Abstract
This study examined whether longitudinal MRI trajectories in medial temporal lobe (MTL) brain regions differed among groups of cognitively normal individuals defined by their cerebrospinal fluid (CSF) levels when they were first enrolled (N = 207; mean clinical follow-up = 13.3 years (max = 20 years), mean MRI follow-up = 2.4 years (max = 8 years)). We first compared atrophy rates among groups defined by CSF amyloid and phosphorylated-tau (p-tau) vs. CSF amyloid and total tau (t-tau). We also examined whether, in the presence of amyloid or tau/p-tau, the atrophy rates differed based on whether the subjects ultimately progressed to a diagnosis of mild cognitive impairment (MCI), as well as whether apolipoprotein ε4 (Apoε4) status had an impact on the longitudinal MRI trajectories. The primary finding was that when the groups were defined using CSF amyloid and p-tau, individuals with low levels of CSF amyloid and high levels of CSF p-tau (referred to as Stage 2) showed a significantly greater rate of atrophy in a composite measure of MTL volumes compared to groups defined by evidence of abnormal CSF levels in only one of the brain proteins (but not both), or no evidence of CSF abnormality. In contrast, there were no differences in rate of MTL atrophy when the groups were defined by levels of CSF amyloid and t-tau (instead of p-tau). Additionally, the rate of MTL atrophy did not differ between subjects who progressed to MCI at follow-up vs. those who remained cognitively normal when CSF levels of amyloid, t-tau, or p-tau were covaried. Lastly, the presence of an APOE ε4 genotype did not modulate the degree of MTL atrophy once baseline levels of CSF amyloid, p-tau or t-tau were accounted for. These results suggest that abnormal levels of CSF amyloid and CSF p-tau (but not t-tau) maximize the likelihood of observing significant MTL atrophy over time among individuals with normal cognition at baseline, and emphasize the importance of differentiating biomarkers that primarily reflect neurofibrillary tangle pathology (CSF p-tau) compared with biomarkers of neuronal injury (CSF t-tau).
Keywords: Preclinical AD, Magnetic resonance imaging, Cerebrospinal fluid, Amyloid, Phosphorylated tau
Highlights
-
•
Examined association between CSF AD biomarkers and medial temporal lobe atrophy
-
•
Abnormal levels of both amyloid and p-tau were associated with greatest atrophy.
-
•
No difference in rate of atrophy based on levels of amyloid and total tau
-
•
Follow-up diagnosis was unrelated to atrophy rate when covarying amyloid and p-tau.
-
•
Levels of CSF amyloid and p-tau were associated with atrophy in preclinical AD.
1. Introduction
The pathological processes underlying Alzheimer's disease (AD) begin years to decades prior to the emergence of clinical symptoms, during the preclinical phase of AD (Sperling et al., 2011). The early development of AD pathology has particular importance for the timing of intervention strategies, as it is hypothesized that interventions will be most successful if initiated prior to the occurrence of substantial neuronal loss, and progression to the symptomatic phase of AD, commonly referred to as Mild Cognitive Impairment (MCI). As a result, considerable effort is being devoted to planning clinical trials for those with preclinical AD. Magnetic resonance imaging (MRI) measures are of particular interest in this regard, since studies among individuals with MCI suggest that MRI may be well suited for tracking the evolution of disease and thus informative with respect to response to treatment. There are, however, few studies that can provide information about MRI measures that might be most useful for tracking disease during the preclinical phase of AD.
A small number of studies have examined regional brain volumes, demonstrating that a set of MRI measures obtained when individuals are cognitively normal are associated with the time to onset of the clinical symptoms of MCI (Csernansky et al., 2005, Soldan et al., 2015, Pettigrew et al., 2016). Several studies have examined the relationship of atrophy rates in various brain regions to the presence of abnormal levels of the primary brain proteins associated with AD, as measured in cerebrospinal fluid (CSF) (Desikan et al., 2011, Pegueroles et al., 2017), or in relation to cut-points derived from brain imaging, such as Flourodeoxyglucose (FDG) (Knopman et al., 2013, Jack et al., 2014, Gordon et al., 2016, Knopman et al., 2016). Taken together, these studies have reported greater atrophy in the medial temporal and temporo-parietal cortical regions among cognitively normal individuals who have evidence of abnormalities based either on CSF protein levels or imaging cut-points.
The primary limitation of these studies is, however, that the follow-up period for the participants has been quite limited, approximately 2–4 years for most studies. Moreover, none of the analyses published to date, to our knowledge, have examined atrophy rates in cognitively normal individuals in relation to their subsequent development of MCI. In the current analyses, we stratified cognitively normal participants based on their CSF levels of AD-related proteins and examined MRI atrophy rates in relation to these subgroups. We also compared these findings between those cognitively normal individuals who remained normal vs. those who progressed to MCI. Additionally we examined the impact of apolipoprotein E (ApoE) genetic status on MRI atrophy rates.
To address these issues, we examined data from a cohort of cognitively normal individuals who have been followed clinically for a mean of 13.3 years (SD = 3.8). The mean MRI follow-up time was 2.4 years, but 81 subjects had an MRI follow-up time of 4–8 years, allowing us to estimate longer MRI trajectories than previous studies. We classified the subjects into subgroups, based on their CSF levels of amyloid and either total tau (t-tau) or phosphorylated tau (p-tau), when they were first enrolled. We based our classification scheme on the hypothetical staging model proposed by the Preclinical AD Workgroup sponsored by the National Institute on Aging and the Alzheimer's Association (NIA-AA) (Sperling et al., 2011). This model proposed that the preclinical phase of AD can be divided into several successive stages: (1) Stage 0 includes individuals with no evidence of either amyloid or tau-related neuronal injury; (2) Stage 1 includes individuals with biomarker evidence of amyloid pathology, but no evidence of neuronal injury; (3) Stage 2 includes individuals with biomarker evidence of both amyloid accumulation and neuronal injury; and (4) Stage 3 includes individuals with evidence of subtle cognitive decline in combination with biomarker evidence of both amyloid pathology and neuronal injury. Subsequently, an additional group of individuals were described, those with no evidence of amyloid pathology but with evidence of tau-related neuronal injury, referred to as suspected non-AD pathology (SNAP) (Jack et al., 2012).
In the present study we compared MRI atrophy rates in several medial temporal lobe (MTL) brain regions in four groups of individuals who were cognitively normal when first enrolled: Stage 0, 1, 2 and SNAP, as defined above. Stage 3 was omitted, since criteria for this stage are not well developed. The substantial sample size in the current study (N = 207) allowed us to address several issues that remain unresolved by prior investigations. First, we compared atrophy rates among groups defined by CSF amyloid and p-tau vs. CSF amyloid and t-tau in order to determine if there were differential rates of atrophy depending on which brain proteins are abnormal. Based on prior analyses of cognitive and imaging data in this cohort (Pettigrew et al., 2016, Soldan et al., 2016b), we hypothesized that the individuals with accumulations of both amyloid and p-tau would show the highest atrophy rates. This issue is also of theoretical importance since a recent A/T/N classification system (Jack et al., 2016) proposes that biomarkers of neurofibrillary tangle pathology (as reflected by CSF p-tau and tau PET imaging) should be classified separately from biomarkers of neuronal injury (e.g., CSF t-tau, FDG PET). Second, we examined whether the atrophy rates differed based on whether the subjects ultimately progressed to a diagnosis of MCI. Previous studies have not been able to address this issue due to the limited duration of follow-up. Third, we examined whether genetic status, specifically ApoE-4 status, had an impact on the longitudinal MRI trajectories (the major genetic risk factor for AD; Corder et al., 1993); we hypothesized that atrophy rates would not differ between those who were ApoE ε4 positive vs. negative, based on prior analyses of cognitive change within this cohort (Albert et al., 2014).
2. Material and methods
2.1. Study design
The data reported here were derived from the BIOCARD study, which was designed to recruit and follow a cohort of cognitively normal individuals who were primarily middle-aged at baseline. The overarching goal was to identify variables among cognitively normal individuals that could predict subsequent development of mild to moderate symptoms of AD. The study was initiated at the National Institutes of Health (NIH) in 1995 and stopped in 2005 for administrative reasons. During the initial study at the NIH, participants were administered a comprehensive neuropsychological battery annually, and magnetic resonance imaging, CSF samples, and blood specimens were obtained approximately every 2 years. In 2009, a research team at the Johns Hopkins School of Medicine was funded to re-establish the cohort, continue annual cognitive and clinical assessments, and evaluate the previously collected MRI scans, and CSF and blood specimens. In 2015, the collection of MRI and CSF biomarkers was re-initiated, and amyloid imaging begun.
2.2. Selection of participants
Recruitment procedures, baseline evaluations, annual clinical and cognitive assessments, and consensus diagnosis procedures have been described in detail previously (Albert et al., 2014). Briefly, recruitment was conducted by the staff of the geriatric psychiatry branch of the intramural program of the National Institute of Mental Health between 1995 and 2005. At baseline, all individuals completed a comprehensive evaluation at the NIH consisting of a physical and neurological examination, an electrocardiogram, standard laboratory studies, and neuropsychological testing. Individuals were excluded from participation if they were cognitively impaired or had significant medical problems, such as severe cerebrovascular disease, epilepsy, or alcohol or drug abuse. A total of 349 cognitively normal individuals were initially enrolled in the study after providing written informed consent. By design, approximately 75% of the participants had a first-degree relative with dementia of the Alzheimer type. The analyses presented herein are based on 207 participants who provided baseline CSF biomarkers within 12 months of their baseline MRI scan (M gap time = 8.4 days between MRI and CSF measures, SD = 37.8). The study was approved by the Johns Hopkins University (JHU) Institutional Review Board.
2.3. Clinical and cognitive assessments
Cognitive and clinical assessments and consensus diagnosis procedures were completed annually at both the NIH and JHU (see Albert et al., 2014, for details). Each participant included in our analyses received a consensus diagnosis by the staff of the JHU BIOCARD Clinical Core. All cases were handled in a manner, comparable with those used in the National Institute on Aging Alzheimer's Disease Centers program: (1) clinical data pertaining to the medical, neurological, and psychiatric status of the individual were examined; (2) reports of changes in cognition by the individual and by collateral sources were reviewed; and (3) decline in cognitive performance, based on review of longitudinal testing from multiple domains, was established. We followed the diagnostic recommendations incorporated in the NIA-AA working group reports for the diagnosis of MCI (Albert et al., 2011) and dementia due to AD (McKhann et al., 2011). The clinical diagnoses were masked to biomarker assessments. Each individual's most recent (i.e., last) diagnosis was coded by a dichotomous indicator variable: 0 if participants have remained cognitively normal over time, or 1 if they have since progressed from normal cognition to clinical symptoms of MCI or dementia due to AD (mean time from baseline MRI to most recent diagnosis = 13.3 years (SD = 3.8)).
2.4. Cerebrospinal fluid assessments
CSF samples were analyzed with the same protocol used in the Alzheimer Disease Neuroimaging Initiative. This protocol used the xMAP-based AlzBio3 kit (Innogenetics, Ghent, Belgium) run on the BioPlex 200 system. The kit contains monoclonal antibodies specific for Aβ1–42 (4D7A3), total tau (t-tau) (AT120), and phosphorylated tau181p (p-tau) (AT270), each chemically bonded to unique sets of color-coded beads, and analyte-specific detector antibodies (HT7 and 3D6). Calibration curves were produced for each biomarker using aqueous buffered solutions that contained the combination of 3 biomarkers at concentrations ranging from 25 to 1555 pg/mL for recombinant tau, 54–1799 pg/mL for synthetic Aβ1–42, and 15–258 pg/mL for a synthetic tau peptide phosphorylated at the threonine 181 position (i.e., the p-tau181p standard). All assays were run in triplicate and each subject had all their samples analyzed on the same plate (see Moghekar et al., 2012, Moghekar et al., 2013 for additional details).
2.5. Magnetic resonance imaging assessments
The MRI scans used in the present study were collected approximately every two years while the study was at the NIH (i.e., 1995–2005). MRI scans acquired at the NIH were obtained using a standard multimodal protocol on a GE 1.5T scanner. MTL regions of interest (ROI) – including the volumes of the hippocampus, entorhinal cortex, and amygdala, and thickness of the entorhinal cortex – were reconstructed from the coronal spoiled gradient echo (SPGR) sequence (TR = 24, TE = 2, FOV = 256 × 256, thickness/gap = 2.0/0.0 mm, flip angle = 20, 124 slices).
MTL ROI volumes were reconstructed using a semi-automated procedure, as previously described (Miller et al., 2013). For each ROI, landmarks were placed manually in each MRI scan to mark the boundaries of the ROI, following previously published protocols. Next, a template was created for the left and right hemispheres of each ROI by hand segmenting these structures in a control subject. The same set of landmarks was placed in this template as in the individual subject scans. After landmarking, region-of-interest large deformation diffeomorphic metric mapping was used to map the template to the individual subject scans, using both landmark matching (Joshi and Miller, 2000) and volume matching (Beg et al., 2005). The resulting segmented binary images were used to calculate the volume of each structure, by hemisphere, by summing the number of voxels within the volume. Entorhinal cortex thickness was modeled by first generating a smooth surface from the segmented gray matter volume. The gray/white surface was then extracted from the closed surface by curvature-based dynamic programming delineation of the extremal boundaries so that the surface closest to the white matter was retained. Laminar thickness was calculated as a single parameter based on the ratio of volume/surface-area (in units of millimeters, mm). Additionally, total intracranial volume (ICV) was calculated using the coronal SPGR scans in Freesurfer 5.1.0 (Segonne et al., 2004). Additional details about the scans acquired under the multimodal protocol, the reconstruction procedures, and their reliability are included in Supplementary Materials I.
The main outcomes of interest were longitudinal rates of change in MTL regions over time, including hippocampus volume, entorhinal cortex volume, amygdala volume, a composite of the MTL volumes, and entorhinal cortex thickness. For each region, at each time point, we first averaged over the left and right hemispheres. The volume measures were then normalized for head size by regressing the left/right averages on ICV, with the standardized residuals from these regression models serving as the volumetric dependent variables. To create the MTL volume composite score, the standardized residuals for the hippocampus, entorhinal cortex, and amygdala were averaged. The dependent variable for entorhinal cortex thickness was the average of the left and right hemispheres, uncorrected for ICV.
2.6. APOE genotyping and coding
APOE genotype was established in all but one of the cohort participants (n = 348). Genotypes were determined by restriction endonuclease digestion of polymerase chain reaction amplified genomic DNA (performed by Athena Diagnostics, Worcester, Massachusetts). APOE ε4 carrier status was coded by a dichotomous indicator variable: ε4 carriers (i.e., individuals with at least one APOE ε4 allele) were coded as 1, and non-carriers were coded as 0. Analyses including APOE carrier status excluded n = 5 individuals with the ε2/ε4 genotype given the ε4 allele increases AD dementia risk (Corder et al., 1993), whereas the ε2 allele decreases AD dementia risk (Corder et al., 1994).
2.7. Statistical analysis
Participants were classified into the four groups based on CSF biomarker levels (i.e., Stage 0, Stage 1, Stage 2, and SNAP) using binary predictors (0, 1) for each group. Because published cut-points for abnormality in CSF AD-biomarkers (e.g., Shaw et al., 2009) cannot be applied to the current study due to considerable inter-laboratory variability even when using the same samples and the same assays (see Mattsson et al., 2011), we applied the same procedures as those reported by Soldan et al. (2016a): biomarker abnormality was defined as CSF Aβ1–42, levels in the lower one-third of the distribution, CSF t-tau in in the upper one-third of the distribution, and CSF p-tau in the upper one-third of the distribution. Tertile cut-points were selected based on the observation that approximately one-third of cognitively normal individuals have evidence of AD pathology (see Soldan et al., 2016a, for more details). The resulting proportion of individuals classified into the four CSF groups was very comparable to prior studies that used clinically validated cut-points (e.g., Jack et al., 2017, Mormino et al., 2014, Vos et al., 2013). These four CSF groups are descriptively referred to as preclinical AD stages (i.e., Stage 0, 1, 2) and SNAP; however, given statistically validated cut-points were not used to define biomarker abnormality, the groups would be more accurately described by their tertile descriptors (e.g., ‘low CSF amyloid and high CSF tau group’, rather than Stage 2). Unless noted otherwise, the results were the same when using more stringent cut-points (i.e., quartiles or quintiles) to classify individuals into the four groups (see Supplementary Materials III). Participants were classified into the groups twice, once according to abnormality in CSF Aβ1–42 and t-tau, and once according to abnormality in CSF Aβ1–42 and p-tau.
Differences in baseline characteristics between individuals in the four CSF groups were first assessed with a global F-test for continuous variables or global chi-square tests for categorical variables. If the global test was significant at p < 0.05, post-hoc t-tests for continuous variables or chi-square tests for dichotomous variables were performed to compare individual groups. Correlations between the baseline CSF and MRI measures were assessed with partial Pearson correlations (covarying age at baseline MRI scan) and p-values were adjusted for multiple comparisons using the Bonferroni-Holm correction.
Linear mixed effect models were used to examine the relationship of baseline Preclinical AD Stages and SNAP to MRI measures over time. These models included linear effects of time, and a random intercept and slope for each participant; all models were run twice – once using CSF groups determined by CSF Aβ1–42 and t-tau, and once using CSF groups determined by Aβ1–42 and p-tau. The outcome variables were the MRI measures over time, including baseline measures and all available follow-up. The predictors were age at baseline MRI, gender (dichotomous), years of education, Stage 1 (dichotomous), Stage 2 (dichotomous), SNAP (dichotomous), time since baseline, and the interaction of each predictor with time. Stage 0, therefore, was treated as the reference group. In these models, the interaction of each stage indicator with time tests if the rate of change in MRI biomarker measures differs between Stage 0 and the other stages.
To determine whether APOE ε4 genetic status or follow-up diagnosis impact longitudinal MRI trajectories, both sets of models were re-run including either the APOE ε4 indicator variable and its interaction with time as additional predictors, or the diagnosis indicator variable and its interaction with time. To test whether the APOE ε4 indicator variable's effects differed by Stage, additional models were run, one for each CSF group (again in reference to Stage 0), that also included the three-way interaction between the Stage indicator, APOE ε4 indicator, and time, as well as the Stage by APOE-e4 interaction. Only one three-way interaction term was tested at a time to limit the total number of terms in the model. Potential three-way interactions between follow-up diagnosis, Stage indicators, and time were not examined in a similar manner because the number of subjects who had progressed to MCI within each CSF group was too small to obtain reliable estimates (range = 5–16 progressors per group).
3. Results
Characteristics of participants at baseline for the entire BIOCARD cohort and for participants in the analysis are shown in Table 1. Table 2 lists baseline characteristics separately for the 4 groups (Stage 0, Stage 1, Stage 2, and SNAP). The groups did not differ in sex, years of education, and baseline MMSE score. However, individuals classified as Stage 2 were older, were more likely to be APOE ε4 carriers, and were more likely to progress to MCI or dementia at follow-up than individuals in Stage 0 (all p < 0.05). Individuals in Stage 1 and SNAP did not differ from Stage 0 on these measures, except that the Stage 1 group had a higher proportion of APOE ε4 carriers than the Stage 0 group. Although individuals classified as Stage 0 tended to have numerically smaller MTL measures than individuals classified as Stage 2, these differences were not significant.
Table 1.
Participant characteristics at baseline. Values reflect means (standard deviations) unless otherwise indicated.
| Variable | Entire BIOCARD cohort | Participants in analyses |
|---|---|---|
| N | 349 | 207 |
| Age, years | 57.3 (10.4) | 56.9 (10.0) |
| Sex, female (%) | 57.6% | 59.4% |
| Ethnicity, Caucasian (%) | 97.1% | 97.6% |
| APOE ε4 carriers (%) | 33.6% | 32.2% |
| Education, years | 17.0 (2.4) | 17.2 (2.3) |
| MMSE score | 29.5 (0.9) | 29.6 (0.8) |
Table 2.
Participant characteristics at baseline for the four CSF groups (Stage 0, 1, 2, and SNAP). Values reflect means (standard deviations) unless otherwise indicated.
| Stage 0 | Stage 1 | Stage 2 | SNAP | |
|---|---|---|---|---|
| Groups defined by CSF Aβ and CSF total tau | ||||
| N | 97 | 41 | 28 | 41 |
| N (%) progressed to MCI or dementia due to AD | 14 (14.4%) | 6 (14.6%) | 16 (57.1%)⁎⁎ | 5 (12.2%) |
| Age, years | 54.9 (10.3) | 55.7 (7.5) | 65.3 (9.6)⁎⁎ | 57.2 (9.2) |
| Sex, female (%) | 61.9% | 56.1% | 50.0% | 63.4% |
| Ethnicity, Caucasian (%) | 96.9% | 97.6% | 100% | 97.6% |
| APOE ε4 carriers (%)a | 22.9% | 40.0% ⁎ | 50.0%⁎ | 35.0% |
| Education, years | 17.2 (2.4) | 16.9 (2.4) | 17.3 (2.2) | 17.2 (2.1) |
| MMSE score | 29.6 (0.7) | 29.4 (0.9) | 29.6 (1.0) | 29.6 (0.7) |
| CSF Aβ, pg/mL | 454.7 (54.5) | 319.3 (38.9)⁎⁎ | 253.7 (73.8)⁎⁎ | 473.6 (54.8) |
| CSF tau, pg/mL | 58.0 (10.5) | 44.8 (13.7) | 115.1 (36.3)⁎⁎ | 93.9 (26.6)⁎⁎ |
| CSF p-tau, pg/mL | 30.8 (8.6) | 27.0 (7.7) | 57.2 (21.8)⁎⁎ | 41.8 (11.8)⁎⁎ |
| Hippocampal volume, standardized residual | 0.02 (0.89) | − 0.03 (1.10) | 0.09 (1.04) | 0.14 (0.95) |
| Entorhinal cortex volume, standardized residual | − 0.08 (0.96) | 0.16 (1.09) | 0.05 (0.89) | 0.17 (0.98) |
| Entorhinal cortex, thickness, mm | 2.13 (0.26) | 2.22 (0.27) | 2.21 (0.24) | 2.22 (0.25) |
| Amygdala volume, standardized residual | 0.11 (0.96) | − 0.03 (0.93) | 0.10 (1.18) | − 0.22 (0.88)⁎ |
| MTL volume composite | 0.02 (0.61) | 0.04 (0.78) | 0.08 (0.79) | 0.03 (0.72) |
| Number of MRI scans (range) | 2.3 (1–5) | 2.3 (1–6) | 2.4 (1–5) | 2. 5 (1–5) |
| MRI follow-up time, years | 2.3 (1.3) | 2.3 (1.5) | 2.4 (1.3) | 2.5 (1.4) |
| N with > 1 MRI scans | 61 | 23 | 17 | 26 |
| Number of MRI scans for subjects with > 1 scan (range) | 3.0 (2–5) | 3.3 (2–6) | 3.4 (2–5) | 3.3 (2–5) |
| MRI follow-up time for subjects with > 1 scan, years | 4.0 (2.3) | 4.2 (1.8) | 4.4 (1.9) | 4.7 (2.2) |
| Groups defined by CSF Aβ and CSF phosphorylated tau | ||||
| N | 95 | 43 | 26 | 43 |
| N (%) progressed to MCI or dementia due to AD | 14 (14.7%) | 6 (14.0%) | 16 (61.5%)⁎⁎ | 5 (11.6%) |
| Age, years | 55.9 (9.5) | 57.0 (8.4) | 63.9 (9.9)⁎⁎ | 54.9 (11.2) |
| Sex, female (%) | 60.0% | 48.8% | 61.5% | 67.4% |
| Ethnicity, Caucasian (%) | 92 (96.8%) | 42 (97.7%) | 26 (100%) | 42 (97.7%) |
| APOE ε4 carriers (%)a | 23.9% | 42.9% ⁎ | 44.0%⁎ | 32.6% |
| Education, years | 17.3 (2.4) | 17.0 (2.4) | 17.2 (2.3) | 17.0 (2.1) |
| MMSE score | 29.6 (0.7) | 29.4 (1.0) | 29.7 (0.7) | 29.7 (0.7) |
| CSF Aβ, pg/mL | 457.7 (57.4) | 309.7 (52.4)⁎⁎ | 264.5 (72.4)⁎⁎ | 466.1 (49.8) |
| CSF tau, pg/mL | 61.8 (17.7) | 49.5 (21.7) | 112.7 (40.6)⁎⁎ | 83.8 (27.8)⁎⁎ |
| CSF p-tau, pg/mL | 28.5 (7.1) | 26.2 (6.3) | 60.8 (19.2)⁎⁎ | 46.2 (7.3)⁎⁎ |
| Hippocampal volume, standardized residual | 0.03 (0.87) | − 0.02 (1.08) | 0.09 (1.06) | 0.12 (1.00) |
| Entorhinal cortex volume, standardized residual | − 0.06 (0.94) | 0.10 (1.10) | 0.15 (0.85) | 0.10 (1.02) |
| Entorhinal cortex, thickness, mm | 2.13 (0.26) | 2.23 (0.27)⁎ | 2.20 (0.24) | 2.21 (0.27) |
| Amygdala volume, standardized residual | 0.08 (1.03) | 0.09 (1.00) | − 0.07 (1.09) | − 0.13 (0.72) |
| MTL volume composite | 0.02 (0.64) | 0.06 (0.76) | 0.06 (0.81) | 0.03 (0.67) |
| Number of MRI scans (range) | 2.5 (1–5) | 2.3 (1–6) | 2.4 (1–5) | 2.0 (1–5) |
| MRI follow-up time, years | 3.0 (2.9) | 2.4 (2.6) | 2.6 (2.5) | 2.0 (2.2) |
| N with > 1 MRI scans | 62 | 24 | 16 | 25 |
| Number of MRI scans for subjects with > 1 scan (range) | 3.3 (2–5) | 3.4 (2–6) | 3.3 (2–5) | 2.7 (2–5) |
| MRI follow-up time for subjects with > 1 scan, years | 4.5 (2.4) | 4.4 (1.8) | 4.2 (1.9) | 3.4 (1.9) |
p ≤ 0.05 for differences between Stage 1, Stage 2, and SNAP relative to Stage 0.
p < 0.001 for differences between Stage 1, Stage 2, and SNAP relative to Stage 0.
Excludes n = 5 participants with APOE ε2/ε4 genotype.
Of the 207 subjects included in the analyses, 38 (18%) were classified in a different CSF group when groups were defined using Aβ1–42 and tau vs. Aβ1–42 and p-tau. The majority of changes in group membership were between Stage 0 and SNAP (n = 30); the remaining changes were between Stages 1 and 2 (n = 8) (see Supplementary Materials II for more details).
There were no significant correlations between the MRI measures and the CSF biomarker values at baseline (all p > 0.05). However, CSF p-tau was correlated with t-tau (r = 0.65, p < 0.001) and with Aβ1–42 (r = − 0.25, p < 0.001).
3.1. Relationship between CSF groups and MRI atrophy over time
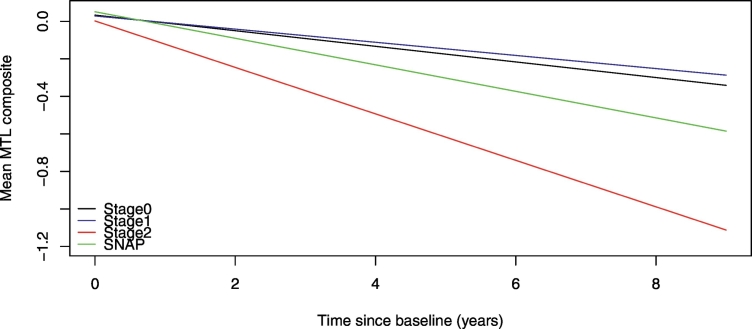
The results from the mixed-effects model examining the association between each Stage and the longitudinal MTL volume composite are summarized in Table 3. When the preclinical AD groups were defined using CSF Aβ1–42 and p-tau, there was a significant interaction between Stage 2 and time (< 0.006), indicating greater atrophy in the MTL volume composite over time for individuals in Stage 2 compared to Stage 0. By comparison, the Stage 1 × time and SNAP × time interactions were not significant (both p > 0.2), suggesting similar rates of change over time in the MTL volume composite for Stages 0, 1, and SNAP. A follow-up mixed-effects model using Stage 2 as the reference group showed that the rate of atrophy of the MTL composite for the Stage 2 group was also greater than for Stage 1 [mean estimate (SE) = 0.09 (0.03), p = 0.0086], but the difference did not reach significance compared to the SNAP group [mean estimate (SE) = 0.05 (0.03), p = 0.128]. These findings are illustrated in Fig. 1. When the preclinical AD groups were defined using Aβ1–42 and t-tau, there were no significant interactions between any of the Stage indicators and time (all p > 0.13), suggesting that the rates of change in the MTL volume composite over the follow-up period did not differ for the four groups. The same pattern of results was obtained using more stringent cut-points (quartiles or quintiles) to classify subjects into the four CSF groups, with one exception: the rate of atrophy of the MTL volume composite of the SNAP group defined using Aβ1–42 and p-tau was significantly greater than for Stages 0 and 1 (p < 0.05, see Supplementary Materials III).
Table 3.
Results of linear mixed effects models for the medial temporal lobe volume composite.
| Groups defined by CSF Aβ and p-tau |
Groups defined by CSF Aβ and total tau |
|||||
|---|---|---|---|---|---|---|
| Model Predictors | Estimate | SE | p-Value | Estimate | SE | p-Value |
| Time | − 0.038 | 0.087 | 0.665 | − 0.007 | 0.093 | 0.941 |
| Baseline age | 0.003 | 0.005 | 0.589 | 0.003 | 0.005 | 0.593 |
| Gender (male) | 0.413 | 0.099 | < 0.0001 | 0.411 | 0.098 | < 0.0001 |
| Education | − 0.012 | 0.021 | 0.578 | − 0.011 | 0.021 | 0.589 |
| Stage 1 | − 0.005 | 0.125 | 0.971 | − 0.112 | 0.127 | 0.930 |
| Stage 2 | − 0.031 | 0.158 | 0.844 | − 0.059 | 0.154 | 0.700 |
| SNAP | 0.018 | 0.124 | 0.886 | − 0.032 | 0.126 | 0.801 |
| Baseline age × time | 0.001 | 0.001 | 0.384 | 0.000 | 0.001 | 0.714 |
| Gender × time | − 0.023 | 0.019 | 0.227 | − 0.015 | 0.019 | 0.455 |
| Education × time | − 0.003 | 0.004 | 0.444 | − 0.003 | 0.004 | 0.427 |
| Stage 1 × time | 0.007 | 0.022 | 0.766 | − 0.009 | 0.025 | 0.719 |
| Stage 2 × time | − 0.082 | 0.029 | 0.006 | − 0.043 | 0.028 | 0.137 |
| SNAP × time | − 0.029 | 0.026 | 0.261 | − 0.017 | 0.022 | 0.439 |
Note: Stage 0 was used as the reference group in these models. Thus, estimates for Stage 1, 2, and SNAP and their interactions with time reflect differences relative to Stage 0.
Fig. 1.
Estimated atrophy rates from linear mixed-effects models predicting longitudinal medial-temporal lobe volume atrophy among individuals classified into the 4 preclinical AD groups (Stage 0, Stage 1, Stage 2, and suspected non–Alzheimer disease pathology [SNAP]) using baseline cerebrospinal fluid Aβ1–42 and phosphorylated tau (p-tau). The estimates are adjusted for baseline age, sex, education, and their interactions with time. There was no difference in atrophy rates between Stage 0, 1, and SNAP (Table 3), but Stage 2 had a greater rate of atrophy than Stages 0 and 1, while the SNAP group had intermediate atrophy levels.
The results for the individual MTL regions were similar to the results using the MTL composite score as the outcome, though less robust, and can be found in the Supplementary Materials IV. Specifically, when the groups were defined using Aβ1–42 and t-tau, there were no interactions between any of the Stage indicators and time (all p > 0.11), suggesting similar rates of change in the individual regions for the four groups. When the groups were defined by Aβ1–42 and p-tau, there was a significant Stage 2 × time interaction for the amygdala (p = 0.035) and a marginally-significant interaction for the entorhinal cortex volume (p = 0.068). For the hippocampus and entorhinal cortex thickness, the Stage 2 × time interactions were in the same direction, but not significant (p = 0.155, and p = 0.260, respectively).
3.2. Effect of APOE ε4 status on relationship between CSF groups and MRI atrophy over time
Follow-up models examining the effects of APOE ε4 status and follow-up diagnosis were only performed for the MTL volume composite. When the APOE ε4 indicator and its interaction with time were added to the model, the results remained the same and neither the effect of APOE ε4 status, nor the APOE ε4 status by time interaction were significant (all p > 0.2 for both Aβ1–42/t-tau and Aβ1–42/p-tau). Furthermore, the three-way interactions between APOE ε4 status, time, and the Stage indicator variables were not significant (all p > 0.8), indicating similar rates of MTL atrophy for APOE ε4 carriers and non-carriers within each of the four groups.
3.3. Effect of follow-up diagnosis on relationship between CSF groups and MRI atrophy over time
Baseline characteristics for individuals who remained normal vs. those who progressed to MCI or dementia at follow-up are shown in Supplementary Materials V. When the follow-up diagnosis indicator and its interaction with time were added to the main models, the results also remained the same. Across all four groups, there was a main effect of diagnosis [mean estimate (SE) = − 0.27 (0.13), p = 0.045 for groups defined by Aβ1–42 and t-tau; mean estimate (SE) = − 0.28 (0.13), p = 0.04 for groups defined by Aβ1–42 and p-tau], but the diagnosis by time interaction was not significant (both p > 0.48). This suggests that individuals who progressed to MCI at follow-up had smaller MTL volume composites at baseline than those who remained cognitively normal, even after accounting for baseline levels of CSF Aβ1–42, t-tau, and p-tau, but the rate of atrophy was the same for progressors and non-progressors. (The three-way interactions between follow-up diagnosis, Stage indicators, and time were not examined due to the small number of progressors in each CSF group (see Table 2).)
4. Discussion
This study compared rates of brain atrophy within several medial temporal brain regions among cognitively normal individuals, categorized on the basis of their CSF biomarker profiles, and determined whether any group differences observed were influenced by whether the participants progressed to MCI over time. The primary finding was that when the groups were defined using CSF Aβ1–42 and p-tau, individuals in Stage 2 (low levels of CSF Aβ1–42 and high levels of CSF p-tau) showed a significantly greater rate of atrophy in the MTL volume composite measure than individuals in Stage 0 and 1, which did not differ from one another. The SNAP group showed intermediate levels of atrophy and did not differ from Stage 0, 1, or 2. When abnormality of CSF p-tau was defined using more extreme cut-points, the SNAP group also showed greater atrophy in the MTL composite than Stages 0 and 1 and did not differ from Stage 2. However, when the groups were defined using levels of CSF Aβ1–42 and CSF t-tau, there were no differences between the four groups at baseline or in the rate of change of the MTL volume composite over time, independent of cut-points.
The long clinical follow-up period of individuals in the current study also allowed us to examine whether follow-up diagnostic status (cognitively normal or MCI) influences the association between the CSF AD biomarkers and rate of change in MTL atrophy. We found that, even after accounting for baseline CSF levels, individuals who progressed to MCI at follow-up had smaller MTL volumes composites at baseline than individuals who remained cognitively normal over time. However, the rate of MTL atrophy did not differ between subjects who progressed to MCI at follow-up vs. those who remained cognitively normal when CSF levels of Aβ1–42, and t-tau or p-tau, were covaried, though we cannot rule out the possibility that we were underpowered to detect an effect. Additionally, future studies are needed to determine whether different rates of atrophy are found among progressors within each of the CSF groups, as the present study did not have the power to examine this question.
These results suggest that individuals who subsequently progressed to MCI had already undergone some degree of MTL atrophy prior to their baseline CSF collection, when they were cognitively normal. This interpretation would be consistent with the finding that baseline measures of MTL structures predict the time to progress from normal cognition to symptom onset of MCI > 6 years prior to symptom onset (Soldan et al., 2015) and show volumetric shape changes between 3 and 10 years before symptom onset (Younes et al., 2014). Another possibility is that individuals who progressed to MCI had smaller MTL volumes prior to the accumulation of amyloid and tau pathology, possibly reflecting genetic or developmental events. Our results also suggest that the additional atrophy in the MTL among those destined to develop MCI is not independent of CSF levels of Aβ1–42, p-tau, and t-tau. Additionally, it is noteworthy that even among subjects in Stage 0, the volume of the MTL composite tended to decrease over time, which may be indicative of atrophy due to non-AD related causes.
Taken together, these findings suggest that abnormal levels of CSF p-tau, particularly in combination with abnormal levels of CSF Aβ1–42, are necessary for observing significant MTL atrophy over time among individuals with normal cognition at baseline. This finding is in line with the results reported by Desikan et al. (2011), indicating that abnormal levels of Aβ1–42 and p-tau appear to be more important for observing AD-related brain atrophy than abnormal levels of Aβ1–42 and t-tau. The finding that individuals with very high levels of p-tau and normal levels of Aβ1–42 may also show elevated MTL atrophy provides some evidence for amyloid-independent, tau-mediated atrophy, though these results need to be replicated given the relatively small number of participants in this group. It is possible that atrophy is directly caused by neurofibrillary tangle pathology (as indexed by CSF p-tau), independent of amyloid, but higher levels of brain amyloid facilitate the accumulation and spread of tangles.
We also found that a composite MTL volume measure provided a more sensitive index of MRI atrophy over time than measures of the individual MTL regions. This finding has implications for AD clinical trials using structural MRI measures for tracking disease progression or response to treatment. Our results suggest that a composite MTL measure would provide a more sensitive endpoint than hippocampal volume or entorhinal cortex volume alone, when tracking atrophy during the preclinical phase of AD. This is likely due to the reduction in measurement error conferred by the use of composite scores (Mosier, 1943), as well as the fact that CSF biomarkers reflect whole brain markers of the underlying disease process and may therefore show stronger associations with more global than local measures of brain atrophy. Future studies are necessary for determining whether MRI composite scores are also more sensitive than individual regional measures when using amyloid and tau PET imaging to classify individuals into preclinical AD stages.
Lastly, we found that although APOE ε4 carriers were more likely to be classified as Stage 1 or 2 than Stage 0 or SNAP, the presence of an APOE ε4 genotype did not modulate the degree of MTL atrophy once baseline levels of CSF Aβ1–42, t-tau, and p-tau were accounted for. Similar findings have been reported for the rate of change in cognition over time among individuals in the four groupings examined here (Soldan et al., 2016a). With respect to the design of clinical trials for AD therapeutics, these findings suggest that while over-recruitment of individuals with the APOE ε4 genotype may be beneficial for ensuring a greater proportion of individuals in Stage 2 (i.e., those at highest risk for AD-related cognitive decline and brain atrophy), the samples do not need to be stratified by ε4 carrier status when baseline levels of amyloid and tau are taken into account.
The results from the current study provide support for the recently proposed A/T/N classification scheme for AD (Jack et al., 2016), which recommends that biomarkers of neurofibrillary tangle pathology (CSF p-tau and tau PET imaging) should be differentiated from biomarkers of neuronal injury (CSF t-tau, structural MRI, and FDG PET imaging). Although CSF p-tau and t-tau were highly correlated in the current study, only elevated p-tau was reliably associated with longitudinal MTL atrophy. This likely reflects the fact that CSF p-tau is very closely related to the AD pathophysiological process, with CSF p-tau concentration levels correlating with neurofibrillary tangle pathology in AD patients (Buerger et al., 2006, Seppala et al., 2012). CSF t-tau levels, while significantly higher among AD-dementia patients than among cognitively normal individuals, can also be seen in other diseases and reflect more general levels of neuronal injury due to both AD and non-AD processes (Zetterberg, 2017). Additionally, it has been hypothesized that CSF p-tau becomes abnormal earlier in the course of AD than CSF t-tau (Jack et al., 2013), so that during the initial phases of the disease, abnormal levels of CSF Aβ1–42 and p-tau may be more strongly associated with MTL atrophy than abnormal levels of CSF Aβ1–42 and t-tau. For example, a study by Gomar et al. (2016) showed that atrophy in the hippocampus was predicted by decreasing levels of Aβ1–42 and increasing levels of p-tau but not t-tau among older, cognitively normal individuals. Similarly, a recent study by Pascoal et al. (2017) reported a significant decline in FDG medial temporal metabolism among cognitively normal individuals with abnormal levels of both amyloid and CSF p-tau, but not among those with abnormal levels of amyloid and t-tau. It is also noteworthy that participants in the current study were relatively young and had a strong family history of AD-dementia; therefore, the atrophy observed among individuals in the current study is more likely to be due to AD than to other causes, which may also explain the p-tau specific association. This interpretation would be consistent with the finding that studies of older individuals, who tend to harbor greater levels of other age-related neurodegenerative pathologies, have observed elevated MTL atrophy among individuals categorized by abnormal levels of amyloid and neuronal injury (i.e., Stage 2, Knopman et al., 2013, Jack et al., 2014, Gordon et al., 2016, Knopman et al., 2016, Pegueroles et al., 2017).
The results from the current study are also consistent with previously reported dissociations between CSF t-tau and p-tau in the BIOCARD cohort. For example, Moghekar et al. (2013) reported that baseline levels of Aβ1–42 and p-tau were associated with the time to progression from normal cognition to MCI due to AD, but t-tau levels were not. Additionally, Pettigrew et al. (2016) found that lower cortical thickness among cognitively normal individuals was associated with p-tau but not with t-tau levels, and that lower cortical thickness predicted the time to progress to symptom onset of MCI. Lastly, Soldan et al. (2016b) found that performance in a visual-spatial episodic memory task was associated with CSF p-tau but not t-tau levels 10 years earlier, when individuals were cognitively normal. Taken together, these results support the view that p-tau levels are more closely related to the cognitive and brain changes associated with AD among middle-aged individuals than t-tau levels. An exception to this pattern of dissociations between t-tau and p-tau is the finding that significant cognitive decline over the course of up 20 years was observed for individuals classified as preclinical Stage 2 using both Aβ1–42/p-tau and Aβ1–42/t-tau (Soldan et al., 2016a). One possibility that may explain this apparent discrepancy is that the long-term cognitive decline observed by Soldan et al. (2016a) among individuals with abnormal levels of Aβ1–42 and t-tau reflected contributions from both AD and non-AD related pathologies, with non-AD processes exerting their effects over a longer time-frame, when individuals were older.
This study must be interpreted within the context of its limitations. First, the BIOCARD cohort is a convenience sample and consists of well-educated, primarily Caucasian participants, the majority of whom have a family history of AD. These factors may limit generalization to the population at large. Second, the mean MRI follow-up time for subjects with > 1 MRI scan was only 4 years and 38% of the cohort only had 1 MRI scan. It is possible, therefore, that MRI atrophy patterns during preclinical AD differ over longer follow-up periods. Third, we did not examine rates of MRI atrophy outside of the MTL; it remains unclear, therefore, whether our results generalize to AD-vulnerable regions outside of the MTL. For example, cortical thinning outside of the MTL has been reported several years prior to the onset of symptoms associated with AD both for late onset and dominantly-inherited AD (e.g., Pettigrew et al., 2016, Weston et al., 2016); it will be important to compare rates of atrophy across these regions in relationship to CSF biomarkers. Fourth, the present study used tertiles (and quintiles) to define the CSF groups, rather than statistically and clinically validated cut-points for determining biomarker abnormality; this method may be highly sample dependent and biased by sample characteristics (such as sample age and family history of dementia). Future studies are therefore necessary to determine if similar findings are obtained using clinically validated CSF cut-points and more diverse samples, and to test whether similar results are obtained when using PET amyloid and tau imaging to classify individuals into preclinical AD stages.
Acknowledgments
Acknowledgements
The BIOCARD Study consists of 7 Cores with the following members: (1) the Administrative Core (Marilyn Albert, Rostislav Brichko); (2) the Clinical Core (Ola Selnes, Marilyn Albert, Anja Soldan, Corinne Pettigrew, Rebecca Gottesman, Ned Sacktor, Scott Turner, Leonie Farrington, Maura Grega, Gay Rudow, Daniel D'Agostino, Scott Rudow); (3) the Imaging Core (Michael Miller, Susumu Mori, Tilak Ratnanather, Timothy Brown, Hayan Chi, Anthony Kolasny, Kenichi Oishi, Thomas Reigel, Laurent Younes); (4) the Biospecimen Core (Abhay Moghekar, Richard O'Brien,); (5) the Informatics Core (Roberta Scherer, Ann Ervin, Jennifer Jones, Matt Toepfner, Alicia Wentz, April Patterson, Aisha Mohammed); (6) the Biostatistics Core (Mei-Cheng Wang, Qing Cai, Daisy Zhu); and (7) the Neuropathology Core (Juan Troncoso, Barbara Crain, Olga Pletnikova, Gay Rudow, and Karen Fisher). The authors are grateful to the members of the BIOCARD Scientific Advisory Board who provide continued oversight and guidance regarding the conduct of the study including: Drs. John Cernansky, David Holtzman, David Knopman, Walter Kukull, and Kevin Grimm, and Drs., John Hsiao, Laurie Ryan, who provide oversight on behalf of the National Institute on Aging (U19-AG03365). The authors thank the members of the BIOCARD Resource Allocation Committee who provide ongoing guidance regarding the use of the biospecimens collected as part of the study, including: Drs. Constantine Lyketsos, Carlos Pardo, Gerard Schellenberg, Leslie Shaw, Madhav Thambisetty, and John Trojanowski.
The authors acknowledge the contributions of the Geriatric Psychiatry Branch of the intramural program of NIMH who initiated the study (Principle investigator: Dr. Trey Sunderland). The authors are particularly indebted to Dr. Karen Putnam, who has provided ongoing documentation of the Geriatric Psychiatry Branch study procedures and the data files received from NIMH.
Disclosure statement
Dr. Pettigrew reports no disclosures.
Dr. Soldan reports no disclosures.
Dr. Sloan reports no disclosures.
Dr. Cai reports no disclosures.
Dr. J. Wang reports no disclosures.
Dr. M.C. Wang reports no disclosures.
Dr. Moghekar reports no disclosures.
Dr. Miller owns a significant equity share in “Anatomy Works”. This arrangement is being managed by the Johns Hopkins University in accordance with its conflict of interest policies.
Dr. Albert is an advisor to Eli Lilly.
Funding sources
This study was supported in part by grants from the National Institutes of Health (U19-AG03365, P50-AG005146).
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nicl.2017.08.022.
Appendix A. Supplementary data
Supplementary material
References
- Albert DeKosky S.T., Dickson D., Dubois B., Feldman H.H., Fox N.C., Gamst A., Holtzman D.M., Jagust W.J., Petersen R.C., Snyder P.J., Carrillo M.C., Thies B., Phelps C.H. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert M., Soldan A., Gottesman R., McKhann G., Sacktor N., Farrington L., Grega M., Turner R., Lu Y., Li S., Wang M.C., Selnes O. Cognitive changes preceding clinical symptom onset of mild cognitive impairment and relationship to ApoE genotype. Curr. Alzheimer Res. 2014;11:773–784. doi: 10.2174/156720501108140910121920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beg M.F., Miller M.I., Trouve A., Younes L. Computing metrics via geodesics on flows of diffeomorphisms. Int. J. Comput. Vis. 2005;61:139–157. [Google Scholar]
- Buerger K., Ewers M., Pirttila T., Zinkowski R., Alafuzoff I., Teipel S.J., DeBernardis J., Kerkman D., McCulloch C., Soininen H., Hampel H. CSF phosphorylated tau protein correlates with neocortical neurofibrillary pathology in Alzheimer's disease. Brain. 2006;129:3035–3041. doi: 10.1093/brain/awl269. [DOI] [PubMed] [Google Scholar]
- Corder E.H., Saunders A.M., Risch N.J., Strittmatter W.J., Schmechel D.E., Gaskell P.C., Jr., Rimmler J.B., Locke P.A., Conneally P.M., Schmader K.E. Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nat. Genet. 1994;7:180–184. doi: 10.1038/ng0694-180. [DOI] [PubMed] [Google Scholar]
- Corder E.H., Saunders A.M., Strittmatter W.J., Schmechel D.E., Gaskell P.C., Small G.W., Roses A.D., Haines J.L., Pericak-Vance M.A. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Csernansky J.G., Wang L., Swank J., Miller J.P., Gado M., McKeel D., Miller M.I., Morris J.C. Preclinical detection of Alzheimer's disease: hippocampal shape and volume predict dementia onset in the elderly. NeuroImage. 2005;25:783–792. doi: 10.1016/j.neuroimage.2004.12.036. [DOI] [PubMed] [Google Scholar]
- Desikan R.S., McEvoy L.K., Thompson W.K., Holland D., Roddey J.C., Blennow K., Aisen P.S., Brewer J.B., Hyman B.T., Dale A.M. Amyloid-beta associated volume loss occurs only in the presence of phospho-tau. Ann. Neurol. 2011;70:657–661. doi: 10.1002/ana.22509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomar J.J., Conejero-Goldberg C., Davies P., Goldberg T.E. Anti-correlated cerebrospinal fluid biomarker trajectories in preclinical Alzheimer's disease. J. Alzheimers Dis. 2016;51:1085–1097. doi: 10.3233/JAD-150937. [DOI] [PubMed] [Google Scholar]
- Gordon B.A., Blazey T., Su Y., Fagan A.M., Holtzman D.M., Morris J.C., Benzinger T.L. Longitudinal beta-amyloid deposition and hippocampal volume in preclinical Alzheimer disease and suspected non-Alzheimer disease pathophysiology. JAMA Neurol. 2016;73:1192–1200. doi: 10.1001/jamaneurol.2016.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack C.R., Jr., Bennett D.A., Blennow K., Carrillo M.C., Feldman H.H., Frisoni G.B., Hampel H., Jagust W.J., Johnson K.A., Knopman D.S., Petersen R.C., Scheltens P., Sperling R.A., Dubois B. A/T/N: an unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology. 2016;87:539–547. doi: 10.1212/WNL.0000000000002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack C.R., Jr., Knopman D.S., Jagust W.J., Petersen R.C., Weiner M.W., Aisen P.S., Shaw L.M., Vemuri P., Wiste H.J., Weigand S.D., Lesnick T.G., Pankratz V.S., Donohue M.C., Trojanowski J.Q. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack C.R., Jr., Knopman D.S., Weigand S.D., Wiste H.J., Vemuri P., Lowe V., Kantarci K., Gunter J.L., Senjem M.L., Ivnik R.J., Roberts R.O., Rocca W.A., Boeve B.F., Petersen R.C. An operational approach to National Institute on Aging-Alzheimer's Association criteria for preclinical Alzheimer disease. Ann. Neurol. 2012;71:765–775. doi: 10.1002/ana.22628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack C.R., Jr., Wiste H.J., Knopman D.S., Vemuri P., Mielke M.M., Weigand S.D., Senjem M.L., Gunter J.L., Lowe V., Gregg B.E., Pankratz V.S., Petersen R.C. Rates of beta-amyloid accumulation are independent of hippocampal neurodegeneration. Neurology. 2014;82:1605–1612. doi: 10.1212/WNL.0000000000000386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack C.R., Jr., Wiste H.J., Weigand S.D., Therneau T.M., Knopman D.S., Lowe V., Vemuri P., Mielke M.M., Roberts R.O., Machulda M.M., Senjem M.L., Gunter J.L., Rocca W.A., Petersen R.C. Age-specific and sex-specific prevalence of cerebral β-amyloidosis, tauopathy, and neurodegeneration in cognitively unimpaired individuals aged 50–95 years: a cross-sectional study. Lancet Neurol. 2017;16:435–444. doi: 10.1016/S1474-4422(17)30077-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S.C., Miller M.I. Landmark matching via large deformation diffeomorphisms. IEEE Trans. Image Process. 2000;9:1357–1370. doi: 10.1109/83.855431. [DOI] [PubMed] [Google Scholar]
- Knopman D.S., Jack C.R., Jr., Lundt E.S., Weigand S.D., Vemuri P., Lowe V.J., Kantarci K., Gunter J.L., Senjem M.L., Mielke M.M., Machulda M.M., Roberts R.O., Boeve B.F., Jones D.T., Petersen R.C. Evolution of neurodegeneration-imaging biomarkers from clinically normal to dementia in the Alzheimer disease spectrum. Neurobiol. Aging. 2016;46:32–42. doi: 10.1016/j.neurobiolaging.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopman D.S., Jack C.R., Jr., Wiste H.J., Weigand S.D., Vemuri P., Lowe V.J., Kantarci K., Gunter J.L., Senjem M.L., Mielke M.M., Roberts R.O., Boeve B.F., Petersen R.C. Selective worsening of brain injury biomarker abnormalities in cognitively normal elderly persons with beta-amyloidosis. JAMA Neurol. 2013;70:1030–1038. doi: 10.1001/jamaneurol.2013.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson N., Andreasson U., Persson S., Arai H., Batish S.D., Bernardini S., Bocchio-Chiavetto L., Blankenstein M.A., Carrillo M.C., Chalbot S., Coart E., Chiasserini D., Cutler N., Dahlfors G., Duller S., Fagan A.M., Forlenza O., Frisoni G.B., Galasko D., Galimberti D., Hampel H., Handberg A., Heneka M.T., Herskovits A.Z., Herukka S.K., Holtzman D.M., Humpel C., Hyman B.T., Iqbal K., Jucker M., Kaeser S.A., Kaiser E., Kapaki E., Kidd D., Klivenyi P., Knudsen C.S., Kummer M.P., Lui J., Llado A., Lewczuk P., Li Q.X., Martins R., Masters C., McAuliffe J., Mercken M., Moghekar A., Molinuevo J.L., Montine T.J., Nowatzke W., O'Brien R., Otto M., Paraskevas G.P., Parnetti L., Petersen R.C., Prvulovic D., de Reus H.P., Rissman R.A., Scarpini E., Stefani A., Soininen H., Schroder J., Shaw L.M., Skinningsrud A., Skrogstad B., Spreer A., Talib L., Teunissen C., Trojanowski J.Q., Tumani H., Umek R.M., Van Broeck B., Vanderstichele H., Vecsei L., Verbeek M.M., Windisch M., Zhang J., Zetterberg H., Blennow K. The Alzheimer's Association external quality control program for cerebrospinal fluid biomarkers. Alzheimers Dement. 2011;7(386–395) doi: 10.1016/j.jalz.2011.05.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G.M., Knopman D.S., Chertkow H., Hyman B.T., Jack C.R., Jr., Kawas C.H., Klunk W.E., Koroshetz W.J., Manly J.J., Mayeux R., Mohs R.C., Morris J.C., Rossor M.N., Scheltens P., Carrillo M.C., Thies B., Weintraub S., Phelps C.H. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M.I., Younes L., Ratnanather J.T., Brown T., Trinh H., Postell E., Lee D.S., Wang M.C., Mori S., O'Brien R., Albert M. The diffeomorphometry of temporal lobe structures in preclinical Alzheimer's disease. Neuroimage Clin. 2013;3:352–360. doi: 10.1016/j.nicl.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghekar A., Goh J., Li M., Albert M., O'Brien R.J. Cerebrospinal fluid Abeta and tau level fluctuation in an older clinical cohort. Arch. Neurol. 2012;69:246–250. doi: 10.1001/archneurol.2011.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghekar A., Li S., Lu Y., Li M., Wang M.C., Albert M., O'Brien R. CSF biomarker changes precede symptom onset of mild cognitive impairment. Neurology. 2013;81:1753–1758. doi: 10.1212/01.wnl.0000435558.98447.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mormino E.C., Betensky R.A., Hedden T., Schultz A.P., Amariglio R.E., Rentz D.M., Johnson K.A., Sperling R.A. Synergistic effect of β-amyloid and neurodegeneration on cognitive decline in clinically normal individuals. JAMA Neurol. 2014;71:1379–1385. doi: 10.1001/jamaneurol.2014.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosier C.I. On the reliability of a weighted composite. Psychometrika. 1943;8:161–168. [Google Scholar]
- Pascoal T.A., Mathotaarachchi S., Mohades S., Benedet A.L., Chung C.O., Shin M., Wang S., Beaudry T., Kang M.S., Soucy J.P., Labbe A., Gauthier S., Rosa-Neto P. Amyloid-beta and hyperphosphorylated tau synergy drives metabolic decline in preclinical Alzheimer's disease. Mol. Psychiatry. 2017;22:306–311. doi: 10.1038/mp.2016.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegueroles J., Vilaplana E., Montal V., Sampedro F., Alcolea D., Carmona-Iragui M., Clarimon J., Blesa R., Lleo A., Fortea J. Longitudinal brain structural changes in preclinical Alzheimer's disease. Alzheimers Dement. 2017;13(5):499–509. doi: 10.1016/j.jalz.2016.08.010. [DOI] [PubMed] [Google Scholar]
- Pettigrew C., Soldan A., Zhu Y., Wang M.C., Moghekar A., Brown T., Miller M., Albert M. Cortical thickness in relation to clinical symptom onset in preclinical AD. Neuroimage Clin. 2016;12:116–122. doi: 10.1016/j.nicl.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segonne F., Dale A.M., Busa E., Glessner M., Salat D., Hahn H.K., Fischl B. A hybrid approach to the skull stripping problem in MRI. NeuroImage. 2004;22:1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Seppala T.T., Nerg O., Koivisto A.M., Rummukainen J., Puli L., Zetterberg H., Pyykko O.T., Helisalmi S., Alafuzoff I., Hiltunen M., Jaaskelainen J.E., Rinne J., Soininen H., Leinonen V., Herukka S.K. CSF biomarkers for Alzheimer disease correlate with cortical brain biopsy findings. Neurology. 2012;78:1568–1575. doi: 10.1212/WNL.0b013e3182563bd0. [DOI] [PubMed] [Google Scholar]
- Shaw L.M., Vanderstichele H., Knapik-Czajka M., Clark C.M., Aisen P.S., Petersen R.C., Blennow K., Soares H., Simon A., Lewczuk P., Dean R., Siemers E., Potter W., Lee V.M., Trojanowski J.Q. Cerebrospinal fluid biomarker signature in Alzheimer's disease neuroimaging initiative subjects. Ann. Neurol. 2009;65:403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldan A., Pettigrew C., Cai Q., Wang M.C., Moghekar A.R., O'Brien R.J., Selnes O.A., Albert M.S. Hypothetical preclinical Alzheimer disease groups and longitudinal cognitive change. JAMA Neurol. 2016;73:698–705. doi: 10.1001/jamaneurol.2016.0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldan A., Pettigrew C., Lu Y., Wang M.C., Selnes O., Albert M., Brown T., Ratnanather J.T., Younes L., Miller M.I. Relationship of medial temporal lobe atrophy, APOE genotype, and cognitive reserve in preclinical Alzheimer's disease. Hum. Brain Mapp. 2015;36:2826–2841. doi: 10.1002/hbm.22810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldan A., Pettigrew C., Moghekar A., Albert M. Computerized cognitive tests are associated with biomarkers of Alzheimer's disease in cognitively normal individuals 10 years prior. J. Int. Neuropsychol. Soc. 2016;22:968–977. doi: 10.1017/S1355617716000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling R.A., Aisen P.S., Beckett L.A., Bennett D.A., Craft S., Fagan A.M., Iwatsubo T., Jack C.R., Jr., Kaye J., Montine T.J., Park D.C., Reiman E.M., Rowe C.C., Siemers E., Stern Y., Yaffe K., Carrillo M.C., Thies B., Morrison-Bogorad M., Wagster M.V., Phelps C.H. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos S.J.B., Xiong C., Visser P.J., Jasielec M.S., Hassenstab J., Grant E.A., Cairns N.J., Morris J.C., Holtzman D.M., Fagan A.M. Preclinical Alzheimer's disease and its outcome: a longitudinal cohort study. Lancet Neurol. 2013;12:957–965. doi: 10.1016/S1474-4422(13)70194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston P.S., Nicholas J.M., Lehmann M., Ryan N.S., Liang Y., Macpherson K., Modat M., Rossor M.N., Schott J.M., Ourselin S., Fox N.C. Presymptomatic cortical thinning in familial Alzheimer disease: a longitudinal MRI study. Neurology. 2016;87:2050–2057. doi: 10.1212/WNL.0000000000003322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younes L., Albert M., Miller M.I. Inferring changepoint times of medial temporal lobe morphometric change in preclinical Alzheimer's disease. Neuroimage Clin. 2014;5:178–187. doi: 10.1016/j.nicl.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetterberg H. Review: tau in biofluids - relation to pathology, imaging and clinical features. Neuropathol. Appl. Neurobiol. 2017;43:194–199. doi: 10.1111/nan.12378. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material