Abstract
In multiple myeloma, despite recent improvements offered by new therapies, disease relapse and drug resistance still occur in the majority of patients. Therefore, there is an urgent need for new drugs that can overcome drug resistance and prolong patient survival after failure of standard therapies. The imipridone ONC201 causes downstream inactivation of ERK1/2 signaling and has tumoricidal activity against a variety of tumor types, while its efficacy in preclinical models of myeloma remains unclear. In this study, we treated human myeloma cell lines and patient-derived tumor cells with ONC201. Treatment decreased cellular viability and induced apoptosis in myeloma cell lines, with IC50 values of 1 to 1.5 μM, even in those with high risk features or TP53 loss. ONC201 increased levels of the pro-apoptotic protein Bim in myeloma cells, resulting from decreased phosphorylation of degradation-promoting Bim Ser69 by ERK1/2. In addition, myeloma cell lines made resistant to several standard-of-care agents (by chronic exposure) were equally sensitive to ONC201 as their drug-naïve counterparts, and combinations of ONC201 with proteasome inhibitors had synergistic anti-myeloma activity. Overall, these findings demonstrate that ONC201 kills myeloma cells regardless of resistance to standard-of-care therapies, making it promising for clinical testing in relapsed/refractory myeloma.
Introduction
Multiple myeloma is a neoplastic plasma-cell disorder characterized by clonal proliferation of malignant CD138+ plasma cells in bone marrow, elevated levels of monoclonal proteins in blood or urine, and severe dysfunction of organs in patients. It accounts for approximately 1% of all neoplastic diseases and 13% of hematological malignancies. In the USA, the annual age-adjusted incidence is 5.6 cases per 100,000 persons. Myeloma treatment has advanced significantly in recent years, and proteasome inhibitors (such as bortezomib and carfilzomib) and immunomodulatory agents have significantly improved outcomes of myeloma patients [1], [2]. However, relapse and drug resistance occur in virtually all responding patients [3], and so this disease remains incurable in the majority of patients, prompting a continued search for additional new therapeutics [4].
ONC201, previously referred to as TIC10, is currently in phase II clinical trials for patients with advanced tumors [5], [6]. This drug is a first-in-class small molecule that was identified in a high-throughput small-molecule library phenotypic screen as an efficacious antitumor therapeutic agent of p53-independent apoptosis [5], [6]. Subsequent mechanism of action studies have shown that ONC201 induces downstream activation of the integrated stress response and inactivation of Akt/ERK signaling in several tumor types [5], [6], [7], [8]. Preclinical studies have shown anti-proliferative and pro-apoptotic activity of ONC201, as a single agent, in numerous solid tumors including human tumor cell lines and patient samples that are refractory to chemotherapy and targeted therapies, but not normal cells. For example, treatment of ONC201 causes cell death of primary tumor cells from patients with colorectal cancer in vitro and has a potent anti-tumor effect in mice bearing cancer cell lines [9]. In glioblastoma, ONC201 treatment is reported to inhibit cell proliferation and induce cell death as well [10]. In hematological malignancies, administration of ONC201 prolongs the median survival of lymphoma-bearing mice in vivo and induces apoptosis in mantle cell lymphoma, acute myeloid leukemia, and T-cell lymphoma in in both cell lines and patient samples in vitro. A large in vitro efficacy screen of human cancer cell lines suggested that ONC201 would be particularly active in multiple myeloma, but this has not been established [6]. In this study, we determined the therapeutic efficacy and action mechanisms of ONC201 in multiple myeloma using both cultured human cell lines and patient-derived malignant plasma cells, supporting application of ONC201 in patients with this disease.
Materials and Methods
Reagents and Antibodies
Except where specified, all chemicals were purchased from Sigma-Aldrich, all antibodies for flow cytometry analysis were purchased from BD Biosciences, and all antibodies for Western blot analysis were purchased from Cell Signaling Technology. ONC201 was manufactured and provided by Oncoceutics Inc. The stock solution of ONC201 was dissolved in DMSO and stored at −80 °C in aliquots. In all experiments, the final concentration of DMSO did not exceed 0.1%. Caspase inhibitor Z-VAD-FMK was purchased from R&D systems.
Cell Lines and Primary Cells
Human myeloma cell lines were purchased from American Type Culture Collection (ATCC), except that ARP-1 and ARK cells were kindly provided by Arkansas Cancer Research Center, AR. The p53 knockout myeloma cells and drug resistant myeloma cells against bortezomib, dexamethasome, or carfilzomib were established as previously described before [11]. Primary myeloma cells were isolated from bone marrow aspirates of myeloma patients using anti-CD138 antibody-coated magnetic beads (Miltenyi Biotec, Inc.). This study was approved by the institutional review board of The University of Texas MD Anderson Cancer Center (Houston, TX). All myeloma cells were cultured in RPMI 1640 medium (Mediatech Cellgro) supplemented with 10% fetal bovine serum (FBS) and antibiotics.
Lentiviral Infection of shRNA In Vitro
Cells were infected with lentivirus containing human Bim shRNAs (Sigma-Aldrich) to knockdown Bim according to the manufacturer's protocol. Stable cell lines were selected with 0.7 μg/ml of puromycin (Sigma-Aldrich) for 4 weeks.
Cell Viability Assay
Cell viability was determined by using CellTiter-Glo® Luminescent Cell Viability Assay (Promega) according to the manufacture's protocol. Briefly, 1 × 104 cells per well in 100 μl volume were aliquoted into a 96-well opaque-walled plate and treated with ONC201 alone or in combination with bortezomib or carfilzomib. Wells without cells and with medium served as background control. After 48 or 72 hours, 100 μl of CellTiter-Glo® Reagent was added into each well, mixed for 2 minutes on an orbital shaker and incubated for 10 minutes at room temperature. The levels of luminescence signal was read by a plate reader spectraMax M5e (Molecular Devices).
Flow Cytometry Analysis
For analysis of apoptosis, cells were treated with ONC201 for 72 hours. In some experiments, 50 μM of Z-VAD-FMK were added simultaneously to the culture. Apoptosis of treated cells (5 × 105 cells/sample) was detected by annexin V–FITC/propidium iodide (PI) staining (Life Technologies). After 20 minutes of incubation at room temperature, cells were measured by a BD LSRFortessa flow cytometer and results were analyzed using Flow Jo software. Apoptotic cells were defined as the annexin V-positive cells. For analysis of levels of phosphorylated or non-phosphorylated ERK1/2 and Bim, primary myeloma cells were treated with or without 2 μM ONC201 for 24 hours. Cells were fixed, permeabilized, and stained with specific antibodies. The stained cells were measured by a BD LSRFortessa flow cytometer and results were analyzed using Flow Jo software.
Western Blot Analysis
Cells were harvested and lysed with 1 × lysis buffer (50 mM Tris, pH 7.5, 140 mM NaCl, 5 mM EDTA, 5 mM NaN3, 1% Triton-X-100, 1% NP-40, and 1 × protease inhibitor cocktail). Cell lysates were subjected to SDS-PAGE, transferred to a polyvinylidene difluoride membrane, and immunoblotted with antibodies against caspase-3, caspase-8, caspase-9, PARP, Bid, Bax, Bak, PUMA, Mcl-1, Noxa (Millipore), Bcl-2, Bcl-xL, GAPDH, phosphorylated or non-phosphorylated Bim, ERK1/2, JNK, p38, and NFκB-p65.
Analysis of drug synergy
Myeloma cells were treated with various combinations of ONC201 and bortezomib or carfilzomib for 48 hours. The cell viabilities were measured and combination index (CI) was calculated using CalcuSyn software (Biosoft version 2.0, Cambridge, United Kingdom). CI<1, =1, and >1 indicates synergism, additive effect and antagonism, respectively [12], [13].
Statistical Analysis
All data are shown as means ± standard deviation for at least three independent experiments performed in triplicate. The Student t test was used to compare experimental groups. A P value <.05 was considered statistically significant.
Results
Administration of ONC201 Reduces Tumor Growth and Viability in Myeloma
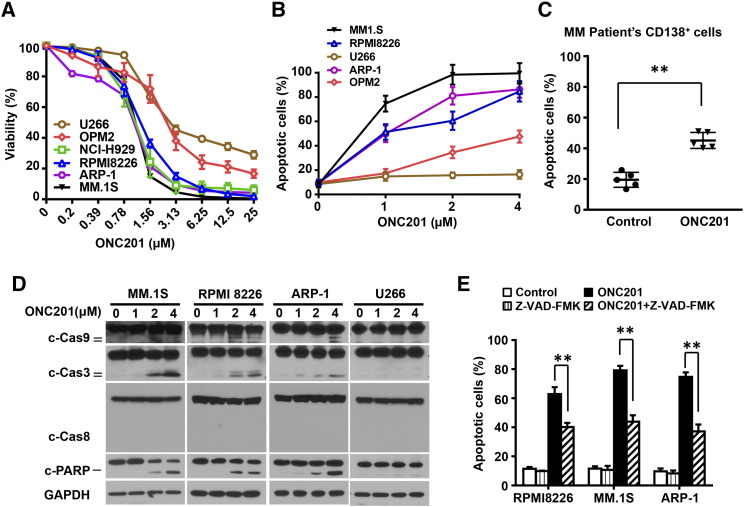
Human myeloma cell lines ARP-1, RPMI8226, MM.1S, U266, NCI-H299, and OPM2 were cultured in medium with various concentrations of ONC201 for 72 hours, and the viability of cultured cells was analyzed using the Cell-TiterGlo luminescent kit. As shown in Figure 1A, treatment of myeloma cells with ONC201 dramatically decreased viable cell number in a dose-dependent manner, and the IC50 values of ONC201 were 1 μM to 1.5 μM in ARP-1, RPMI8226, MM.1S, NCI-H299, and OPM2 cells and were 3.25 μM in U266 cells, indicating that the majority of myeloma cells are sensitive to ONC201 treatment at a concentration that is achievable based on pharmacokinetics in Phase I (Stein et al., Clinical Cancer Research, In Press).
Figure 1.
Treatment of ONC201 reduces cell viability and induces apoptosis in both patient-derived myeloma cells and cell lines. Myeloma cells were cultured with gradually increased concentrations of ONC201 for 72 hours. After cultures, the cells were subjected to the Cell-Titer Glo Luminescent kit for assessing cell viability and the annexin-V binding assay for assessing cell apoptosis. (A) Shown is the percentage of cell viability in myeloma cell lines U266, OPM2, NCI-H929, RPMI8226, ARP-1, and MM.1S cells treated with different doses of ONC201. The IC50 of ONC201 treatment is 1 μM to 1.5 μM in the tested cells. (B-C) Annexin-V binding assay shows the percentage of apoptotic cells in (B) myeloma cell lines MM.1S, ARP-1, RPMI8226, OPM2, and U266 treated with 0 μM, 1 μM, 2 μM, or 4 μM of ONC201, and (C) CD138+ malignant plasma cells isolated from bone marrow aspirates of five patients, treated without (Control) or with 2 μM of ONC201. (D) Western blot analysis shows the cleaved (c) levels of caspases (Cas) 9, 3, and 8, and PARP in MM.1S, ARP-1, RPMI8226, and U266 cells treated with 0 μM, 1 μM, 2 μM, or 4 μM of ONC201. The levels of GAPDH served as protein loading controls. (E) Flow cytometry analysis shows the percentage of apoptotic cells in the myeloma cell lines MM.1S, ARP-1, and RPMI8226, treated with ONC201 without or with the pan-caspase inhibitor Z-VAD-FMK (50 μM). Results shown are representative of three to five independent experiments. **P < .01.
An annexin V-binding assay showed that ONC201 induced apoptosis in MM.1S, ARP-1, RPMI8226, and OPM2 cells in a concentration-dependent manner, but had less of an effect in U266 cells (Figure 1B). Similarly, in cultures of CD138+ primary malignant plasma cells, isolated from the bone marrow aspirates of five patients with newly diagnosed multiple myeloma, ONC201 induced a higher percentage of apoptotic cells than in control cultures (Figure 1C). As shown in Figure 1D, ONC201 treatment up-regulated the levels of cleaved capase-9, -3, and PARP, but it did not change the levels of cleaved caspase-8 and GAPDH proteins, in MM.1S, RPMI8226, and ARP-1 cells with dose dependence. The treatment had less effect on caspase cleavage in U266 cells (Figure 1D). Addition of the pan-caspase inhibitor Z-VAD-FMK significantly protected myeloma cell lines ARP-1, MM.1S, and RPMI8226 from ONC201-induced apoptosis (Figure 1E).
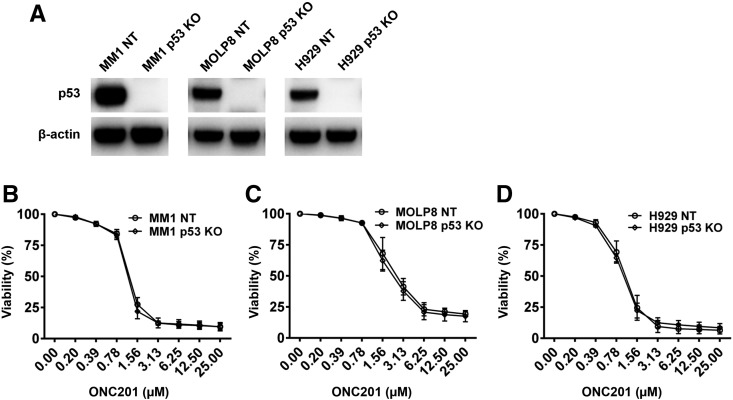
The status of TP53 has been shown to be important for myeloma cells in response to treatment. However, there was no difference in the effect of ONC201 on myeloma cell viability between p53 wild-type cell lines (ARP-1, NCI-H929 and MM.1S) and lines with mutant p53 (OPM2 and RPMI8226) (Figure 1A). Furthermore, when we used the CRISPR/Cas9 system to create isogenic p53 knockout forms of MM1, MOLP8, and NCI-H929 (Figure 2A), they were equally sensitive to ONC201 (Figure 2, B–D). In line with prior reports in other tumor types, these findings indicate that ONC201 acts in myeloma in a TP53-independent manner.
Figure 2.
ONC201 acts in a TP53-independent manner. (A) Western blot analysis shows the protein levels of p53 in the wild-type (NT) and TP53 knockout (p53 KO) myeloma cell lines MM1, MOLP8, and H929. The levels of β-actin served as protein loading controls. (B-D) The paired myeloma cell lines with NT or p53 KO were incubated with gradually increased concentrations of ONC201 in a 72 hour-culture. Shown is the percentage of cell viability in NT or p53 KO of myeloma cells MM1 (B), MOLP8 (C), and H929 (D). Results shown are representative of three independent experiments.
ONC201 Treatment Activates Bim-Mediated Apoptotic Signaling
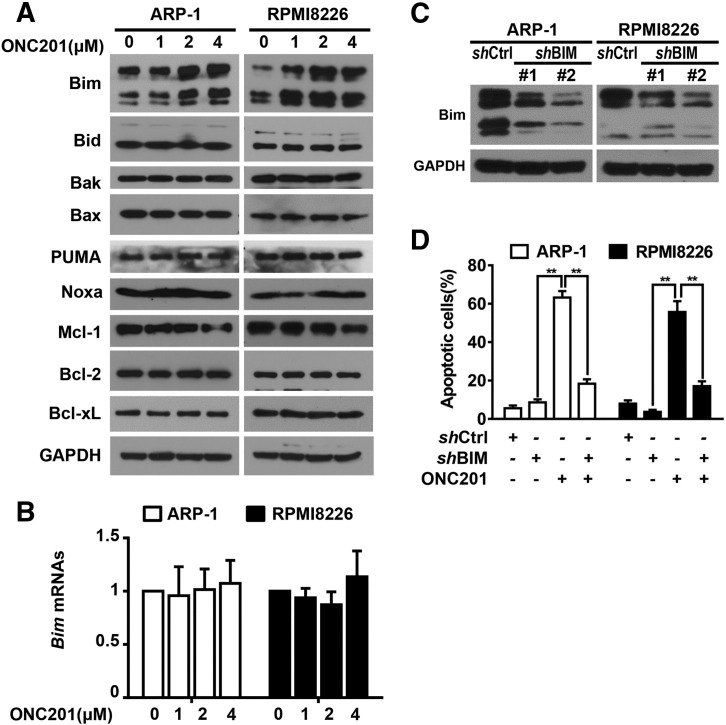
Because members of the Bcl-2 family can modulate mitochondrial permeability, thereby regulating caspase-9 dependent apoptosis in tumor cells, we examined whether ONC201 treatment regulates the expression of pro- or anti-apoptotic members of the Bcl-2 protein family. As shown in Figure 3A, ONC201 treatment significantly up-regulated the protein levels of the pro-apoptotic protein Bim, but did not change the expression of other members, in ARP-1 and RPMI8226 cells. However, we did not observe obvious change of Bim mRNAs in ONC201-treated myeloma cells as compared to those in untreated cells (Figure 3B). When we knocked down the expression of Bim in ARP-1 or RPMI8226 cells, using two clones of shRNAs specific for human Bim (Figure 3C), we found that treatment of ONC201 induced lower percentages of apoptosis than in non-targeted shRNA cells (Figure 3D). These data indicate that Bim up-regulation mediates ONC201-induced myeloma cell apoptosis.
Figure 3.
ONC201 treatment induces myeloma cell apoptosis through up-regulation of the expression of pro-apoptotic protein Bim. (A) Myeloma cells ARP-1 and RPMI8226 were cultured with 0 μM, 1 μM, 2 μM, or 4 μM of ONC201 for 24 hours, and then subjected to Western blot analysis on the levels of pro-apoptotic and anti-apoptotic proteins. The protein expression of Bim was increased in ARP-1 and RPMI8226 cells after ONC201 treatment. (B) Quantitative real-time PCR analysis shows Bim mRNAs in the myeloma cell lines ARP-1 and RPMI8226 treated with or without ONC201 (2 μM) in a 24 hour-culture. (C) Western blotting shows the reduced levels of Bim proteins in ARP-1 and RPMI8226 cells that were infected with a lentivirus carrying with the shRNAs of human Bim (shBim) when compared to those with non-targeted shRNAs (shCtrl). Two clones of shBim were examined. (D) Annexin-V binding assay shows the low percentage of apoptotic cells in ARP-1 and RPMI8226 cells with shBim after ONC201 treatment compared with that in shCtrl cells. The levels of GAPDH served as protein loading controls. Results shown are representative of three to five independent experiments. **P < .01.
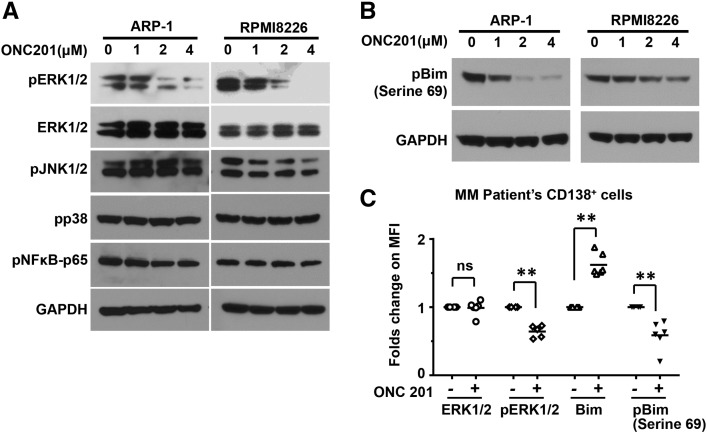
Phosphorylation of Bim at Ser69 by ERK1/2 has been shown to promote proteasomal degradation of Bim. We found that ONC201 treatment dramatically down-regulated the levels of phosphorylated ERK1/2, but did not affect the phosphorylation of other signaling kinases including NF-κB, JNK1/2, and p38 MAPK in ARP-1 and RPMI8226 cells (Figure 4A). We also observed concentration-dependent reduction in phosphorylated Bim at Ser69 (Figure 4B). Similar results were also obtained from primary myeloma cells isolated from bone marrow aspirates of patients (Figure 4C). These findings suggest that ONC201 enhances Bim expression through inhibition of the ERK1/2 signaling pathway and reduction of Bim phosphorylation.
Figure 4.
ONC201 treatment inhibits the ERK1/2 signaling and reduces the phosphorylation of Bim in myeloma cells. Western blotting analysis shows (A) the non-phosphorylated or phosphorylated (p) levels of ERK1/2, and the phosphorylated (p) levels of other kinases JNK1/2, p38, and NFκB, and (B) the phosphorylated (p) levels of Bim at Serine 69 in ARP-1 and RPMI8226 cells 24 hours after the treatment of 0 μM, 1 μM, 2 μM, or 4 μM of ONC201. The levels of GAPDH served as protein loading controls. (C) Flow cytometry analysis shows the levels of phosphorylated (p) or non-phosphorylated ERK or Bim in primary myeloma cells treated without or with ONC201 (2 μM) in a 24 hour-culture. Primary myeloma cells were isolated from bone marrow aspirates of myeloma patients (n = 6) and sorted with anti-CD138 antibody coated magnetic beads. Results shown are representative of three independent experiments.
ONC201 Overcomes Drug Resistance and Has a Synergistic Anti-Myeloma Activity
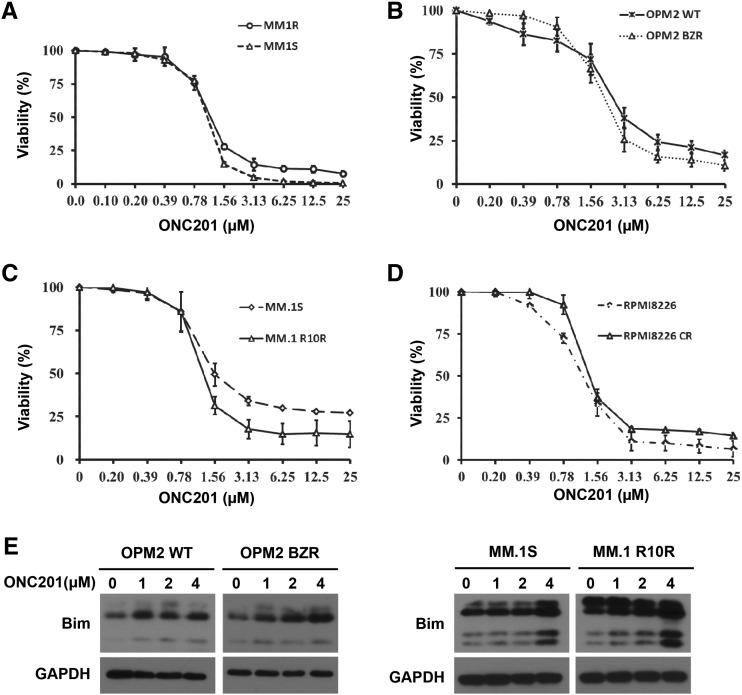
Proteasome inhibitors bortezomib and carfilzomib and the classical agent dexamethasome are commonly used in myeloma, but resistance against these drugs eventually develops in the majority of patients. ONC201 was tested in parental and drug-resistant paired forms of myeloma cell lines, and found to be equally effective against dexamethasome-resistant cells of MM.1 (Figure 5A), bortezomib-resistant cells of OPM2 (Figure 5B) and carfilzomib-resistant cells of MM.1 (Figure 5C) and RPMI8226 (Figure 5D). Furthermore, we observed that ONC201 treatment increased Bim protein levels in both parental and resistant myeloma cells (Figure 5E).
Figure 5.
ONC201 is effective in myeloma cells resisted to the commonly used chemo-therapeutic drugs. Chemo-resistant myeloma cell lines and their respective wild-type cells were cultured with different doses of ONC201 for 72 hours. There were no significant difference in the cell viability of MM cells between (A) dexamethasone-sensitive MM.1S and -resistant MM.1R, (B) bortezomib-sensitive OPM2 and -resistant OPM2 BZR, (C) carfilzomib-sensitive MM.1S and -resistant MM.1 R10R, and (D) carfilzomib-sensitive RPMI8226 and -resistant RPMI8226 CR. (E) Western blot analysis shows the protein levels of Bim in bortezomib-sensitive OPM2 and -resistant OPM2 BZR, and carfilzomib-sensitive MM.1S and -resistant MM.1 R10R. The levels of GAPDH served as protein loading controls. Results shown are representative of five independent experiments.
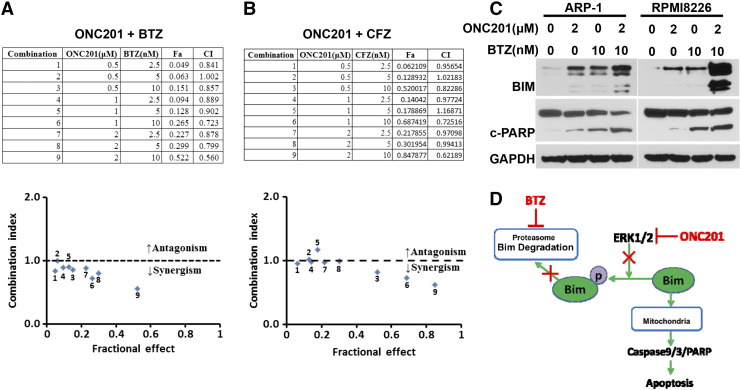
To determine whether combination with ONC201 improves the anti-myeloma activity of chemotherapy, myeloma cells ARP-1 and RPMI8226 were cultured with bortezomib or carfilzomib alone or in combination with ONC201. The antagonism or synergism of the combinations was evaluated using Chou-Talalay analysis. At most dose combinations, ONC201 displayed synergy with bortezomib (Figure 6A) or carfilzomib (Figure 6B). We also found that combination treatment with bortezomib and ONC201 induced higher levels of Bim proteins and cleaved PARP in ARP-1 or RPMI8226 cells than treatment with bortezomib alone (Figure 6C). These results suggest that combination with ONC201 may increase the efficacy of chemotherapy drugs such as proteasome inhibitors on myeloma cells.
Figure 6.
Combinations of bortezomib or carfilzomib with ONC201 have a synergistic anti-myeloma effect. The myeloma cell line ARP-1 was treated with a combination of ONC201 with either bortezomib (A) or carfilzomib (B) for 48 hours to evaluate their cell viabilities. Upper tables show a combination of ONC201 with bortezomib (BTZ) or carfilzomib (CFZ), and lower panels show the antagonism or synergism of the combinations. (C) Myeloma cells ARP-1 and RPMI8226 were cultured without (0) or with 2 μM of ONC201 (ONC) or 10 nM of bortezomib (BTZ) single or in a combination for 24 hours, and the cultured cells were then subjected to Western blot analysis. Shown are the levels of Bim proteins, cleaved (c) PARP, and GAPDH proteins in the tested ARP-1 or RPMI8226 cells. Results shown are representative of three to five independent experiments. (D) A depiction of the signaling mechanism for induction of apoptosis in myeloma cells by combination of ONC201 and bortezomib (BTZ).
Discussion
The imipridone ONC201 has been reported to reduce tumor growth and prolong survival in solid tumors [5] and some hematological malignancies including leukemia and lymphomas [8]. We found that ONC201 treatment induced p53-independent apoptosis of human myeloma cell lines and patient-derived primary CD138+ malignant plasma cells. We also determined that ONC201 inactivated the ERK1/2 signaling pathway, inhibiting Bim phosphorylation and consequently up-regulating its expression, leading to caspase-9/-3/PARP-mediated apoptosis in myeloma cells. Thus, our study provides a strong rationale for the application of ONC201 as a robust anticancer drug in myeloma.
Treatment of ONC201 has been reported to regulate tumor growth independently of p53 status in some tumors. In line with the previous results, we revealed that in myeloma, treatment of ONC201 could reduce cellular proliferation of both wild-type cells (ARP-1, NCI-H929 and MM.1S) and the cells with mutant p53 (OPM2 and RPMI8226), and knockout of p53 expression in myeloma cells did not change the effect of ONC201 on myeloma cells, indicating the action of ONC201 on myeloma cells in an additional apoptotic mechanism. Previous studies have shown that apoptosis can be triggered by a variety of stimuli through the death receptor pathway (the ‘extrinsic apoptosis pathway’) or the mitochondrial pathway (the ‘intrinsic apoptosis pathway’). In the extrinsic or death receptor pathway, apoptosis is initiated through the binding of death ligands of the tumor necrosis factor (TNF) superfamily of cytokines, the TNF-related apoptosis-inducing ligand (TRAIL), and the CD95 (APO-1/Fas) ligand to their respective receptors, TNFR1, DR4/5, and Fas and consequently activating the caspase-8 and caspase-3 cascades [14], [15]. Kline et al. reported that in solid tumors, ONC201 triggered PERK-independent activation of the integrated stress response and activated the DR5/Foxo3a/TRAIL signaling pathway, leading to caspase-8 dependent apoptosis in tumor cells [7]. In hematological malignancies, Talekar et al. reported that treatment of non-Hodgkin's lymphoma cells with ONC201 induces tumor cell death through TRAIL [16]. However, Ishizawa et al. reported that TRAIL/DR5-mediated apoptotic signaling pathway was clearly not involved in AML or MCL [8]. In line with their data, we also found that the expression of TRAIL was not significantly changed in myeloma cells in response to ONC201, indicating that the mechanism of ONC201 action can differ between cancer types and the TRAIL-independent signaling pathway is equally responsible for ONC201 action in myeloma cells.
When myeloma cells were treated with ONC201, we did not observe activation of caspase-8. In line with our observation, recent studies showed that application of the caspase-8 inhibitor Z-IETD-FMK did not abrogate ONC201 effects on apoptosis in mantle cell lymphoma and acute myeloid leukemia [8]. These findings suggest that in hematological malignancies including multiple myeloma, apparently different from solid tumors, apoptosis induced by ONC201 occurs via the mitochondrial pathway, in which apoptotic stimuli induce permeabilization of the outer mitochondrial membrane, which triggers release of cytochrome c into cytoplasm, leading to cleavage of caspase-9 in apoptosome and subsequent activation of downstream executioner caspases such as caspase-3 and PARP [17]. Indeed, we observed that ONC201 treatment for 24 hours concentration-dependently up-regulated the cleavage of capase-9, caspase-3, and subsequent PARP, indicating that ONC201 triggers myeloma cell death through the caspase-9 dependent mitochondrial mediated apoptosis.
Mitochondrial outer membrane permeabilization is controlled by the balance of Bcl-2 family proteins [18], composed of pro-apoptotic proteins Bax, Bim, Bad, Bid, and Puma, and anti-apoptotic proteins such as Bcl-2, Mcl-1, and Bcl-XL [19], [20]. These proteins have been shown to be involved in ONC201-induced tumor cell apoptosis [10]. In addition, Kline et al. indicated a dose-dependent decrease of the anti-apoptotic protein XIAP in myeloma cells in response to ONC201 treatment [7]. In this study, we discovered a new action mechanism of ONC201 in inducing apoptosis in myeloma cells through activation of Bim-mediated apoptotic signaling. Alterations suppressing the pro-apoptotic protein Bim are reported to contribute to resistance to various standard or new chemotherapeutic agents. Bim deficiency represents a novel mechanism of adaptive bortezomib resistance in myeloma cells [21], and Bim loss induced by activation of the MAPK signaling pathway may predict a fundamental mechanism of resistance to histone deacetylase inhibitors [22], [23]. In addition, decreased Bim expression predicts a significantly reduced sensitivity of myeloma cells to ABT-737 treatment [24], and also correlates with poor survival in patients with multiple myeloma [25].
On the other hand, up-regulation of Bim by pharmacologic means may be an effective anti-cancer strategy. Bim expression is regulated by MAPK signaling, and inhibitors of MEK and ERK can reduce Bim phosphorylation at Ser69, resulting in a decrease of proteasome-mediated Bim degradation [26] We found that ONC201 significantly up-regulated the expression of Bim proteins in myeloma cells in a concentration-dependent manner, but did not alter that of other Bcl-2 family members including Bid, Bak, Bax, PUMA, Bcl-2, and Bcl-xL. Knockdown of Bim in myeloma cells using lentivirus-carried shRNAs remarkably reduced ONC201 effects on apoptosis, suggesting a critical role of Bim in induction of apoptosis by ONC201. Our results showed that ONC201 treatment down-regulated the phosphorylation of Bim and ERK1/2 in myeloma cells, but not other MAPK kinases such as JNK1/2 and p38 MAPK, or other signaling molecules such as NF-κB. Hence, up-regulation of Bim protein expression by ONC201 appears to result from loss of Bim phosphorylation through suppression of the ERK1/2 signaling (Figure 6D).
Previous studies have shown that inhibition of Bim degradation in the proteasome by the inhibitor bortezomib is a key mechanism for the accumulation of Bim protein, thereby enhancing Bim-mediated apoptosis [27]. Our data demonstrated that the combination of ONC201 with the proteasome inhibitors significantly increased Bim expression and PARP cleavage in myeloma cells, and had a synergistic effect on anti-myeloma activity. In contrast, ONC201 may increase Bim in non-proteasomal locations through inactivation of ERK signaling and Bim phosphorylation. Myeloma cells that resist bortezomib or carfilzomib do so by maintaining proteasome activity, and therefore Bim does not accumulate if Bim phosphorylation remains intact. Since ONC201 inhibits ERK signaling and Bim phosphorylation in bortezomib- or carfilzomib-resistant cells, Bim accumulates and leads to apoptosis (Figure 6D). Although more evidences are needed to prove the action mechanism for the combination treatment, our results indicate that targeting this pro-apoptotic molecule using two agents with different action mechanisms boosts therapeutic efficacy, providing a rationale for combining ONC201 with a proteasome inhibitor in myeloma therapy. The unusually benign safety profile of ONC201 in preclinical models and clinical studies [28], [29] should facilitate its clinical evaluation in heavily-pretreated myeloma patients who are frail and permit combination therapy.
Acknowledgements and Grant Supports
We would like to thank our Departmental Myeloma Tissue Bank for providing patient samples. This work was supported by National Cancer Institute MDACC SPORE in Multiple Myeloma [grant number P50 CA142509]; National Cancer Institute UTMDACC SPORE in Multiple Myeloma Career Development Award [grant number CDP-060315]; and National Cancer Institute UTMDACC SPORE in Developmental Research Program [grant number DRP-00013585].
Authors' Contributions
Y.T. and J.Y. designed all experiments and wrote the manuscript; Y.T., J.H., and H.L. performed all experiments and statistical analyses; H.C.L and H.W established the TP53KO cell lines as well as drug resistant cell lines; J.E.A. provided the supply of ONC201; J.I., M.A., R.E.D., R.Z.O., and J.E.A. provided critical suggestions. All authors reviewed the final manuscript.
Disclosure of Potential Conflicts of Interest
The authors have no competing financial interests.
Contributor Information
Richard E. Davis, Email: REDavis1@mdanderson.org.
Jing Yang, Email: jiyang@mdanderson.org.
References
- 1.Munshi NC, Anderson KC. New strategies in the treatment of multiple myeloma. Clin Cancer Res. 2013;19:3337–3344. doi: 10.1158/1078-0432.CCR-12-1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bianchi G, Richardson PG, Anderson KC. Promising therapies in multiple myeloma. Blood. 2015;126:300–310. doi: 10.1182/blood-2015-03-575365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar SK, Lee JH, Lahuerta JJ, Morgan G, Richardson PG, Crowley J, Haessler J, Feather J, Hoering A, Moreau P. Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: a multicenter international myeloma working group study. Leukemia. 2012;26:149–157. doi: 10.1038/leu.2011.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364:1046–1060. doi: 10.1056/NEJMra1011442. [DOI] [PubMed] [Google Scholar]
- 5.Allen JE, Krigsfeld G, Mayes PA, Patel L, Dicker DT, Patel AS, Dolloff NG, Messaris E, Scata KA, Wang W. Dual inactivation of Akt and ERK by TIC10 signals Foxo3a nuclear translocation, TRAIL gene induction, and potent antitumor effects. Sci Transl Med. 2013;5:171ra117. doi: 10.1126/scitranslmed.3004828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allen JE, Kline CL, Prabhu VV, Wagner J, Ishizawa J, Madhukar N, Lev A, Baumeister M, Zhou L, Lulla A. Discovery and clinical introduction of first-in-class imipridone ONC201. Oncotarget. 2016 doi: 10.18632/oncotarget.11814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kline CL, Van den Heuvel AP, Allen JE, Prabhu VV, Dicker DT, El-Deiry WS. ONC201 kills solid tumor cells by triggering an integrated stress response dependent on ATF4 activation by specific eIF2alpha kinases. Sci Signal. 2016;9:ra18. doi: 10.1126/scisignal.aac4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishizawa J, Kojima K, Chachad D, Ruvolo P, Ruvolo V, Jacamo RO, Borthakur G, Mu H, Zeng Z, Tabe Y. ATF4 induction through an atypical integrated stress response to ONC201 triggers p53-independent apoptosis in hematological malignancies. Sci Signal. 2016;9:ra17. doi: 10.1126/scisignal.aac4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prabhu VV, Allen JE, Dicker DT, El-Deiry WS. Small-molecule ONC201/TIC10 targets chemotherapy-resistant colorectal cancer stem-like cells in an Akt/Foxo3a/TRAIL-dependent manner. Cancer Res. 2015;75:1423–1432. doi: 10.1158/0008-5472.CAN-13-3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karpel-Massler G, Ba M, Shu C, Halatsch ME, Westhoff MA, Bruce JN, Canoll P, Siegelin MD. TIC10/ONC201 synergizes with Bcl-2/Bcl-xL inhibition in glioblastoma by suppression of Mcl-1 and its binding partners in vitro and in vivo. Oncotarget. 2015;6:36456–36471. doi: 10.18632/oncotarget.5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee HC, Wang H, Baladandayuthapani V, Lin H, He J, Jones RJ, Kuiatse I, Gu D, Wang Z, Ma W. RNA polymerase I inhibition with CX-5461 as a novel therapeutic strategy to target MYC in multiple myeloma. Br J Haematol. 2017;177:80–94. doi: 10.1111/bjh.14525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70:440–446. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 13.Zhang N, Fu JN, Chou TC. Synergistic combination of microtubule targeting anticancer fludelone with cytoprotective panaxytriol derived from panax ginseng against MX-1 cells in vitro: experimental design and data analysis using the combination index method. Am J Cancer Res. 2016;6:97–104. [PMC free article] [PubMed] [Google Scholar]
- 14.Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 15.Peter ME, Krammer PH. The CD95(APO-1/Fas) DISC and beyond. Cell Death Differ. 2003;10:26–35. doi: 10.1038/sj.cdd.4401186. [DOI] [PubMed] [Google Scholar]
- 16.Talekar MK, Hall J, Wertheim GB, Martinez D, Allen JE, Grupp SA. Effect of ONC201 and bortezomib on apoptosis in non-Hodgkin's™ lymphoma (NHL) xenografts. J Clin Oncol. 2017;35:e19016. [Google Scholar]
- 17.Scorrano L, Korsmeyer SJ. Mechanisms of cytochrome c release by proapoptotic BCL-2 family members. Biochem Biophys Res Commun. 2003;304:437–444. doi: 10.1016/s0006-291x(03)00615-6. [DOI] [PubMed] [Google Scholar]
- 18.Chipuk JE, Fisher JC, Dillon CP, Kriwacki RW, Kuwana T, Green DR. Mechanism of apoptosis induction by inhibition of the anti-apoptotic BCL-2 proteins. Proc Natl Acad Sci U S A. 2008;105:20327–20332. doi: 10.1073/pnas.0808036105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 20.Moldoveanu T, Follis AV, Kriwacki RW, Green DR. Many players in BCL-2 family affairs. Trends Biochem Sci. 2014;39:101–111. doi: 10.1016/j.tibs.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen S, Zhang Y, Zhou L, Leng Y, Lin H, Kmieciak M, Pei XY, Jones R, Orlowski RZ, Dai Y. A Bim-targeting strategy overcomes adaptive bortezomib resistance in myeloma through a novel link between autophagy and apoptosis. Blood. 2014;124:2687–2697. doi: 10.1182/blood-2014-03-564534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zaanan A, Okamoto K, Kawakami H, Khazaie K, Huang S, Sinicrope FA. The mutant KRAS gene up-regulates BCL-XL protein via STAT3 to confer apoptosis resistance that is reversed by BIM protein induction and BCL-XL antagonism. J Biol Chem. 2015;290:23838–23849. doi: 10.1074/jbc.M115.657833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hubner A, Barrett T, Flavell RA, Davis RJ. Multisite phosphorylation regulates Bim stability and apoptotic activity. Mol Cell. 2008;30:415–425. doi: 10.1016/j.molcel.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Del Gaizo Moore V, Brown JR, Certo M, Love TM, Novina CD, Letai A. Chronic lymphocytic leukemia requires BCL2 to sequester prodeath BIM, explaining sensitivity to BCL2 antagonist ABT-737. J Clin Invest. 2007;117:112–121. doi: 10.1172/JCI28281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romagnoli M, Seveno C, Wuilleme-Toumi S, Amiot M, Bataille R, Minvielle S, Barille-Nion S. The imbalance between Survivin and Bim mediates tumour growth and correlates with poor survival in patients with multiple myeloma. Br J Haematol. 2009;145:180–189. doi: 10.1111/j.1365-2141.2009.07608.x. [DOI] [PubMed] [Google Scholar]
- 26.Luciano F, Jacquel A, Colosetti P, Herrant M, Cagnol S, Pages G, Auberger P. Phosphorylation of Bim-EL by Erk1/2 on serine 69 promotes its degradation via the proteasome pathway and regulates its proapoptotic function. Oncogene. 2003;22:6785–6793. doi: 10.1038/sj.onc.1206792. [DOI] [PubMed] [Google Scholar]
- 27.Tan TT, Degenhardt K, Nelson DA, Beaudoin B, Nieves-Neira W, Bouillet P, Villunger A, Adams JM, White E. Key roles of BIM-driven apoptosis in epithelial tumors and rational chemotherapy. Cancer Cell. 2005;7:227–238. doi: 10.1016/j.ccr.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 28.Allen JE, Crowder RN, El-Deiry WS. First-in-class small molecule ONC201 induces DR5 and cell death in tumor but not normal cells to provide a wide therapeutic index as an anti-cancer agent. PLoS One. 2015;10:e0143082. doi: 10.1371/journal.pone.0143082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stein MN, Bertino JR, Kaufman HL, Mayer T, Moss R, Silk A, Chan N, Malhotra J, Rodriguez L, Aisner J. First-in-human clinical trial of oral ONC201 in patients with refractory solid tumors. Clin Cancer Res. 2017 doi: 10.1158/1078-0432.CCR-16-2658. [DOI] [PMC free article] [PubMed] [Google Scholar]