Abstract
Insomnia is the most prevalent sleep disorder in the United States and has high comorbidity with a number of cardiovascular diseases (CVDs). In the past decade, a number of observational studies have demonstrated an association between insomnia and incident cardiovascular disease (CVD) morbidity and mortality, including hypertension (HTN), coronary heart disease (CHD), and heart failure (HF). Despite some inconsistencies in the literature, likely due to variations in how insomnia is defined and measured, the existing data suggest that insomnia, especially when accompanied by short sleep duration, is associated with increased risk for HTN, CHD and recurrent acute coronary syndrome, and HF. Purported mechanisms likely relate to dysregulation of the hypothalamic-pituitary axis, increased sympathetic nervous system activity, and increased inflammation. This paper reviews the most recent studies of insomnia and CVD and the potential pathophysiological mechanisms underlying this relationship and highlights the need for randomized trials to further elucidate the nature of the relationship between insomnia and CVD.
Key Words: cardiovascular disease, insomnia, sleep medicine
Abbreviations: ACS, acute coronary syndrome; CHD, coronary heart disease; CRP, C-reactive protein; CVD, cardiovascular disease; HPA, hypothalamic-pituitary-adrenal; MI, myocardial infarction; PSG, polysomnography; SNS, sympathetic nervous system
Insomnia is the most commonly encountered sleep disorder in the United States, with prevalence rates of 15% to 24%.1, 2, 3 The American Academy of Sleep Medicine defines the disorder as a perceived difficulty with sleep initiation, consolidation, duration, or quality, despite an adequate opportunity to sleep, coupled with subsequent daytime impairment,4 and as chronic when lasting at least 3 months. Daytime impairment includes a wide spectrum of symptoms, such as problems with attention, concentration, or memory; fatigue, malaise, or energy reduction; errors or accidents at work, while driving, or any other form of social or vocational dysfunction; headaches or GI symptoms due to sleep loss; daytime sleepiness; mood disturbances; irritability; or specific worrying about sleep.5 In the past decade there has been increasing evidence associating insomnia with hypertension (HTN),6, 7, 8, 9 coronary heart disease (CHD),10, 11, 12, 13, 14, 15, 16 and heart failure (HF),17, 18 as well as subclinical cardiovascular disease (CVD)19 and CVD mortality.20, 21, 22 Because of the wide variations in how insomnia is defined and measured, however, there are conflicting data, and caution must be exercised when comparing studies and interpreting results. Nonetheless, the existing data suggest that insomnia is an important risk factor for CVD.23, 24 Notably, in 2016, the American Heart Association reviewed evidence linking sleep disorders and CVD and published a scientific statement asking health organizations to develop evidence-based sleep recommendations for a number of sleep disorders, including insomnia.25 Overall existing data support the need for further research to address causal mechanisms and whether treatment of insomnia improves HTN and coronary disease, reduces risk of recurrent acute coronary syndrome (ACS), and improves quality of life and mood in patients with CVD.
Defining and Measuring Insomnia
Several diagnostic systems have used varying definitions of insomnia, which may have contributed to inconsistencies in clinical practice and research. The current classification of insomnia in the newest editions of both the International Classification of Sleep Disorders, 3rd edition, and the Diagnostic and Statistical Manual of Mental Disorders, 5th edition, have attempted to clarify past nosologies by simplifying the insomnia criteria. The previous classification systems had differentiated insomnia as a “primary” vs “secondary” disorder and included multiple diagnostic subtypes of primary insomnia despite many overlapping symptoms. With removal of “secondary” insomnia as a diagnosis as well as the multiple diagnostic subtypes of “primary insomnia,” the International Classification of Sleep Disorders, 3rd edition, and the Diagnostic and Statistical Manual of Mental Disorders, 5th edition, have consolidated these insomnia diagnoses under a single disorder, “chronic insomnia disorder.” These definitions are largely congruent with the American Academy of Sleep Medicine insomnia definition.26, 27 These changes are intended to improve consistency in diagnosing insomnia while highlighting the importance of treating insomnia as a separate entity given that comorbid medical and mental health disorders are often bidirectional and warrant independent attention and treatment.4, 28 Additionally, recognizing that variations in defining and measuring insomnia hamper insomnia research, the American Academy of Sleep Medicine developed operational research diagnostic criteria for insomnia to facilitate more consistent research in the field.4 Further, quantitative research diagnostic criteria specify a minimum length of sleep onset duration (≥ 30 min) or periods of waking after sleep onset (≥ 30 min at least three times per week) to provide more clarity and consistency in research trials.29, 30
There is wide variability in how insomnia is ascertained in the research community, and a variety of measurement tools exist to identify and describe pertinent features of this disorder, including objective (polysomnography [PSG], actigraphy) and self-reporting tools (sleep diaries, insomnia questionnaires). Each type of instrument may capture different aspects of sleep and provide different insights into pathologic sleep conditions. Sleep diaries, which ask the individual to record daily bed and waking times and estimate sleep latency and awakenings, are often used to capture salient aspects of sleep patterns typical of insomnia and are considered a gold standard assessment tool by some.31, 32 Although commonly used in specialized sleep clinic settings, in research settings, these instruments present challenges related to the need for multiple-day data collection, including missing or misspecified data. There is a paucity of large research studies evaluating the association between insomnia and CVD using sleep diaries.4, 5 The most common approach for assessing insomnia in research has been from questionnaires administered at a single point in time, either including one or more individual questions regarding insomnia symptoms (difficulties initiating or maintaining sleep or early morning awakenings, or both) or from insomnia screening instruments, such as the Insomnia Severity Index.22 PSG, which is not recommended as a diagnostic or prognostic tool in insomnia, in part due to the wide discrepancies between objective recording and habitual sleep experience, has been used in research to characterize associated sleep duration and sleep quality. Insomnia may also be identified in research based on self-reporting of a prior diagnosis. However, given the underdiagnosis of this condition, this definition likely is not sensitive.
Short sleep duration is often related to but distinct from insomnia. In the Sleep Heart Health Study, a prospective study of 631 participants with insomnia symptoms, 48% of participants also had a sleep duration of < 6 h on PSG.33 Considering sleep duration as a distinct but important modifier of insomnia is an area that has received variable recognition. Although the definition of insomnia does not include criteria related to sleep duration, the occurrence of short sleep duration with insomnia appears to identify a phenotype with distinct clinical features. Unfortunately, only some studies describe insomnia regarding its association with short or normal sleep duration, potentially introducing heterogeneity into the literature. Many of the pathophysiological pathways that increase CVD risk may be separately influenced by insomnia and short sleep duration, and the two entities occurring together may have additive or multiplicative “effects” on CVD risk. A previous review suggests a specific phenotype of insomnia with objective short sleep duration that activates the stress system and is associated with adverse cardiometabolic health as opposed to insomnia with normal sleep duration. Authors recommend the use of actigraphy as opposed to PSG to quantify objective habitual sleep duration.34
Insomnia and CVD Pathophysiology: Underlying Mechanisms
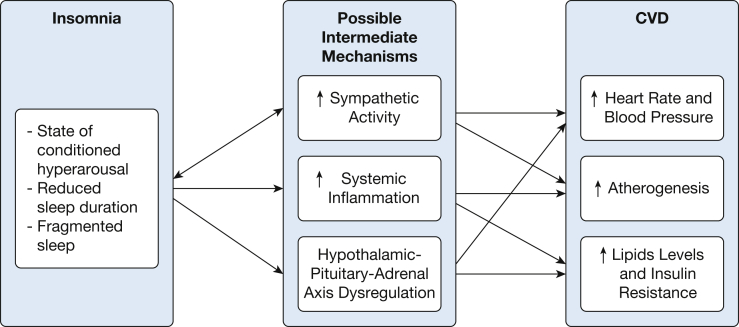
Although the pathogenesis of insomnia and CVD is not fully understood, there are multiple mechanisms underlying this relationship, including dysregulation of the hypothalamic-pituitary axis (HPA) axis,24, 35, 36, 37, 38 abnormal modulation of the autonomic nervous system, and increased sympathetic nervous system (SNS) activity,39 increased systemic inflammation,40, 41 and increased atherogenesis19, 42 (Fig 1). Chronic insomnia is considered a state of conditioned hyperarousal43 and thought to be related to increases in SNS activity and hormones implicated in arousal and sleeplessness, such as cortisol. There is evidence that both adrenocorticotropic hormone and cortisol secretion are increased in patients with insomnia, particularly those with objective short sleep duration, suggesting increased HPA axis activity.36, 37, 38, 44 Chronic activation or dysregulation of the HPA axis not only may lead to increased risk of CVD but also to insulin resistance, diabetes, and mental health disorders such as anxiety and depression. To that end, insomnia is associated with impaired glucose metabolism45 and diabetes,39 which may serve as mediators on the pathway to CVD.
Figure 1.
Flow diagram regarding possible pathophysiological mechanisms underlying the relationship between insomnia and cardiovascular disease (CVD).
Studies also demonstrate increased SNS activity, with elevated levels of plasma and urine norepinephrine in both short sleepers and those with insomnia compared with normal control subjects,39, 46 as well as increased heart rate and altered or blunted heart rate variability,47, 48 reflecting underlying autonomic dysregulation. SNS activity is an integral part of cardiovascular homeostasis and plays a critical role in the pathogenesis of HTN, arrhythmias, CHD, and HF.49 A recent cross-sectional study demonstrated an association between difficulty falling asleep and reduced parasympathetic tone (measured by 1-min heart rate recovery from maximal exercise test) in patients undergoing cardiac rehabilitation who had moderate to severe insomnia.50 In this study, insomnia severity was ascertained using the Insomnia Severity Index, a well-validated22, 51 questionnaire commonly used to screen and grade insomnia. A study of > 500 participants in the Multiethnic Study of Atherosclerosis showed that participants who slept ≤ 7 hours per night and reported insomniac symptoms had greater heart rate orthostatic reactivity and a high frequency of heart rate variability reactivity to mental stress than participants who slept > 7 hours per night with no insomniac symptoms.52
Although not directly addressing insomnia, experiments with sleep curtailment and sleep disruption show subsequent increases in blood pressure and elevation of inflammatory cytokines, including those implicated in atherogenesis,42, 53 such as C-reactive protein (CRP)42, tumor necrosis factor-α,54 and interleukin 6.54, 55 Epidemiologic data have also suggested an association between insomnia and elevated levels of CRP.41 To this end, Parthasarathy et al40 demonstrated a link between chronic persistent insomnia and cardiopulmonary mortality associated with increased CRP levels. Additionally, a large epidemiologic study (N = 4,011) showed an association between moderate to severe insomnia and elevated CRP levels in men.56
Another study of subclinical vascular disease (considered to reflect vascular inflammation) separately assessed short sleep duration (< 5 h) and insomnia (using standard clinical diagnostic criteria but for 1 month's duration) and found that both were significantly associated with increased carotid intima media thickness in 86 healthy volunteers.57 The Coronary Artery Disease in Young Adults Study (CARDIA) demonstrated a significant inverse association between short sleep duration and incident coronary calcification (a marker of subclinical CVD) that was independent of apneic risk, lipid levels, blood pressure, BMI, diabetes, and depression, among other confounders and mediators.19 The extent to which these findings reflected insomnia vs short sleep duration is not clear.
Insomnia has high comorbidity with a number of psychiatric diseases, particularly depression, and there is considerable overlap between the two disorders, which likely have a bidirectional relationship. Although insomnia is sometimes considered to be secondary to depression or other affective disorders, there is also evidence suggesting that insomnia may contribute to incident depression.58 Given the evidence that affective disorders, particularly depression, increase the risk of CVD,59, 60 there is opportunity to further dissect the contributing role of insomnia through direct or indirect (ie, depression) paths in increasing CVD.
Hypertension
Existing research mostly supports an association between insomnia and incident HTN.6, 7, 8, 9, 61 One of the largest prospective epidemiologic studies, from the Penn State cohort, followed 786 individuals with chronic insomnia who were free of baseline HTN for 7½ years. Participants were recruited from a random general community sample and underwent PSG at baseline. Chronic insomnia was defined as a complaint of insomnia for ≥ 1 year. After adjustment for numerous potential confounders, chronic insomnia was significantly associated with more than a twofold increased risk of HTN (OR, 2.24; 95% CI, 1.19-4.19; P = .010). When coupling chronic insomnia with objective short sleep duration defined by PSG (< 6 h), the odds of HTN increased to almost fourfold (OR, 3.75; 95% CI, 1.58-8.95; P = .012),6 A meta-analysis pooling 58,924 subjects from 17 prospective cohort studies with a 1-year minimum follow-up showed a significantly increased incidence of HTN among subjects with individual symptoms of insomnia and a reduced, although still significantly increased, risk of HTN when combining multiple symptoms of insomnia.62 Other cross-sectional studies have also supported a significant association between insomnia occurring with reduced objective (but not subjective) sleep duration and increased risk of HTN.7, 21 One of the studies specifically compared patients with insomnia (defined using clinical criteria) with and without objectively measured and subjectively measured short sleep duration. They found that insomnia with objectively measured short sleep duration (< 6 h per night) was significantly associated with HTN compared with insomnia with objectively measured longer sleep (> 6 h). Additionally, they found no significant associations between insomnia and HTN when using subjectively measured sleep duration.21 It is not clear if these differences reflect greater measurement error for subjective sleep reports compared with PSG. Alternatively, it is possible that sleep duration measured by PSG identifies individuals who do not sleep well during laboratory monitoring and have a phenotype that associates with HTN risk
Coronary Heart Disease
A number of prospective observational studies have demonstrated that insomnia is associated with increased risk of CHD, recurrent ACS, and mortality.10, 11, 12, 13, 14, 15, 16 Further, there is a high prevalence of insomnia among patients with comorbid CHD: an astounding one third of patients presenting with initial ACS during and after hospitalization report insomnia.15
One of the first large prospective population studies associating insomnia with incident CHD was the Nord-Trondelag Health Study (the HUNT study). Laugsand et al10 collected information on difficulty initiating or maintaining sleep or having nonrestorative sleep and whether these symptoms impaired work performance; they subsequently followed 52,610 men and women for 11.4 years for a first acute myocardial infarction (MI). Depending on the specific symptom of insomnia, there was a 27% to 45% (statistically significant) increased risk of acute MI after adjusting for confounders. The single symptom most strongly associated with incident MI was difficulty initiating sleep. When combining symptoms, a dose-dependent association was demonstrated between insomnia and MI (test for trend P = .003).10 The second largest prospective study used data from the Taiwan National Health Insurance Research Database, matching 22,040 people with a diagnosis of insomnia by age, sex, and comorbidity to 22,040 people without insomnia. Participants were followed for 10 years for acute MI or stroke. Those with insomnia had a 68% increased risk of experiencing an incident MI (95% CI, 1.31-2.16) and an 85% increased risk of stroke (95% CI, 1.62-2.12) after adjusting for confounders.63 These prospective data support insomnia as an antecedent risk factor for CHD and cerebrovascular disease.
A number of trials have examined subjective sleep duration with CHD morbidity and mortality.16, 64 A large prospective observational trial in Taiwan included 392,164 adults who reported their sleep duration at a health check-up program and were followed for CHD mortality using a death registry. The authors demonstrated a U-shaped relationship between self-reported sleep duration and death from CHD. When compared with those with “normal” sleep duration (6-8 h per night) those sleeping < 4 h had a 34% increased risk of death from CHD (95% CI, 1.11-1.65), whereas those sleeping > 8 h had a 35% increased risk of death from CHD (95% CI, 1.11-1.65).64 Of note, however, the collection of sleep duration data through a single question at a general check-up appointment is a serious limitation to this study. A meta-analysis pooling 15 prospective studies (24 cohorts totaling 474,684 men and women) assessed sleep duration by questionnaire and CVD outcomes and death by certification and registries and also demonstrated a U-shaped association, but it was statistically significant for both short-duration and long-duration sleepers. The relative risk of the development of or death from CHD was 1.48 (95% CI, 1.22-1.80; P < .0001) for short sleep duration (< 5-6 h) and 1.38 (95% CI, 1.15-1.66; P = .0005) for long sleep duration (> 8-9 h).16 Insomnia was not specifically reported in these papers, precluding assessment of the extent to which associations reflected a sleep-duration specific finding or reflected a phenotype related to insomnia.
Heart Failure
There is a high prevalence of insomniac symptoms among patients with HF, ranging from 23% to 73%. There are many reasons insomnia may be a result of HF, such as disease-related anxiety and depression, medications, and Cheyne-Stokes respiration. There are also longitudinal data from some,17, 65 although not all, studies66 that have reported insomnia to precede the onset of HF. In a middle-aged prospective cohort of 2,322 men, self-reported symptoms of insomnia predicted increased risk of incident HF after adjusting for baseline risk factors and confounders so that for any sleep disturbance, the hazard ratio (HR) for incident HF was 1.52 (95% CI, 1.16-1.99).65 The largest prospective study to examine the association between insomnia and incident HF is once again the Nord-Trondelag Health Study (HUNT study). In this sample of 54,279 Norwegian men and women free of known HF at baseline and followed for 11.3 years, incident HF was associated with symptoms of insomnia. Moreover, associations persisted after adjusting for depression and anxiety and other confounders and showed a dose-dependent increase in risk of incident HF with an increasing number of cumulative symptoms of insomnia. In the fully adjusted model, those with three symptoms had an HR of 5.25 (95% CI, 2.25-12.22) compared with 1.43 (95% CI, 0.76-2.69) with those with only one symptom. Once again, difficulty initiating sleep was most strongly associated with incident HF.17
Of note, all these studies used subjective means (questionnaire) to determine symptoms of insomnia, which is sufficient to diagnose insomnia in a clinical setting. However, once again, the definitions varied, as assessment of daytime impairment was not collected by all the studies and various symptoms were used in analysis to capture poor sleep.
Insomnia and Overall CVD Risk and Mortality
Several studies have also reported that insomnia is associated with both CVD risk and CVD mortality. A systematic review and meta-analysis of prospective cohort studies showed that 122,501 subjects free of baseline CVD disease with insomnia (assessed using a questionnaire) had a 45% increased risk (95% CI, 1.29-1.62) of the development of or death from CVD during a 3- to 20-year follow-up.67 Another meta-analysis of 17 cohort studies with 311,260 subjects free of baseline CVD demonstrated a 33% increased relative risk (95% CI, 1.13-1.57) of CVD mortality among subjects with insomnia.23 Subsequently, Parthasarathy et al40 also demonstrated an increased risk of mortality from heart disease and cardiopulmonary mortality among 1,409 patients with chronic persistent insomnia (HR, 2.02; P = .02 and HR, 2.11; P = .004, respectively) that was associated with increased CRP levels.
Unanswered Questions
Variations in the measurement and definition of insomnia continue to be a research challenge despite attempts at expert consensus regarding the best means of identifying and defining insomnia for research purposes. There is a need to further clarify the roles of short sleep duration vs poor sleep quality on CVD and to better define subgroups at increased risk. Future research may benefit from attempting to dissect the distinct as well as overlapping influences of insomnia and short sleep duration on risk of CVD. This research need requires that each set of exposures are measured using valid tools, that the potential influences of confounding sleep disorders such as periodic limb movement disorder and sleep apnea are addressed by controlling for these factors or through subject selection, and that samples that are studied are sufficiently large to distinguish distinct subgroups. Observational studies may improve our understanding of whether individuals with a phenotype characterized by “insomnia/short sleep duration” differs from the “insomnia/normal sleep duration” phenotype. However, experimental studies (whereby one or the other sleep disturbance is treated or manipulated) are also needed to further elucidate the pathophysiological mechanisms by which variations in sleep duration may modify risk associated with insomnia and vice versa.
There is also a need to further clarify the roles of insomnia vs psychiatric disorders, particularly depression, in increased risk of CVD. The two disorders are distinct entities deserving separate management, yet they have many overlapping symptoms and a likely bidirectional relationship.
Given how common insomnia is in patients with CVD and its impact on quality of life, there is potential value in routinely screening patients with CVD for insomnia and further defining the role of screening and treatment for insomnia among patients with CVD. Although observational data and biological plausibility suggest a relationship between insomnia and CVD, little is known about how treatment of insomnia may impact CVD, and causality is difficult to ascertain from observational studies. Thus, there is a need for randomized controlled trials to determine whether insomnia is in fact on the causal pathway for CVD, whether it is a mediator for depression or other risk factors that cause CVD, and whether treatment of insomnia may improve outcomes in this vulnerable population. In particular, cognitive behavioral therapy for insomnia is a safe and effective treatment that may be very promising in patients with comorbid CVD.68 Further dissecting the mechanisms underlying the relationship between insomnia and CVD is another important research target. Specific mechanisms of interest include autonomic nervous system activity, BP and vascular changes, and systemic inflammation and neurohumoral changes. Another question of interest is whether an association exists between insomnia and cardiac arrhythmias, particularly atrial fibrillation, a relatively unstudied area to date.
In summary, the literature demonstrates a high prevalence of insomnia, a disorder of conditioned hyperarousal, among patients with a variety of CVDs and HF (Table 1). There are emerging data linking disturbed sleep (including insomnia and short sleep duration) with intermediate mechanisms for heart disease, such as elevations in proinflammatory biomarkers and BP. Insomnia also appears to increase the incidence of depression, which can independently contribute to CVD. Prospective data suggest that insomnia is associated with an increased incident risk of HTN, HF, and CHD in patients with insomnia, particularly when coupled with objective measurements of short sleep duration. There is a need to further refine measurements of insomnia phenotypes, elucidate the role of short sleep duration in insomnia-associated CVD risk, and address whether individuals with a predominance of problems with prolonged sleep latency compared with sleep maintenance or early awakenings have different prognoses. Mechanistic studies and randomized controlled trials, as well as studies of large well-characterized samples, are all needed to achieve a better understanding of the mechanisms by which insomnia contributes to CVD and to define which patients to most effectively target for sleep interventions.
Table 1.
Summary of Current Data
| Study/Year | Type | Outcome | No. | Main Findings | Confounders |
|---|---|---|---|---|---|
| Fernandez-Mendoza et al6/2012 | Prospective, population-based | HTN | 786 | Twofold ↑ odds HTN, almost fourfold ↑ odds with objective short sleep and insomnia | Baseline + depression, SDB, DM, BP |
| Bathgate et al21/2016 | Cross-sectional, clinic-based | HTN | 255 | 3.59 ↑ risk HTN in patients with insomnia with < 6 h sleep duration | Baseline + use of hypnotic agents, ESS, AHI, DM, cholesterol, depression |
| Vgontzas et al7/2009 | Cross-sectional, population-based | HTN | 1,741 | 5.1 ↑ odds HTN in patients with insomnia with < 5 hours sleep duration | Baseline + DM, SDB, depression, sampling weight |
| Suka et al8/2003 | Cross-sectional, clinic-based | HTN | 255 | Almost twofold ↑ odds HTN with DIS or DMS | Age, BMI, tobacco or alcohol use, job stress |
| Meng et al62/2013 | Prospective, meta-analysis | HTN | 58,924 | 5% ↑ relative risk of HTN | … |
| Laugsand et al10/2011 | Prospective, population-based | CHD | 52,610 | 1.45 ↑ odds acute MI with DIS and 1.3 ↑ odds acute MI with DMS | Baseline (except race) + marital status, education, shift work, BP, lipid levels, DM, physical activity |
| Leineweber et al11/2003 | Prospective, hospital-based | CHD | 292, female only | Poor sleep quality associated with 2.5 ↑ risk recurrent CHD | Age, BMI, HF symptoms, HTN, DM, HDL, triglyceride levels, education, depression |
| Li et al12/2014 | Prospective, community-based sample of male health professionals | CHD | 23,447, men only | 25% ↑ risk mortality with DIS, 9% ↑ with DMS | Baseline + physical activity, Alternate Healthy Eating Index, marriage status, living status, anxiety, medications, sleep duration, snoring frequency, cholesterol level, HTN, DM, MI, stroke, LUTS |
| Mallon et al13/2002 | Prospective, population-based | CHD | 1,870, men only | 3.1 ↑ relative risk of CHD in men but not in women with DIS | Age, marriage, living alone, smoking, BMI > 28, HTN, cardiac disease, respiratory disease, DM, joint pain, GI disease, depression, sleep duration, DIS, DMS, snoring, sleeping aid use, urogenital disease |
| Meisinger et al14/2007 | Prospective, population-based | CHD | 6,896 | 12% ↑ relative risk in men and 53% ↑ in women with DMS, 16% ↑ in men and 30% in women with DIS | Baseline + lipid levels, education, family history of MI, physical activity, HTN, DM, menopause status |
| Coryell et al15/2013 | Cross-sectional, hospital-based | CHD | 102 | Prevalence of moderate or severe insomnia in patients with ACS = 37% | Descriptive study |
| Cappuccio et al16/2011 | Prospective, meta-analysis | CHD | 474,684 | Short sleep duration ↑ risk CHD by 48% | … |
| Hsu et al63/2015 | Prospective, population-based | CHD | 44,080 | 68% ↑ risk of future MI, 85% ↑ risk of stroke, ↑ 81% ↑ risk composite CVD | Age, sex, HTN, DM, lipid levels, PAD, CKD, CAD, CHF, chronic pulmonary disease |
| Ingelsson et al65/2007 | Prospective, population-based cohort | HF | 2,322, men only | 52% ↑ risk HF overall and 58% ↑ risk HF in overweight but not normal-weight participants | BP, DM, LVH by ECG, smoking, BMI |
| Laugsand et al17/2014 | Prospective, population-based cohort | HF | 54,279 | 4.53-fold ↑ risk HF in those with 3 symptoms of insomnia compared with those with no symptoms | Baseline + education, shift work, marital status, MI, BP, physical activity, lipid levels |
| Sofi et al67/2014 | Prospective, meta-analysis | CVD mortality | 122,501 | +45% ↑ risk of development of or death from CVD | … |
| Li et al23/2014 | Prospective, meta-analysis | CVD mortality | 311,260 | 41% ↑ risk MI, 28% ↑ risk CHD, 55% ↑ risk stroke, 33% ↑ risk CVD mortality | … |
| Parthasarathy et al40/2015 | Prospective, population-based | CVD mortality | 1,409 | 58% ↑ risk death in persistent, but not intermittent, insomnia | Baseline + physical activity, sedative use |
Baseline: age, race, sex, tobacco use, alcohol use, BMI.
ACS = acute coronary syndrome; AHI = apnea-hypopnea index; CAD = coronary artery disease; CHD = coronary heart disease; CKD = chronic kidney disease; CVD = cardiovascular disease; DIS = difficulty initiating sleep; DM = diabetes mellitus; DMS = difficulty maintaining sleep; ESS = Epworth Sleepiness Scale; HF = heart failure; HTN = hypertension; LUTS = lower urinary tract symptoms; LVH = left ventricular hypertrophy; MI = myocardial infarction; PAD = peripheral arterial disease; SDB = sleep disordered breathing.
Acknowledgments
Financial/nonfinancial disclosures: None declared.
Footnotes
FUNDING/SUPPORT: This work was supported by National Institutes of Health Grant [NIH 5T32HL007901].
References
- 1.Ford E.S., Cunningham T.J., Giles W.H., Croft J.B. Trends in insomnia and excessive daytime sleepiness among U.S. adults from 2002 to 2012. Sleep Med. 2015;16(3):372–378. doi: 10.1016/j.sleep.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roth T., Coulouvrat C., Hajak G. Prevalence and perceived health associated with insomnia based on DSM-IV-TR; International Statistical Classification of Diseases and Related Health Problems, Tenth Revision; and Research Diagnostic Criteria/International Classification of Sleep Disorders, Second Edition criteria: results from the America Insomnia Survey. Biol Psychiatry. 2011;69(6):592–600. doi: 10.1016/j.biopsych.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 3.Pearson N.J., Johnson L.L., Nahin R.L. Insomnia, trouble sleeping, and complementary and alternative medicine: Analysis of the 2002 national health interview survey data. Arch Intern Med. 2006;166(16):1775–1782. doi: 10.1001/archinte.166.16.1775. [DOI] [PubMed] [Google Scholar]
- 4.Edinger J.D., Bonnet M.H., Bootzin R.R. Derivation of research diagnostic criteria for insomnia: report of an American Academy of Sleep Medicine Work Group. Sleep. 2004;27(8):1567–1596. doi: 10.1093/sleep/27.8.1567. [DOI] [PubMed] [Google Scholar]
- 5.Schutte-Rodin S., Broch L., Buysse D., Dorsey C., Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4(5):487–504. [PMC free article] [PubMed] [Google Scholar]
- 6.Fernandez-Mendoza J., Vgontzas A.N., Liao D. Insomnia with objective short sleep duration and incident hypertension: the Penn State cohort. Hypertension. 2012;60(4):929–935. doi: 10.1161/HYPERTENSIONAHA.112.193268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vgontzas A.N., Liao D., Bixler E.O., Chrousos G.P., Vela-Bueno A. Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep. 2009;32(4):491–497. doi: 10.1093/sleep/32.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suka M., Yoshida K., Sugimori H. Persistent insomnia is a predictor of hypertension in Japanese male workers. J Occup Health. 2003;45(6):344–350. doi: 10.1539/joh.45.344. [DOI] [PubMed] [Google Scholar]
- 9.Gangwisch J.E., Malaspina D., Posner K. Insomnia and sleep duration as mediators of the relationship between depression and hypertension incidence. Am J Hypertens. 2010;23(1):62–69. doi: 10.1038/ajh.2009.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laugsand L.E., Vatten L.J., Platou C., Janszky I. Insomnia and the risk of acute myocardial infarction: a population study. Circulation. 2011;124(19):2073–2081. doi: 10.1161/CIRCULATIONAHA.111.025858. [DOI] [PubMed] [Google Scholar]
- 11.Leineweber C., Kecklund G., Janszky I., Akerstedt T., Orth-Gomer K. Poor sleep increases the prospective risk for recurrent events in middle-aged women with coronary disease. The Stockholm Female Coronary Risk Study. J Psychosom Res. 2003;54(2):121–127. doi: 10.1016/s0022-3999(02)00475-0. [DOI] [PubMed] [Google Scholar]
- 12.Li Y., Zhang X., Winkelman J.W. The association between insomnia symptoms and mortality: a prospective study of U.S. men. Circulation. 2014;129(7):737–746. doi: 10.1161/CIRCULATIONAHA.113.004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mallon L., Broman J.E., Hetta J. Sleep complaints predict coronary artery disease mortality in males: a 12-year follow-up study of a middle-aged Swedish population. J Intern Med. 2002;251(3):207–216. doi: 10.1046/j.1365-2796.2002.00941.x. [DOI] [PubMed] [Google Scholar]
- 14.Meisinger C., Heier M., Lowel H., Schneider A., Doring A. Sleep duration and sleep complaints and risk of myocardial infarction in middle-aged men and women from the general population: the MONICA/KORA Augsburg cohort study. Sleep. 2007;30(9):1121–1127. doi: 10.1093/sleep/30.9.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coryell V.T., Ziegelstein R.C., Hirt K., Quain A., Marine J.E., Smith M.T. Clinical correlates of insomnia in patients with acute coronary syndrome. Int Heart J. 2013;54(5):258–265. doi: 10.1536/ihj.54.258. [DOI] [PubMed] [Google Scholar]
- 16.Cappuccio F.P., Cooper D., D'Elia L., Strazzullo P., Miller M.A. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J. 2011;32(12):1484–1492. doi: 10.1093/eurheartj/ehr007. [DOI] [PubMed] [Google Scholar]
- 17.Laugsand L.E., Strand L.B., Platou C., Vatten L.J., Janszky I. Insomnia and the risk of incident heart failure: a population study. Eur Heart J. 2014;35(21):1382–1393. doi: 10.1093/eurheartj/eht019. [DOI] [PubMed] [Google Scholar]
- 18.Javaheri S., Blackwell T., Ancoli-Israel S., Ensrud K.E., Stone K.L., Redline S. Sleep-disordered breathing and incident heart failure in older men. Am J Respir Crit Care Med. 2016;193(5):561–568. doi: 10.1164/rccm.201503-0536OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.King C.R., Knutson K.L., Rathouz P.J., Sidney S., Liu K., Lauderdale D.S. Short sleep duration and incident coronary artery calcification. JAMA. 2008;300(24):2859–2866. doi: 10.1001/jama.2008.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phillips B., Mannino D.M. Do insomnia complaints cause hypertension or cardiovascular disease? J Clin Sleep Med. 2007;3(5):489–494. [PMC free article] [PubMed] [Google Scholar]
- 21.Bathgate C.J., Edinger J.D., Wyatt J.K., Krystal A.D. Objective but not subjective short sleep duration associated with increased risk for hypertension in individuals with insomnia. Sleep. 2016;39(5):1037–1045. doi: 10.5665/sleep.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morin C.M., Belleville G., Belanger L., Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601–608. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li M., Zhang X.W., Hou W.S., Tang Z.Y. Insomnia and risk of cardiovascular disease: a meta-analysis of cohort studies. Int J Cardiol. 2014;176(3):1044–1047. doi: 10.1016/j.ijcard.2014.07.284. [DOI] [PubMed] [Google Scholar]
- 24.Grandner M.A., Alfonso-Miller P., Fernandez-Mendoza J., Shetty S., Shenoy S., Combs D. Sleep: important considerations for the prevention of cardiovascular disease. Curr Opin Cardiol. 2016;31(5):551–565. doi: 10.1097/HCO.0000000000000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.St-Onge M.P., Grandner M.A., Brown D. Sleep duration and quality: impact on lifestyle behaviors and cardiometabolic health: a scientific statement from the American Heart Association. Circulation. 2016;134(18):e367–e386. doi: 10.1161/CIR.0000000000000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.American Academy of Sleep Medicine . 3rd ed. American Academy of Sleep Medicine; Darien, IL: 2014. International Classification of Sleep Disorders. [Google Scholar]
- 27.American Psychiatric Assciation . 5th ed. American Psychiatric Publishing; Arlington, VA: 2013. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- 28.Sateia M.J. International classification of sleep disorders-third edition: highlights and modifications. Chest. 2014;146(5):1387–1394. doi: 10.1378/chest.14-0970. [DOI] [PubMed] [Google Scholar]
- 29.Buysse D.J., Ancoli-Israel S., Edinger J.D., Lichstein K.L., Morin C.M. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29(9):1155–1173. doi: 10.1093/sleep/29.9.1155. [DOI] [PubMed] [Google Scholar]
- 30.Lichstein K.L., Durrence H.H., Taylor D.J., Bush A.J., Riedel B.W. Quantitative criteria for insomnia. Behav Res Ther. 2003;41(4):427–445. doi: 10.1016/s0005-7967(02)00023-2. [DOI] [PubMed] [Google Scholar]
- 31.Buysse D.J. Insomnia. JAMA. 2013;309(7):706–716. doi: 10.1001/jama.2013.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carney C.E., Buysse D.J., Ancoli-Israel S. The consensus sleep diary: standardizing prospective sleep self-monitoring. Sleep. 2012;35(2):287–302. doi: 10.5665/sleep.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bertisch S GD, Buysse D, Rueschman M, Wang R, Redline S. Prospective study of insomnia with objective short sleep duration and risk of incident cardiovascular disease: Sleep Heart Health Study [abstract]. J Sleep Sleep Disorders Research. 2015;38. http://www.journalsleep.org/Resources/Documents/2015AbstractSupplement.pdf. Accessed March 14, 2017.
- 34.Fernandez-Mendoza J., Vgontzas A.N. Insomnia and its impact on physical and mental health. Curr Psychiatry Rep. 2013;15(12):418. doi: 10.1007/s11920-013-0418-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Castro-Diehl C., Roux A.V., Redline S., Seeman T., Schrager S., Shea S. Association of sleep duration and quality with alterations in the hypothalamic-pituitary adrenocortical axis: the multi-ethnic study of atherosclerosis (MESA) J Clin Endocrinol Metab. 2015;100(8):3149–3158. doi: 10.1210/jc.2015-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vgontzas A.N., Bixler E.O., Lin H.M. Chronic insomnia is associated with nyctohemeral activation of the hypothalamic-pituitary-adrenal axis: clinical implications. J Clin Endocrinol Metab. 2001;86(8):3787–3794. doi: 10.1210/jcem.86.8.7778. [DOI] [PubMed] [Google Scholar]
- 37.Vgontzas A.N., Tsigos C., Bixler E.O. Chronic insomnia and activity of the stress system: a preliminary study. J Psychosom Res. 1998;45(1):21–31. doi: 10.1016/s0022-3999(97)00302-4. [DOI] [PubMed] [Google Scholar]
- 38.Floam S., Simpson N., Nemeth E., Scott-Sutherland J., Gautam S., Haack M. Sleep characteristics as predictor variables of stress systems markers in insomnia disorder. J Sleep Res. 2015;24(3):296–304. doi: 10.1111/jsr.12259. [DOI] [PubMed] [Google Scholar]
- 39.Vgontzas A.N., Liao D., Pejovic S., Calhoun S., Karataraki M., Bixler E.O. Insomnia with objective short sleep duration is associated with type 2 diabetes: a population-based study. Diabetes Care. 2009;32(11):1980–1985. doi: 10.2337/dc09-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parthasarathy S., Vasquez M.M., Halonen M. Persistent insomnia is associated with mortality risk. Am J Med. 2015;128(3):268–275.e262. doi: 10.1016/j.amjmed.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Irwin M.R. Why sleep is important for health: a psychoneuroimmunology perspective. Annu Rev Psychol. 2015;66:143–172. doi: 10.1146/annurev-psych-010213-115205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meier-Ewert H.K., Ridker P.M., Rifai N. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. 2004;43(4):678–683. doi: 10.1016/j.jacc.2003.07.050. [DOI] [PubMed] [Google Scholar]
- 43.Riemann D. Hyperarousal and insomnia: state of the science. Sleep Med Rev. 2010;14(1):17. doi: 10.1016/j.smrv.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 44.Rodenbeck A., Cohrs S., Jordan W., Huether G., Ruther E., Hajak G. The sleep-improving effects of doxepin are paralleled by a normalized plasma cortisol secretion in primary insomnia. A placebo-controlled, double-blind, randomized, cross-over study followed by an open treatment over 3 weeks. Psychopharmacology (Berl) 2003;170(4):423–428. doi: 10.1007/s00213-003-1565-0. [DOI] [PubMed] [Google Scholar]
- 45.Knutson K.L., Van Cauter E., Zee P., Liu K., Lauderdale D.S. Cross-sectional associations between measures of sleep and markers of glucose metabolism among subjects with and without diabetes: the Coronary Artery Risk Development in Young Adults (CARDIA) sleep study. Diabetes Care. 2011;34(5):1171–1176. doi: 10.2337/dc10-1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang J., Ma R.C., Kong A.P. Relationship of sleep quantity and quality with 24-hour urinary catecholamines and salivary awakening cortisol in healthy middle-aged adults. Sleep. 2011;34(2):225–233. doi: 10.1093/sleep/34.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bonnet M.H., Arand D.L. Heart rate variability in insomniacs and matched normal sleepers. Psychosom Med. 1998;60(5):610–615. doi: 10.1097/00006842-199809000-00017. [DOI] [PubMed] [Google Scholar]
- 48.Johansson J.K., Kronholm E., Jula A.M. Variability in home-measured blood pressure and heart rate: associations with self-reported insomnia and sleep duration. J Hypertens. 2011;29(10):1897–1905. doi: 10.1097/HJH.0b013e32834abccd. [DOI] [PubMed] [Google Scholar]
- 49.Manolis A.J., Poulimenos L.E., Kallistratos M.S., Gavras I., Gavras H. Sympathetic overactivity in hypertension and cardiovascular disease. Curr Vasc Pharmacol. 2014;12(1):4–15. doi: 10.2174/15701611113119990140. [DOI] [PubMed] [Google Scholar]
- 50.Horsley K.J., Rouleau C.R., Garland S.N. Insomnia symptoms and heart rate recovery among patients in cardiac rehabilitation. J Behav Med. 2016;39(4):642–651. doi: 10.1007/s10865-016-9725-y. [DOI] [PubMed] [Google Scholar]
- 51.Gagnon C., Belanger L., Ivers H., Morin C.M. Validation of the Insomnia Severity Index in primary care. J Am Board Fam Med. 2013;26(6):701–710. doi: 10.3122/jabfm.2013.06.130064. [DOI] [PubMed] [Google Scholar]
- 52.Castro-Diehl C., Diez Roux A.V., Redline S. Sleep duration and quality in relation to autonomic nervous system measures: the Multi-Ethnic Study of Atherosclerosis (MESA) Sleep. 2016;39(11):1927–1940. doi: 10.5665/sleep.6218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mullington J.M., Simpson N.S., Meier-Ewert H.K., Haack M. Sleep loss and inflammation. Best Pract Res Clin Endocrinol Metab. 2010;24(5):775–784. doi: 10.1016/j.beem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shearer W.T., Reuben J.M., Mullington J.M. Soluble TNF-alpha receptor 1 and IL-6 plasma levels in humans subjected to the sleep deprivation model of spaceflight. J Allergy Clin Immunol. 2001;107(1):165–170. doi: 10.1067/mai.2001.112270. [DOI] [PubMed] [Google Scholar]
- 55.Patel S.R., Zhu X., Storfer-Isser A. Sleep duration and biomarkers of inflammation. Sleep. 2009;32(2):200–204. doi: 10.1093/sleep/32.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liukkonen T., Rasanen P., Ruokonen A. C-reactive protein levels and sleep disturbances: observations based on the Northern Finland 1966 Birth Cohort study. Psychosom Med. 2007;69(8):756–761. doi: 10.1097/PSY.0b013e318157cb96. [DOI] [PubMed] [Google Scholar]
- 57.Nakazaki C., Noda A., Koike Y., Yamada S., Murohara T., Ozaki N. Association of insomnia and short sleep duration with atherosclerosis risk in the elderly. Am J Hypertens. 2012;25(11):1149–1155. doi: 10.1038/ajh.2012.107. [DOI] [PubMed] [Google Scholar]
- 58.Baglioni C., Spiegelhalder K., Nissen C., Riemann D. Clinical implications of the causal relationship between insomnia and depression: how individually tailored treatment of sleeping difficulties could prevent the onset of depression. EPMA J. 2011;2(3):287–293. doi: 10.1007/s13167-011-0079-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rugulies R. Depression as a predictor for coronary heart disease. a review and meta-analysis. Am J Prev Med. 2002;23(1):51–61. doi: 10.1016/s0749-3797(02)00439-7. [DOI] [PubMed] [Google Scholar]
- 60.Jiang W., Alexander J., Christopher E. Relationship of depression to increased risk of mortality and rehospitalization in patients with congestive heart failure. Arch Intern Med. 2001;161(15):1849–1856. doi: 10.1001/archinte.161.15.1849. [DOI] [PubMed] [Google Scholar]
- 61.Ogunbanjo B.O. Sexually transmitted diseases in Nigeria. A review of the present situation. West Afr J Med. 1989;8(1):42–49. [PubMed] [Google Scholar]
- 62.Meng L., Zheng Y., Hui R. The relationship of sleep duration and insomnia to risk of hypertension incidence: a meta-analysis of prospective cohort studies. Hypertens Res. 2013;36(11):985–995. doi: 10.1038/hr.2013.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hsu C.Y., Chen Y.T., Chen M.H. The association between insomnia and increased future cardiovascular events: a nationwide population-based study. Psychosom Med. 2015;77(7):743–751. doi: 10.1097/PSY.0000000000000199. [DOI] [PubMed] [Google Scholar]
- 64.Strand L.B., Tsai M.K., Gunnell D., Janszky I., Wen C.P., Chang S.S. Self-reported sleep duration and coronary heart disease mortality: a large cohort study of 400,000 Taiwanese adults. Int J Cardiol. 2016;207:246–251. doi: 10.1016/j.ijcard.2016.01.044. [DOI] [PubMed] [Google Scholar]
- 65.Ingelsson E., Lind L., Arnlov J., Sundstrom J. Sleep disturbances independently predict heart failure in overweight middle-aged men. Eur J Heart Fail. 2007;9(2):184–190. doi: 10.1016/j.ejheart.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 66.Strand L.B., Laugsand L.E., Dalen H., Vatten L., Janszky I. Insomnia and left ventricular function—an echocardiography study. Scand Cardiovasc J. 2016;50(3):187–192. doi: 10.3109/14017431.2016.1157205. [DOI] [PubMed] [Google Scholar]
- 67.Sofi F., Cesari F., Casini A., Macchi C., Abbate R., Gensini G.F. Insomnia and risk of cardiovascular disease: a meta-analysis. Eur J Prev Cardiol. 2014;21(1):57–64. doi: 10.1177/2047487312460020. [DOI] [PubMed] [Google Scholar]
- 68.Conley S., Redeker N.S. Cognitive Behavioral therapy for insomnia in the context of cardiovascular conditions. Curr Sleep Med Rep. 2015;1(3):157–165. doi: 10.1007/s40675-015-0019-7. [DOI] [PMC free article] [PubMed] [Google Scholar]