359.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P., Ozer H. L. Temperature-sensitive mutants of simian virus 40: infection of permissive cells. J Virol. 1971 Oct;8(4):516–524. doi: 10.1128/jvi.8.4.516-524.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisbrod S. Active chromatin. Nature. 1982 May 27;297(5864):289–295. doi: 10.1038/297289a0. [DOI] [PubMed] [Google Scholar]
- White B. A., Bancroft F. C. Cytoplasmic dot hybridization. Simple analysis of relative mRNA levels in multiple small cell or tissue samples. J Biol Chem. 1982 Aug 10;257(15):8569–8572. [PubMed] [Google Scholar]