Abstract
We previously observed that aquaporin-3 and aquaporin-10 are upregulated in the epidermis of hand dermatitis patients (Med. Hypotheses, 84, 2015, 498). To address the functional relevance of this upregulation, we overexpressed AQP3/AQP10 in mice using the human K1 promoter. Combining imiquimod with detergent-containing water challenge, a common trigger in hand and other dermatitis, resulted in an increase in acanthosis in mice overexpressing AQP3 or AQP3 and AQP10. Aquaporin overexpression also drove a trend towards greater weight loss in these animals. These data support a role for cutaneous aquaporins in the pathogenesis of dermatitis and as a potential target in their treatment.
Keywords: aquaporin-3, aquaporin-10, dermatitis, filaggrin, imiquimod
1 | BACKGROUND
Despite increased insights regarding the immunobiology of atopic dermatitis (AD) and dermatitis in general, these diseases remain chronic, incurable afflictions whose underlying pathogenesis is poorly understood but appears exacerbated by humidity gradients.[1] Indeed, in quality-of-life measures, dermatitis (AD and others) is more debilitating than psoriasis and non-melanoma skin cancer[2] and accounts for up to $3.8 billion US dollars in annual care.[3] However, the exact cause of these conditions remains incomplete. One of the underlying mechanisms involved in sustaining AD and dyshidrosis is the increase in transepidermal water loss (TEWL). TEWL is the loss of water via the skin. Aquaporins are membrane channel proteins that increase the permeability of cell membranes to the bidirectional, osmotically driven passage of small uncharged molecules, including water, glycerol and urea. Interestingly, recent publications have shown that aquaporin-3 (AQP3) and aquaporin-10 (AQP10) are the main aquaglyceroporins expressed in human keratinocytes.[4,5] In healthy skin, AQP3 expression has been reported to be low, with the majority of expression in the stratum basale,[4] although others have observed AQP3 expression in suprabasal keratinocytes as well, see supplementary references s1–4. In atopic dermatitis patients, AQP3 expression was reportedly increased in both the stratum basale and the stratum spinosum, potentially contributing to the increase in TEWL. AQP3 overexpression in keratinocytes has also been shown to promote proliferation.[6] Interestingly, UVB-treated skin, therapeutic for AD, exhibits a decrease in AQP3 expression.[7] Finally, AQP3 water and glycerol conducting properties are activated by water and inhibited in an acidic environment (pH<7), which constitutes the normal pH in the stratum corneum of healthy skin.
2 | QUESTIONS ADDRESSED
We previously demonstrated the overexpression of AQP3 and AQP10 protein in human eczematous as compared to healthy skin[8] corroborating previous studies measuring AQP3.[4–6] These findings are consistent with inflammatory skin models in mice whereby increased AQP3 expression was found after ovalbumin or oxazolone challenge.[6]
The functional relevance of augmented AQP3 expression in these inflammatory settings was unclear. We hypothesized that overexpression of AQP3±AQP10 may contribute to the severity and/or chronicity of inflammatory skin disease. Therefore, we overexpressed epidermal aquaporins in mice similar to the levels found in human skin disease states to determine their contribution to overall disease metrics (weight loss, skin thickening and skin differentiation).
3 | EXPERIMENTAL DESIGN
We overexpressed murine AQP3 (the major epidermal aquaporin) as well as human AQP10 (exclusive to human skin) in the epidermis of mice, using the human K1 promoter (Figure 1A,B, respectively). Using primers specific to spliced mRNAs of AQP3 and AQP10, we confirmed mRNA overexpression of AQP3 and APQ10 (Figure 1C). We then observed Mendelian rations of both single-and double-transgenic mice using primers specific to the transgenic DNA constructs of both AQP3 and AQP10 (Figure 1D). Corresponding protein overexpression in the epidermis was determined by immunofluorescent microscopy (Fig. S1). These mice were than examined for overall weight loss, acanthosis and epidermal differentiation of keratinocytes. We employed a variation of the imiquimod mouse model which cannot exactly recapitulate human pathologies such as AD and psoriasis but has been very well characterized and can still provide valuable insights into these human diseases.
FIGURE 1.
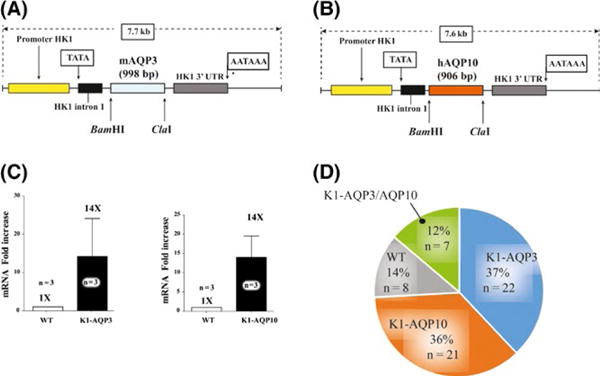
AQP3-and AQP10-transgenic mice are viable and express both transgenes in the skin. (A) AQP3 and (B) AQP10 expression construct maps under the control of the human HK1 promoter are shown. AQP3 and AQP10 coding sequences were cloned into the previously reported expression plasmid.[9] (C) mRNA was detected for both AQP3 and AQP10 construct using specific primers (P=.3, P=.03, respectively). (D) Mendelian distribution of transgene expression for progeny from AQP3×AQP10 mating. Mean and SEM are displayed
4 | RESULTS
We measured daily weights of WT and aquaporin-overexpressing mice during imiquimod challenge for 5 days followed by 4 days of detergent-containing water challenge.
We observed that single AQP3 overexpression as well as AQP3×AQP10 overexpression resulted in a trend towards greater weight loss, but did not meet statistical significance (Figure 2A,B).
FIGURE 2.
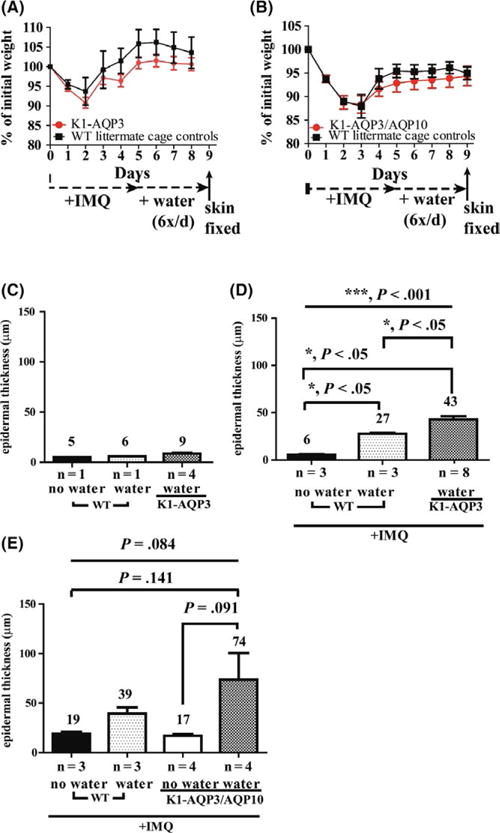
Imiquimod and detergent-containing water challenge-induced acanthosis is augmented by AQP3/AQP10 overexpression. (A) Daily weights of AQP3 transgenic compared to wild-type (WT) littermate controls recorded over the 10-day experimental protocol and similarly performed for (B) AQP3/10 transgenic mice. (C) Clinical and histological epidermal inflammation and thickening observed in AQP3 and AQP3/AQP10 transgenic mice were increased. (D) Acanthosis in AQP3 transgenic mice was similar to WT mice for water only challenge. (E) AQP3 transgenic and (F) AQP3/10 transgenic mice display increased acanthosis 4 days after cessation of IMQ application when submitted to daily detergent-containing water exposure as described in Data S1. Student’s t test was performed for pairwise comparisons in A & B, and ANOVAs were performed in C-E. Mean and SEM are displayed
Next, we examined acanthosis in these AQP3- and AQP3/AQP10-overexpressing mice after 5 days of imiquimod (IMQ) challenge and 4 days of detergent-containing water challenge. Dorsal skin harvested on day 10 appeared thicker (more acanthotic histologically) in the presence (vs absence) of detergent-containing water challenge (Figures 2C, and S2). Detergent-containing water and imiquimod-treated skin also demonstrated slightly increased acanthosis in both AQP3- and AQP3/AQP10-overexpressing mice (Figure 2D,E) that was not observed in detergent-containing water only interventions (Figure 2D,E). In AQP3-overexpressing mice (Figure 2D), there was a significant difference among three conditions overall (P<.001). Further pairwise comparisons show there was significant difference between transgenic and WT (P<.05), between transgenic and WT plus detergent-containing water (P<.05) and between WT and WT plus detergent-containing water (P<.05). In AQP3/AQP10-overexpressing mice (Figure 2E), the difference among the four conditions was P=.084. Further pairwise comparisons show a difference between transgenic and transgenic plus detergent-containing water (P=.091) and between transgenic plus detergent-containing water and WT (P=.141). P-values for other pairs were above .469.
As the skin differentiation markers filaggrin, K10 and loricrin are important for proper skin barrier function, we evaluated the expression of these proteins and transcripts. We observed a blunting of water-based induction of filaggrin upon immunohistochemical staining (Fig. S3A,B,G). No statistically significant difference in mRNA expression of filaggrin, K10 or loricrin was found using RT-qPCR (Fig. S3B,D,F). Quantification of filaggrin immunofluorescence seen in Fig. S3A was performed showing a decrease in filaggrin fluorescence albeit not statistically significant (WT plus detergent-containing water vs AQP3/AQP10 plus detergent-containing water).
5 | CONCLUSION
Increased expression of AQP3 and AQP10 in murine skin with the concomitant presence of an inflammatory stimulus (IMQ) and contact with detergent-containing water increases skin acanthosis and affects the biology of keratinocytes. A model for the proposed contribution of these factors is diagrammed in Fig. S4.
Supplementary Material
FIGURE S1 Immunohistochemistry of AQP3 and AQP10.
FIGURE S2 Clinical and gross histological epidermal inflammation and thickening observed in AQP3 and AQP3/AQP10 transgenic mice.
FIGURE S3 Induction of skin differentiation markers filaggrin, K10 and loricrin in AQP3/AQP10 overexpressing mice.
FIGURE S4 A proposed model of AQP3 and AQP10 upregulation during inflammation contributing to dysfunctional skin barrier.
DATA S1 Materials and methods.
DATA S2 Supplementary references.
Acknowledgments
This study was supported in part by the Skin Diseases Research Center Core D P30AR039750 (KDC, DLP), Start-up Institutional Funds (DLP), CTSC Core Utilization Award UL1TR000439 (DCS, TSM, DLP), ASA Carson Scholar Award (DLP), VA Merit Award IBX002719A (DLP). These studies were conducted after IACUC approval of protocol #2014-0161 in full compliance.
Footnotes
CONFLICT OF INTEREST
The authors have declared no conflicting interests.
AUTHOR CONTRIBUTIONS
DCS, ADG, KDC, TSM and DLP had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. DCS and DLP involved in study concept and design. DCS and DLP involved in analysis and interpretation of data. DCS, TSM and DLP involved in drafting of the manuscript. DCS, TSM and DLP involved in critical revision of the manuscript for important intellectual content. DCS, ADG, KDC, TSM, DLP involved in administrative, technical or material support. DLP involved in study supervision.
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the supporting information tab for this article.
References
- 1.Lofgren SM, Warshaw EM. Dermatitis. 2006;17(4):165. doi: 10.2310/6620.2006.05021. [DOI] [PubMed] [Google Scholar]
- 2.Ben-Gashir MA, Seed PT, Hay RJ. Br J Dermatol. 2004;150:284. doi: 10.1111/j.1365-2133.2004.05776.x. [DOI] [PubMed] [Google Scholar]
- 3.Ellis CN, Drake LA, Prendergast MM, Abramovits W, Boguniewicz M, Daniel CR, Lebwohl M, Stevens SR, Whitaker-Worth DL, Cheng JW, Tong KB. J Am Acad Dermatol. 2002;46:361. doi: 10.1067/mjd.2002.120528. [DOI] [PubMed] [Google Scholar]
- 4.Olsson M, Broberg A, Jernås M, Carlsson L, Rudemo M, Suurküla M, Svensson PA, Benson M. Allergy. 2006;61(9):1132. doi: 10.1111/j.1398-9995.2006.01151.x. [DOI] [PubMed] [Google Scholar]
- 5.Boury-Jamot M, Sougrat R, Tailhardat M, Le Varlet B, Bonté F, Dumas M, Verbavatz JM. Biochim Biophys Acta. 2006;1758(8):1034. doi: 10.1016/j.bbamem.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 6.Nakahigashi K, Kabashima K, Ikoma A, Verkman AS, Miyachi Y, Hara-Chikuma M. J Invest Dermatol. 2011;131:865. doi: 10.1038/jid.2010.395. [DOI] [PubMed] [Google Scholar]
- 7.Shan SJ, Xiao T, Chen J, Geng SL, Li CP, Xu X, Hong Y, Ji C, Guo Y, Wei H, Liu W, Li D, Chen HD. Int J Mol Med. 2012;29:625. doi: 10.3892/ijmm.2011.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soler DC, Bai X, Ortega L, Pethukova T, Nedorost ST, Popkin DL, Cooper KD, McCormic TS. Med Hypotheses. 2015;84(5):498. doi: 10.1016/j.mehy.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qin H, Zheng X, Zhong X, Shetty AK, Elias PM, Bollag WB. Arch Biochem Biophys. 2011;508(2):138. doi: 10.1016/j.abb.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1 Immunohistochemistry of AQP3 and AQP10.
FIGURE S2 Clinical and gross histological epidermal inflammation and thickening observed in AQP3 and AQP3/AQP10 transgenic mice.
FIGURE S3 Induction of skin differentiation markers filaggrin, K10 and loricrin in AQP3/AQP10 overexpressing mice.
FIGURE S4 A proposed model of AQP3 and AQP10 upregulation during inflammation contributing to dysfunctional skin barrier.
DATA S1 Materials and methods.
DATA S2 Supplementary references.


