Abstract
Enzymatic hydrolysis of seaweed biomass was studied using xylanase produced from marine bacteria Bacillus sp. strain BT21 through solid-state fermentation of wheat bran. Three types of seaweeds, Ahnfeltia plicata, Padina tetrastromatica and Ulva lactuca, were selected as representatives of red, brown, and green seaweeds, respectively. Seaweed biomass was pretreated with hot water. The efficiency of pretreated biomass to release reducing sugar by the action of xylanase as well as the type of monosaccharide released during enzyme saccharification of seaweed biomass was studied. It was seen that pretreated biomass of seaweed A. plicata, U. lactuca, and P. tetrastroma, at 121 °C for 45 min, followed by incubation with 50 IU xylanase released reducing sugars of 233 ± 5.3, 100 ± 6.1 and 73.3 ± 4.1 µg/mg of seaweed biomass, respectively. Gas chromatography analysis illustrated the release of xylose, glucose, and mannose during the treatment process. Hot water pre-treatment process enhanced enzymatic conversion of biomass into sugars. This study revealed the important role of xylanase in saccharification of seaweed, a promising feedstock for third-generation bioethanol production.
Keywords: Enzyme, Xylanase, Pre-treatment, Hydrolysis, Seaweed, Bacillus sp.
Introduction
Xylan, a major component of hemicelluloses, is a heterogeneous molecule having a linear backbone consisting of β-(1,4)-linked d-xylosyl residues and several side branches of different groups attached to the main chain. Due to heterogeneous nature of xylan, its hydrolysis requires complex enzyme system in which group of enzymes work together synergistically, which mainly includes the main-chain enzyme and side-chain enzyme. In general, depolymerisation of xylan is accomplished by the action of endo-xylanases and β-xylosidases. Endo-1,4-β-xylanases (EC 3.2.1.8) hydrolyze β-1,4-glycosidic linkages of the xylan backbone to produce short-chain xylooligosaccharides of various lengths. Hence, endo-xylanases are the crucial enzyme components of the microbial xylanolytic systems (Frederick et al. 1981; Beg et al. 2001).
From the biotechnological point of view, xylanases have applications in animal feed, aroma, fruit juices, baking, textile, paper industries, ethanol (Polizeli et al. 2005; Khandeparkar and Bhosle 2006b; Khandeparker et al. 2017), and human health (Harris and Ramalingam 2010). Production of fuel ethanol from renewable lignocellulosic materials has been extensively studied in the last decades (Eriksson et al. 2002; Sun and Cheng 2002). Currently, bioethanol is mainly derived from sucrose and starch crops (e.g., sugarcane and corn) as well as lignocellulosic materials (e.g., rice straw and switchgrass). Major drawback faced here is limited cultivable lands, as well as the high costs involved in converting lignocellulosic materials into ethanol due to the presence of lignin. In view of these problems, algae have recently been considered as a third-generation feedstock for biofuel production (Nigam and Singh 2010). Algal feedstocks have several advantages over other types of feedstock. These include high area productivity, no competition with conventional agriculture for land, utilization of different water sources (e.g., seawater, brackish water, saline water, and wastewater), recycling of carbon dioxide, and compatibility with integrated production of fuels and co-products within bio refineries. Hence, algal feedstocks are considered one of the most promising non-food feedstocks for biofuels (Wijffels and Barbosa 2010; Wang et al. 2011; Borines et al. 2011; Wei et al. 2013). However, seaweed polysaccharides are structurally complex and diverse in chemical composition and differ from land plants with respect to the abundance of matrix and skeletal components. Thus, an efficient hydrolysis for sustainable production of biofuels from different macroalgal feedstocks is required (Trivedi et al. 2013). The objective of this study is to study the efficiency of hot water pre-treatment process to enhance enzymatic conversion of seaweed biomass using xylanase.
Materials and methods
Microorganism, culture condition, and chemicals
Bacterial culture was isolated from Chorao island of Mandovi estuary, Goa, India. The culture was grown at room temperature in basal salt solution (BSS) supplemented with xylan (0.5%) as sole carbon source. The composition of the BSS medium (w/v) was as follows: NaCl, 30.00 g; KCL, 0.75 g; MgSO4, 7.00 g; NH4Cl, 1.00 g; K2HPO4 (10%), 7.00 mL; KH2PO4 (10%), 3.00 mL; trace metal solution, 1.00 mL; distilled water, 1000 mL; the pH of the medium was adjusted using 1 N NaOH. Trace metal solution has the following composition: H3B03, 2.85 g; MnCl2·7H2O, 1.80 g; FeS04·7H20, 2.49 g; Na-tartrate, 1.77 g; CuCl2, 0.03 g; ZnCl2, 0.02 g; CoCl2, 0.04 g; Na2MoO4·2H2O, 0.02 g; distilled water, 1000 mL (Khandeparkar and Bhosle 2006a). Xylan (beech wood) and 3,5-dinitrosalicylic acid, and gas chromatography (GC) standards were purchased from Sigma-Aldrich Co., St Louis, MO, USA.
Production and preparation of enzyme
The culture was grown for 48 h in the BSS as above; this was used to inoculate 500-mL flasks each containing 30 mL of BSS medium and 10 g (substrate to moisture ratio 1:3) of wheat bran. The flasks were incubated at room temperature. The culture was harvested in the stationary growth phase, i.e., after 4 days. The content of the flask was suspended in 100 mL of 50 mM glycine–NaOH buffer (pH 9) vortexed thoroughly and centrifuged (10,000 rpm for 10 min, 4 °C). The enzyme was precipitated from the culture supernatant by adding ammonium sulfate to 80% saturation. This was left overnight and the precipitate was collected by centrifugation at 10,000 rpm for 10 min. The precipitate obtained was dissolved in phosphate buffer (50 mM, pH 8.0) and dialyzed against the same buffer for 24 h. Dialysis was carried out using cellulose tubing (molecular weight cut-off 13,000 Da). The enzyme was partially purified using ion-exchange chromatography (Khandeparkar and Bhosle 2006b).
Enzyme assay
Xylanase assay was carried out using 3,5-dinitrosalicylic acid method (DNS method) (Miller 1959). 1% solution of xylan was used as substrate for xylanase. The reducing sugars released due to enzymatic hydrolysis were measured at 510 nm using spectrophotometer.
Estimation of carbohydrate
10 mg of dried seaweed powder was taken and boiled for 2 h in 1 mL of 2.5 N HCl in water bath. The mixture was then cooled and centrifuged. The supernatant was used for carbohydrate estimation using 5% phenol solution and concentrated sulphuric acid (Dubois et al. 1956). The color intensity was measured at 490 nm. Sugar content was calculated by referring to a standard d-glucose and the results have been expressed as μg/mg sugar.
Collection of seaweed sample
Seaweeds such as A. plicata, P. tetrastromatica, and U. lactuca were collected from the coast of Goa (15.5809°N, 73.7448°E), India. The seaweed samples were washed thoroughly with fresh water to remove salts and debris, and were dried at 50 °C temperature. After drying, the seaweed samples were powdered using a grinder.
Pre-treatment of seaweed
Seaweed pre-treatment was carried out with 10% macroalgal biomass at 121 °C for 45 min. Pre-treatment was performed as per the reports of Yazdani et al. (2015). After the pre-treatment, solids were separated from the solutions by vacuum filtration and washed several times with distilled water.
Optimization of hydrolysis condition for effective saccharification of seaweed
Xylanase doses and incubation period were optimized for enzymatic hydrolysis of seaweed. Dried seaweed biomass (1%) was hydrolyzed with different concentrations of xylanase from 10 to 50 U/mg dry wt. Seaweed biomass was incubated for different time intervals from 0 to 8 h at 30 °C on an orbital shaker with a speed of 140 rpm. Samples were taken out periodically after an interval of 2 h each and centrifuged. The reducing sugar was measured spectrophotometrically using 3,5-dinitrosalicylic acid (DNS) method (Miller 1959).
Enzymatic hydrolysis of seaweed
Pretreated and untreated macroalgal biomass was added to a phosphate buffer solution (0.05 M), pH 7, and the enzymatic hydrolysis was initiated by adding xylanase (50 IU/mg) to seaweed biomass. The hydrolysis was performed at 30 °C for 6 h. The product was centrifuged and RS (reducing sugar) from hydrolysate was analyzed by DNS method which was then further processed for GC analysis.
GC analysis of hydrolytic product
The monosaccharide composition of seaweed hydrolysis product after hot water treatment and after enzyme treatment was studied using gas chromatography (GC) method described in Khodse et al. (2008). Briefly, the sample was treated with 12 M H2SO4 at room temperature for 2 h. It was diluted with 1.2 M H2SO4 using cold distilled water, flushed with N2, sealed and hydrolyzed for 3 h at 100 °C. After cooling, an internal standard (inositol) was added. The sample was neutralized, treated with NaBH4, acetylated and analyzed using a Shimadzu GC Model-GC-2010 equipped with a flame ionization detector (FID), a programmable on-column injector and a fused silica column coated with CPSil-88 (25 m, i.d. 0.32 mm). The response factors were calculated using standard sugar alditol acetates and myoinositol as an internal standard and were used for the quantification of the results.
Result
Identification of the bacterial isolate
Morphological and biochemical analysis showed that isolate BT21 was similar to members of the genus Bacillus. 16S rRNA analysis showed that isolate BT21 was equidistantly related to Bacillus tequilensis strain IARI-BHI-20 and members of the representatives of the Bacillus subtilis cluster (similarity value of 99%). Our results are very similar to that of Gatson et al. (2006) who had also reported that B. tequilensis is closely related to B. subtilis, but could be differentiated on the basis of DNA homology. Given this, we are unable to report on its precise taxonomic position and will refer to it as Bacillus sp. strain BT21. The 16S rRNA gene sequence has been submitted to GenBank under accession number KF797798.
Carbohydrate content of seaweed
The carbohydrate content of seaweeds is listed in Table 1. A. plicata red seaweed had a total carbohydrate content of 43.05 ± 0.7%. P. tetrastromatica brown seaweed showed 14.2 ± 0.8%, and U. lactuca green seaweed had carbohydrate content of 39.2 ± 0.8% on dry weight basis.
Table 1.
Carbohydrate composition of seaweeds during biomass processing (mean ± SD)
| Seaweeds | Total carbohydrate (μg/mg) | |||
|---|---|---|---|---|
| Untreated biomass | 30-min pre-treatment | 45-min pre-treatment | 60-min pre-treatment | |
| P. tetrastromatica | 142.1 ± 8.32 | 139.07 ± 4.56 | 136.36 ± 5.12 | 129.12 ± 5.78 |
| A. plicata | 430.5 ± 7.56 | 419 ± 6.33 | 406.00 ± 3.92 | 390 ± 4.68 |
| Ulva lactuca | 393.4 ± 12.31 | 357 ± 8.02 | 343.00 ± 6.88 | 306 ± 5.11 |
Optimization of enzymatic treatment on seaweed biomass
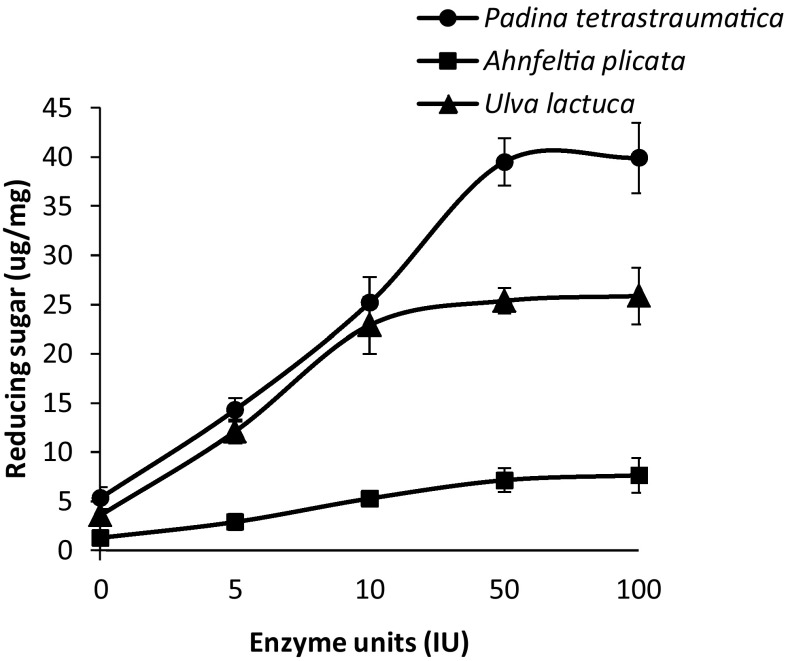
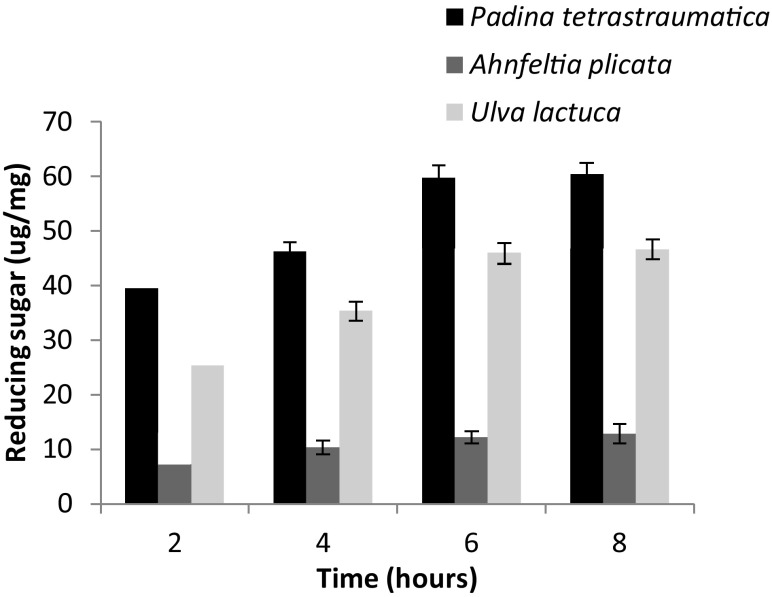
Dry biomass of P. tetrastromatica, A. plicata, and U. lactuca was saccharified by incubating it with different doses of enzyme (0–100 U). Treatment resulted in attaining maximum reducing sugar yield of 39.5 ± 2.4, 9.16 ± 1.9, and 25.36 ± 1.3 µg/mg, respectively, at an enzyme dose of 50 U for 2 h of incubation (Fig. 1). The enzyme dosage of 50 U/mg was employed for the hydrolysis of biomass in subsequent optimization of incubation period. All three seaweeds showed increase in reducing sugar yield with increase in incubation period from 2 to 6 h, while it remained steady for the next 2 h. Reducing sugar release from P. tetrastromatica increased from 39.5 ± 2.4 to 59.56 ± 2.9 µg/mg when incubated for 6 h, while A. plicata showed release of reducing sugars from 9.16 ± 1.9 to 12.16 ± 2.4 µg/mg, and reducing sugar yield from U. lactuca indicated to increase from 25.36 ± 1.3 to 45.84 ± 3.4 µg/mg after 6 h of incubation (Fig. 2).
Fig. 1.
Saccharification of untreated solid waste (1%) of A. plicata (filled square), P. tetrastromatica (filled circle), and U. lactuca (filled triangle) using different concentrations (10–50 U/g biomass) of Bacillus sp. strain BT21 xylanase at 30 °C. Error bars indicate the standard deviation of three replicates
Fig. 2.
Optimization of enzymatic hydrolysis of A. plicata, P. tetrastromatica, and U. lactuca with respect to different incubation periods (2–8 h) at 30 °C, pH 7, using 50 U of Bacillus sp. strain BT21 xylanase. Error bars indicate the standard deviation of three replicates
Saccharification by optimizing pre-treatment conditions on seaweed biomass
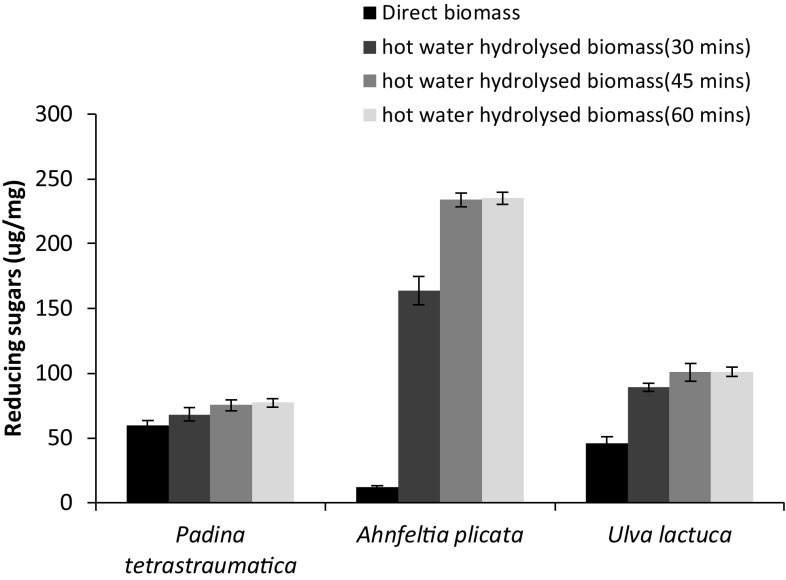
In this study, macroalgal biomass was pretreated with hot water and the effect of pre-treatment on reducing sugar yield was studied. It was seen that the amount of reducing sugar release by all three seaweeds was maximum when the seaweed was pretreated with hot water for 45 min and with further increase in pre-treatment time, sugar yield remained almost constant (Fig. 3).
Fig. 3.
The effect of hot water pre-treatment for different time intervals (30, 45, and 60 min) on the yield of reducing sugars from biomass of A. plicata, P. tetrastromatica, and U. lactuca. Pre-treatment was followed by enzymatic hydrolysis for 6 h and using 50 U of Bacillus sp. strain BT21 xylanase per gram of biomass at 30 °C, pH 7. Error bars indicate the standard deviation of three replicates
It was seen that hot water-pretreated dry biomass of A. plicata showed drastic increase in reducing sugar (RS) yields of 233 ± 5.3 µg/mg (Fig. 3) when treated with 50 IU of xylanase, while untreated dry biomass of A. plicata showed 12.17 ± 0.3 µg/mg of sugar release after enzyme treatment. Carbohydrate content of pretreated A. plicata biomass was 406 ± 3.92 μg/mg on dry weight basis (Table 1) and it was seen that the enzymatic hydrolysis released around 57% of RS from this macroalgae. Dry biomass of U. lactuca showed reducing sugar (RS) yields of 100 ± 6.1 µg/mg (Fig. 3), while untreated dry biomass of U. lactuca showed 45.8 ± 2.4 µg/mg of sugar release with enzyme treatment. Carbohydrate content of pretreated U. lactuca biomass was 343 ± 6.88 μg/mg on dry weight basis (Table 1), here the release of 29% of RS was observed. RS yield from dry biomass of P. tetrastromatica was seen to be 73.3 ± 4.1 µg/mg (Fig. 3), while untreated dry biomass of P. tetrastromatica showed 59.5 ± 2.3 µg/mg of sugar release. Carbohydrate content of pretreated P. tetrastromatica biomass was 136.36 ± 5.12 μg/mg on dry weight basis (Table 1), thus 53% of RS was released due to enzyme treatment.
Monosaccharide composition of sugars released during hydrolysis
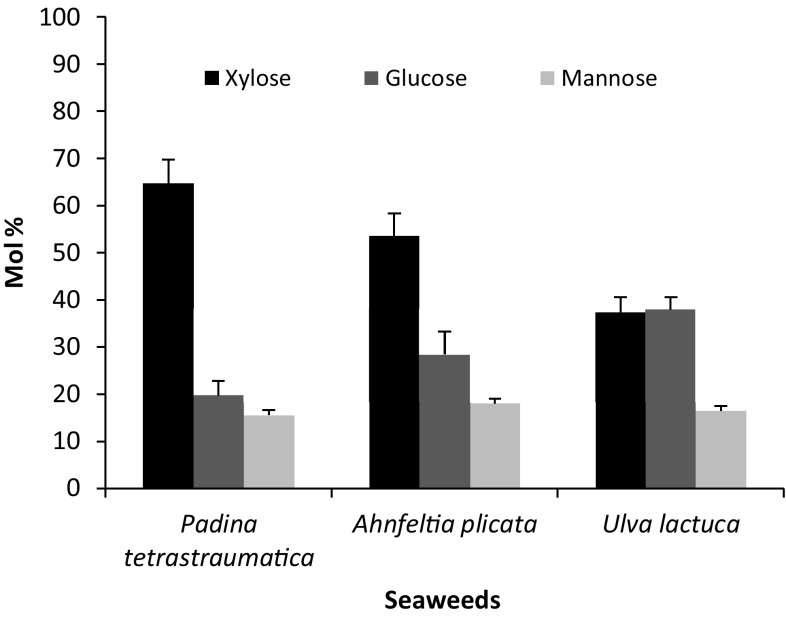
The monomeric sugar yield in the hydrolyzate derived from hot water-pretreated biomass was analyzed by gas chromatography, results are shown in Fig. 4. It was observed that xylanase released xylose, glucose, and mannose from the hot water-pretreated biomass. It was noticed that the amount of xylose monomers released during hydrolysis was more in Padina sp. (64%) and Ahnfeltia sp. (53%), while Ulva sp. released 37% of xylose and glucose in hydrolysate along with small amount of mannose. It is observed that P. pavonia contain fucose (35%) and xylose (8%) as major monosaccharides in cell wall followed by glucose (5%), mannose (2%), and galactose (1%) (Fig. 4).
Fig. 4.
Variation in the average monosaccharide composition (as Mol%) released due to enzymatic hydrolysis of Bacillus sp. strain BT21 xylanase on hot water-pretreated biomass (pretreated for 45 min) of A. plicata, P. tetrastromatica, and U. lactuca at 30 °C, pH 7, for 6 h. Error bars indicate the standard deviation of three replicates
Discussion
In our study, red seaweed had higher carbohydrate content compared to green seaweed, while brown seaweed had least carbohydrates. Carbohydrate content of seaweeds ranging from 10.63 to 28.58% is reported by Parthiban et al. (2013), authors reported maximum carbohydrate content in the green seaweed Enteromorpha intestinalis and the brown seaweed Dictyota dichotoma was recorded with minimum value. Dhargalkar et al. (1980) from Maharashtra coast and Sobha et al. (2001) from Kovalam coast noted maximum value of carbohydrate content in Rhodophycean members than in Phaeophycean and Chlorophycean members. Kumar et al. (2011) reported slightly higher yields of carbohydrates ranging from 46 to 57% on dry weight basis in different taxa of Ulva from tropical seaweeds. Carbohydrate content of Hawaiian seaweeds reported by McDermid and Stuercke (2003) showed 20% carbohydrate content in Ulva sp., while other green seaweeds ranged from 4.5 to 39.9% of carbohydrate. They also reported Ahnfeltiopsis sp. to have carbohydrate content around 30–35%, while in other red seaweeds carbohydrate content ranged from 10 to 35%. Brown seaweeds reported in this study had least carbohydrates ranging from 7 to 12%. The variations in the carbohydrate contents in seaweeds may be attributed to species difference and to the differences in their habitat and metabolic preferences (Pádua et al. 2004).
The saccharification of seaweed is an essential unit operation for ethanol fermentation and has been widely studied in recent years. Various physical, chemical, and biological pre-treatment have been shown to increase saccharification efficiency (Lu et al. 2010). One of the primary steps to increase the hydrolysis rate of the macroalgal biomass is to apply a pre-treatment to enhance the bio-digestibility of the seaweed, thus increasing accessibility of biomass to hydrolytic enzymes. Okuda et al. (2008) showed that hydrothermal pre-treatment can improve the rate of enzymatic hydrolysis of glucan in red and green macroalgae.
Enzymatic pre-treatment of seaweed is mainly influenced by the biochemical composition, physiological structure, life-cycle period, and type of seaweed. It was noticed that the amount of xylose monomers released during hydrolysis was more in Ahnfeltia sp. and Padina sp, while Ulva sp. released similar amount of xylose and glucose in hydrolysate along with small amount of mannose. Ahnfeltia sp. and Padina sp. also released substantial amount of glucose and mannose. A high percentage of xylose release is quite natural as xylanase was used for hydrolysis which cleaves xylose from xylan backbone. We have not found any traces of fucose in enzymatic hydrolysate of Padina sp., may be xylanase was not effective in releasing fucose from cell wall polysaccharides. We have reported U. lactuca, green algae, releases 37% of glucose and xylose during enzymatic action. U. lactuca has been reported to contain 44% of glucose and 31% of xylose monomer (Jiao et al. 2012). In this study, action of xylanase releasing xylose as well as substantial amount of glucose during enzyme hydrolysis indicates the possibility of xyloglucan-like structure in seaweeds, mainly in U. lactuca which shows 37% of glucose release. Xyloglucan has a backbone of β1 → 4-linked glucose residues, most of which are substituted with 1–6-linked xylose side chains. Roelofsen et al. (1953) proposed the idea of xyloglucan as a polysaccharide in cell wall of algae while working on Halicystis osterhouti as they found both xylose and glucose in alkaline extract of these algae. Glucose and xylose yields of 93.2 and 79.5% at 15 FPU/g cellulose, respectively, are also reported when corn stover was pretreated with lime and saccharified with cellulase (Kim and Holtzapple 2005). They termed the polysaccharides in corn stover as holocellulose (cellulose and hemicellulose). Xylan is a main constituent of seaweed. Algal biomass has been reported to have high hemicelluloses (16–20%) content compared to cellulose (7–9%) (Ververis et al. 2007; Yaich et al. 2011), but there are no reports on enzyme hydrolysis of seaweeds by hemicellulases, thus it becomes mandatory to focus on the role of xylanase in the conversion of biomass to sugars and further to bioethanol.
Conclusion
Algae are emerging as one of the most promising long-term, sustainable sources of biomass for fuel, food, feed, and other co-products. Improved saccharification of seaweed will help in producing high concentrations of ethanol. Algal biomass has high hemicellulose content compared to cellulose. This research and previous study suggest that enzymatic hydrolysis followed by hot water pre-treatment of seaweed biomass could be effectively employed for higher yield of reducing sugar if we use cocktail of cellulase and xylanase. Further focus on using enzyme cocktail for potential utilization of seaweed biomass as feedstock for sustainable energy is required.
Acknowledgements
The authors are grateful to the Director, National Institute of Oceanography (CSIR), Goa (India) for providing necessary facilities and Dr. N. Ramaiah for encouragement and support. Authors thank Ram Murti Meena for gene sequencing. Authors thank DST, New Delhi and CSIR-funded project PSC0206 for financial support. This is National Institute of Oceanography (NIO) contribution number 8328.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Beg QK, Kapoor M, Mahajan L, Hoondal GS. Microbial xylanases and their industrial applications: a review. Appl Microbiol Biotechnol. 2001;56:326–338. doi: 10.1007/s002530100704. [DOI] [PubMed] [Google Scholar]
- Borines MG, de Leon RL, McHenry MP. Bioethanol production from farming non-food macroalgae in Pacific island nations: chemical constituents, bioethanol yields, and prospective species in the Philippines. Renew Sustain Energy Rev. 2011;15(9):4432–4435. doi: 10.1016/j.rser.2011.07.109. [DOI] [Google Scholar]
- Dhargalkar VK, Jatap TJ, Untawale AG. Biochemical constituents of seaweeds along the Maharashtra coast. Indian J Mar Sci. 1980;9(4):297–299. [Google Scholar]
- Dubois M, Gilles K, Hamilton J, Rebers P, Smith F. Colorimetric method for determination of sugars and related substances. Anal Biochem. 1956;28(3):350–356. [Google Scholar]
- Eriksson T, Borjesson J, Tjerneld F. Mechanism of surfactant effect in enzymatic hydrolysis of lignocelluloses. Enzyme Microb Technol. 2002;31(3):353–364. doi: 10.1016/S0141-0229(02)00134-5. [DOI] [Google Scholar]
- Frederick MM, Frederick JR, Fratzke AF, Reilly PJ. Purification and characterization of a xylobiose- and xylose-producing endo-xylanase from Aspergillus niger. Carbohydr Res. 1981;97(1):87–103. doi: 10.1016/S0008-6215(00)80527-3. [DOI] [Google Scholar]
- Gatson JW, Benz BF, Chandrasekaran C, Satomi M, Venkateswaran K, Hart ME. Bacillus tequilensis sp. nov., isolated from a 2000-year-old Mexican shaft-tomb, is closely related to Bacillus subtilis. Int J Syst Evol Microbiol. 2006;56:1475–1484. doi: 10.1099/ijs.0.63946-0. [DOI] [PubMed] [Google Scholar]
- Harris AD, Ramalingam C. Xylanases and its application in food industry: a review. J Exp Sci. 2010;1(7):01–11. [Google Scholar]
- Jiao GL, Yu GL, Wang W, Zhao XL, Zhang JZ, Stephen HE. Properties of polysaccharides in several seaweeds from Atlantic Canada and their potential anti-influenza viral activities. J Ocean Univ China. 2012;11:205–212. doi: 10.1007/s11802-012-1906-x. [DOI] [Google Scholar]
- Khandeparkar R, Bhosle N. Purification and characterization of thermoalkalophilic xylanase isolated from the Enterobacter sp. MTCC 5112. Res Microbiol. 2006;157:315–325. doi: 10.1016/j.resmic.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Khandeparkar R, Bhosle N. Isolation, purification and characterization of the xylanase produced by Arthrobacter sp. MTCC 5214 when grown in solid-state fermentation. Enzyme Microb Technol. 2006;39:732–742. doi: 10.1016/j.enzmictec.2005.12.008. [DOI] [Google Scholar]
- Khandeparker R, Parab P, Amberkar U. Recombinant xylanase from Bacillus tequilensis BT21: biochemical characterisation and its application in the production of xylobiose from agricultural residues. Food Technol Biotechnol. 2017;55(2):164–172. doi: 10.17113/ftb.55.02.17.4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodse VB, Fernandes L, Bhosle NB, Sardessai S. Carbohydrates, uronic acids and alkali extractable carbohydrates in contrasting marine and estuarine sediments: distribution, size fractionation and partial chemical characterization. Org Geochem. 2008;39:265–283. doi: 10.1016/j.orggeochem.2008.01.003. [DOI] [Google Scholar]
- Kim S, Holtzapple MT. Lime pretreatment and enzymatic hydrolysis of corn stover. Bioresour Technol. 2005;96:1994–2006. doi: 10.1016/j.biortech.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Kumar M, Trivedi N, Reddy CR, Jha B. Toxic effects of imidazolium ionic liquids on the green seaweed Ulva lactuca: oxidative stress and DNA damage. Chem Res Toxicol. 2011;24:1882–1890. doi: 10.1021/tx200228c. [DOI] [PubMed] [Google Scholar]
- Lu C, Wang H, Luo Y, Guo L. An efficient system for pre-delignification of gramineous biofuel feedstock in vitro: application of a laccase from Pycnoporus sanguineus H275. Protein Biochem. 2010;45:1141–1147. doi: 10.1016/j.procbio.2010.04.010. [DOI] [Google Scholar]
- McDermid KJ, Stuercke B. Nutritional composition of edible Hawaiian seaweeds. J Appl Phycol. 2003;15:513–524. doi: 10.1023/B:JAPH.0000004345.31686.7f. [DOI] [Google Scholar]
- Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31(3):426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- Nigam PS, Singh A. Production of liquid biofuels from renewable resources. Prog Energy Combust Sci. 2010;37:42–68. [Google Scholar]
- Okuda K, Oka K, Onda A, Kajiyoshi K, Hiraoka M, Yanagisawa K. Hydrothermal fractional pretreatment of sea algae and its enhanced enzymatic hydrolysis. J Chem Technol Biotechnol. 2008;83:836–841. doi: 10.1002/jctb.1877. [DOI] [Google Scholar]
- Pádua MD, Fontoura PSG, Mathias AB. Chemical composition of Ulvaria oxysperma (Kützing) Bliding, Ulva lactuca (Linnaeus) and Ulva fasciata (Delile) Braz Arch Biol Technol. 2004;47(1):49–55. doi: 10.1590/S1516-89132004000100007. [DOI] [Google Scholar]
- Parthiban C, Saranya C, Girija K, Hemalatha A, Suresh M, Anantharaman P. Biochemical composition of some selected seaweeds from Tuticorin coast. Adv Appl Sci Res. 2013;4(3):362–366. [Google Scholar]
- Polizeli MLTM, Rizzatti ACS, Monti R, Terenzi HF, Jorge JA, Amorim DS. Xylanases from fungi: properties and industrial applications. Appl Microbiol Biotechnol. 2005;67:577. doi: 10.1007/s00253-005-1904-7. [DOI] [PubMed] [Google Scholar]
- Roelofsen PA, Dalitz VC, Wijnman CF. Constitution, submicroscopic structure and degree of crystallinity of the cell wall of Halicystis osterhoutii. Biochem Biophys Acta. 1953;11:344–352. doi: 10.1016/0006-3002(53)90054-7. [DOI] [PubMed] [Google Scholar]
- Sobha V, Bindu VK, Bindu MS, Unnikrishnan P. Biochemical studies of algae along the southern Kerala coast with special reference to fibre content. Seaweed Res Util. 2001;23(1&2):65–73. [Google Scholar]
- Sun Y, Cheng J. Hydrolysis of lignocellulosic materials for ethanol production: a review. Biores Technol. 2002;83(1):1–11. doi: 10.1016/S0960-8524(01)00212-7. [DOI] [PubMed] [Google Scholar]
- Trivedi N, Gupta V, Reddy CRK, Jha B. Enzymatic hydrolysis and production of bio ethanol from common macrophytic green alga Ulva fasciata Delile. Bioresour Technol. 2013;150:106–112. doi: 10.1016/j.biortech.2013.09.103. [DOI] [PubMed] [Google Scholar]
- Ververis C, Georghiou K, Danielidis D, Hatzinikolaou D, Santas P, Santas R, Corleti V. Cellulose, hemicelluloses, lignin and ash content of some organic materials and their suitability for use as paper pulp supplements. Bioresour Technol. 2007;98:296–301. doi: 10.1016/j.biortech.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Wang X, Liu X, Wang G. Two-stage hydrolysis of invasive algal feedstock for ethanol fermentation. J Integr Plant Biol. 2011;53(3):246–252. doi: 10.1111/j.1744-7909.2010.01024.x. [DOI] [PubMed] [Google Scholar]
- Wei N, Quarterman J, Jin YS. Marine macroalgae: an untapped resource for producing fuels and chemicals. Trends Biotechnol. 2013;31(2):70–77. doi: 10.1016/j.tibtech.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Wijffels RH, Barbosa MJ. An outlook on microalgal biofuels. Science. 2010;329:796–799. doi: 10.1126/science.1189003. [DOI] [PubMed] [Google Scholar]
- Yaich H, Garna H, Besbes S, Paquot M, Blecker C, Attia H. Chemical composition and functional properties of Ulva lactuca seaweed collected in Tunisia. Food Chem. 2011;128:895–901. doi: 10.1016/j.foodchem.2011.03.114. [DOI] [Google Scholar]
- Yazdani P, Zamani A, Karimi K, Taherzadeh MJ. Characterization of Nizimuddinia zanardini macroalgae biomass composition and its potential for biofuel production. Bioresour Technol. 2015;176:196–202. doi: 10.1016/j.biortech.2014.10.141. [DOI] [PubMed] [Google Scholar]