Abstract
We aimed to investigate the integrity and clinical relevance of striatal dopamine receptor type-2 (D2R) availability in Parkinson's disease (PD) patients. We studied 68 PD patients, spanning from early to advanced disease stages, and 12 healthy controls. All participants received one [11C]raclopride PET scan in an OFF medication condition for quantification of striatal D2R availability in vivo. Parametric images of [11C]raclopride non-displaceable binding potential were generated from the dynamic [11C]raclopride scans using implementation of the simplified reference tissue model with cerebellum as the reference tissue. PET data were interrogated for correlations with clinical data related to disease burden and dopaminergic treatment. PD patients showed a mean 16.7% decrease in caudate D2R and a mean 3.5% increase in putaminal D2R availability compared to healthy controls. Lower caudate [11C]raclopride BPND correlated with longer PD duration. PD patients on dopamine agonist treatment had 9.2% reduced D2R availability in the caudate and 12.8% in the putamen compared to PD patients who never received treatment with dopamine agonists. Higher amounts of lifetime dopamine agonist therapy correlated with reduced D2Rs availability in both caudate and putamen. No associations between striatal D2R availability and levodopa treatment and dyskinesias were found. In advancing PD the caudate and putamen D2R availability are differentially affected. Chronic exposure to treatment with dopamine agonists, but no levodopa, suppresses striatal D2R availability, which may have relevance to output signaling to frontal lobes and the occurrence of executive deficits, but not dyskinesias.
Abbreviations: AIMS, Abnormal Involuntary Movement Scale; BDI-II, Beck Depression Inventory; BPND, non-displaceable binding potential; D2R, dopamine receptor type-2; H&Y, Hoehn and Yahr staging; LED, levodopa-equivalent-dose; MMSE, Mini-Mental State Examination; MRI, magnetic resonance imaging; PD, Parkinson's disease; PET, position emission tomography; ROI, region of interest; UPDRS, Unified Parkinson's Disease Rating Scale
Keywords: Parkinson's disease, Basal ganglia, PET, Dopamine D2 receptors, Dopamine agonists
Highlights
-
•
D2R in caudate and putamen are differentially affected in PD.
-
•
Loss of D2R in caudate correlates with longer disease duration.
-
•
Dopamine agonists treatment, but not levodopa, suppresses caudate and putamen D2Rs.
-
•
No association between striatal D2R availability, levodopa treatment and dyskinesia.
1. Introduction
Postsynaptic striatal dopamine type-2 receptors (D2Rs) modulate the striatopallidal and the striatocortical signaling pathways in the basal ganglia output nuclei. In Parkinson's disease (PD), stimulation of these pathways is affected due to the progressive degeneration of dopaminergic neurons (Samii et al., 2004, Herz et al., 2015), leading to alterations in synaptic dopamine levels and the development of motor symptoms (Pavese et al., 2006), cognitive symptoms (Sawamoto et al., 2008a, Sawamoto et al., 2008b), and dyskinesias (Politis et al., 2014). Although long-term changes in D2R availability may be also implicated (Antonini et al., 1997), it is unclear how these changes are affected by the progression of the disease and the dopaminergic treatment in patients with PD.
[11C]raclopride PET is a selective marker and a valuable tool for investigating D2R availability in vivo (Laruelle, 2000). [11C]raclopride PET studies in de novo PD patients have reported an increase in D2R availability in the putamen contralateral to the more affected limbs, while the D2R availability in the caudate remains intact (Rinne et al., 1993, Turjanski et al., 1997). A combined PET study with [18F]fluoro-l-dopa (F-DOPA), a marker of pre-synaptic dopamine storage capacity, and [11C]raclopride reported inverse correlations between reduced F-DOPA and increased post-synaptic D2R in de novo PD (Sawle et al., 1993). Therefore, in early denovo PD, upregulation of post-synaptic D2R in the putamen could be a compensatory mechanism in response to depletion of pre-synaptic dopaminergic terminals and synaptic dopamine levels. However, as PD progresses D2R availability normalizes in the putamen, but availability in the caudate is reduced (Antonini et al., 1997, Turjanski et al., 1997, Brooks et al., 1992, Dentresangle et al., 1999). As the disease progresses and increasing degeneration of nigrostriatal dopamine neurons occurs this compensatory mechanism fails; leading to decreased D2R availability in caudate. Furthermore, after 3–5 years of levodopa treatment, [11C]raclopride binding is significant reduced in the putamen and caudate compared to baseline (Antonini et al., 1997). These findings suggest that down-regulation of striatal D2R in PD may be induced by chronic dopaminergic treatment. Alterations in D2R function have been also suggested to play a role in mechanisms underlying dyskinesias in PD (Boyce et al., 1990, Graham et al., 1993).
Here we used [11C]raclopride PET to explore the integrity of striatal D2R ligand binding and its relevance to the development of clinical symptoms and the chronic exposure to dopaminergic treatment in a group of patients with PD.
2. Materials and methods
2.1. Participants
We studied 68 patients with idiopathic PD according to the UK Brain Bank clinical diagnostic criteria. All PD patients were receiving dopamine replacement therapy. To assess the effect of dopamine agonist treatment, PD patients were divided into two groups, those who were receiving dopamine agonists (n = 46; either Ropinerole or Pramipexole, not in extended release) and patients who had never received treatment with dopamine agonists (n = 22). Apart from levodopa and dopamine agonists, PD patients were not on any other medication with known action on dopamine receptors. Twelve healthy individuals matched for age and gender, with no history of neurological or psychiatric illness served as the healthy control group. All subjects were non-demented (Table 1).
Table 1.
Clinical characteristics of Parkinson's disease patients and healthy controls.
| Parkinson's disease patients | Healthy controls | |
|---|---|---|
| No of subjects | 68 | 12 |
| Sex | 52 M/16F | 10 M/2F |
| Age (years ± SD) | 62.8 (± 9.1) | 63.3 (± 7.0) |
| Disease duration (years ± SD)a | 10.71 (± 6.4) | – |
| H&Y OFF (mean ± SD) | 2.8 (± 0.94) | – |
| UPDRS Part-III OFF (mean ± SD) | 42.0 (± 13.0) | – |
| UPDRS Part-III ON (mean ± SD) | 20.8 (± 8.9) | – |
| MMSE (mean ± SD) | 29.2 (± 1.4) | 29.4 (± 0.7) |
| BDI-II (mean ± SD) | 13.7 (± 8.7) | 3.1 (± 2.6) |
| Duration of dopaminergic therapy (years ± SD) | 6.5 (± 4.1) | – |
| Duration of dopamine agonist (DA) treatment (years ± SD) | 4.3 (± 3.7) | – |
| Duration of levodopa (l-DOPA) treatment (years ± SD) | 5.5 (± 4.2) | – |
| Daily LEDl-DOPA (mg ± SD) | 739.0 (± 31.9) | – |
| Daily LEDDA (mg ± SD) | 173.8 (± 180.4) | – |
| Daily LEDTOTAL (mg ± SD) | 911.0 (± 721.6) | – |
| Lifetime LEDl-DOPA (g ± SD) | 991.5 (± 057.6) | – |
| Lifetime LEDDA (g ± SD) | 299.4 (± 391.4) | – |
| Lifetime LEDTOTAL (g ± SD) | 1291.0 (± 1250.8) | – |
Disease duration has been accounted from time of first appearance of PD symptoms; LEDl-DOPA = levodopa equivalent dose; LEDDA = dopamine agonists equivalent dose; H&Y = Hoehn and Yahr; MMSE = Mini Mental State Examination; BDI-II = Beck Depression inventory; HRSD = Hamilton Rating Scale for Depression. SD = Standard Deviation.
2.2. Clinical assessments
PD subjects were staged for disease severity with the Hoehn and Yahr (H&Y) scale and were clinically assessed with the Unified Parkinson's Disease Rating Scale (UPDRS), Abnormal Involuntary Movement Scale (AIMS), Mini-Mental State Examination (MMSE) and Beck Depression Inventory (BDI-II). Daily and lifetime dopamine agonist equivalent dose (LEDDA) and daily and lifetime levodopa equivalent dose (LEDl-DOPA) unit calculations were based on theoretical equivalence to levodopa as described previously (Politis et al., 2014, Politis et al., 2010).
2.3. Scanning procedures
All subjects had [11C]raclopride PET and 1.5-Tesla (T) volumetric MRI in an OFF medication condition after 18 h withdrawal of dopaminergic medications. Long acting dopaminergic medications were withdrawn for 72 h prior to the PET scan. PET imaging was performed at the Cyclotron Unit (Hammersmith Hospital) and [11C]raclopride radiotracer was supplied by Hammersmith Imanet plc, London, UK. All subjects were scanned using an ECAT HR+ (CTI/Siemens 962) 3D PET tomography scanner, with 15.5 cm total axial field of view (FOV). This camera has a mean image transaxial resolution (3D mode) over a 10 cm radius FOV of 6.0 + 0.5 mm and an axial resolution of 5.0 + 0.8 mm (Brix et al., 1997). All subjects were scanned following administration of [11C]raclopride as an intravenous bolus, mean dose 250 MBq; PET images were obtained as 20 time frames acquired over 60 min. Volumetric T1-weighted MRI scans were acquired using a 1.5 T MRI (Picker Eclipse, Picker International Inc., Highlands Heights, OH, USA). MRI was performed on the same day as the PET for co-registration of functional images.
The study received ethical approval from the Ethics Committee of Hammersmith, Queen Charlotte's and Chelsea and Acton Hospitals. All subjects gave informed written consent in accordance with the Declaration of Helsinki.
2.4. [11C]raclopride PET image analysis
2.4.1. Movement correction
Head movement correction was carried out for all PET images using frame-by-frame realignment (Montgomery et al., 2006) with in-house software (c-wave) implemented in Matlab 8.2 (The MathWorks Inc.). Non-attenuated corrected images were denoised using a level 2, order 64 Battle Lemarie wavelet filter (Turkheimer et al., 1999) followed by realignment using a mutual information algorithm (Studholme et al., 1997). The first three frames were excluded and frame 10 was chosen as the reference frame due to good signal-to-noise ratio. Frames 4–20 of the original time series were resliced and reassembled into a movement-corrected dynamic scan. Decay-corrected time-activity curves were derived and compared to those without movement correction. The amount and timing of any movements were assessed graphically and compared with intrascan notes. Motion correction was applied to all PET data, however since patients were scanned off medication, the effect of head movement has to be considered when interpreting the findings.
2.4.2. Parametric images
Parametric [11C]raclopride non-displaceable binding potential (BPND) images were generated from the dynamic [11C]raclopride scans using a basis function implementation of the simplified reference tissue model, with the cerebellum as the reference region for non-specific binding (Gunn et al., 1997). PET images were coregistered and resliced to corresponding volumetric T1-weighted MR images using the Mutual Information Registration algorithm in the Statistical Parametric Mapping (SPM, version 8) software package (Wellcome Department of Cognitive Neuroscience, Institute of Neurology, London, UK) implemented in Matlab 8.2.
2.4.3. Region of interest analysis
Regions-of-interests (ROIs) were manually delineated on individual co-registered volumetric T1-weigthed MRI images and sampled on parametric PET images, using ANALYZE medical imaging software (Mayo Foundation, Rochester, MN, USA, Version 10). A reliable, robust and repeatable technique was used for manual delineation of ROIs using the Talairach and Tournoux (Talairach and Tournous, 1988) stereotaxic atlas in combination with Duvernoy 3-dimensional sectional atlas (Duvernoy, 1999) on individual coregistered MRIs. The anterior border for the cerebellum was defined by the inferior semilunar lobule, the posterior border and the lateral border was defined by the transverse sinus and the medial border by the cerebellar falx. The anterior border for the caudate nucleus was defined by the lateral ventricle, the posterior border by the internal capsule, the medial border by the lateral ventricle and fornix, and the lateral border was defined by the external capsule. The anterior border for the putamen was defined by the anterior limb of the internal capsule, the posterior border by the posterior limb of the internal capsule, the lateral border by the external capsule/claustrum, and the medial border by the lamina medullaris lateralis. For each subject ROIs were manually delineated in both hemispheres. To minimize possible partial volume effects, anatomical data from high resolution MRI was used to manually delineate specific ROIs ensuring the volume for each ROI was standardized so there was no significant difference in ROI volume across groups. Therefore, reducing the effects of partial volume difference between groups which could account for changes in binding potential.
2.5. MRI volumetric analysis
FreeSurfer's image analysis suite (version 6) was used to process individual MRI scans to derive measures of subcortical volumes using automated procedures previous described (Fischl et al., 2002). Subcortical volumes were normalized for intracranial volume using validated methods within the FreeSufer toolkit (Buckner et al., 2004).
2.6. Statistics
Statistical analysis and graph illustrations were performed with SPSS (version 22) and GraphPad Prism (version 6.0) for MAC OS X, respectively. For all variables, variance homogeneity and Gaussianity were tested with Bartlett and Kolmogorov-Smirnov tests. Repeated-measures analysis of covariance was used to interrogate the association between medication condition and clinical characteristics with D2 binding where and [11C]raclopride BPND in ROIs was the repeated measure. If a significant interaction was found between a medication and clinical variable and the repeated-measure variable ROI, univariate tests were then carried out on the individual ROIs and resulting p values were corrected for multiple comparisons.
We compared [11C]raclopride BPND in the caudate and putamen between PD patients and healthy controls. We assessed the effect of medication on caudate and putamen [11C]raclopride BPND in PD patients receiving dopamine agonists (n = 46) compared to PD patients not receiving dopamine agonists (n = 22). To assess the effect of dyskinesias on the integrity of striatal D2Rs, PD subjects were categorized into two groups depending on having dyskinesias (n = 38) or not having dyskinesias (n = 30).
[11C]raclopride BPND in the caudate and putamen were checked for associations with (a) disease duration, (b) UPDRS Part-III motor scores in ON and OFF medication state, (c) AIMS scores (d) BDI-II scores, (e) dopamine agonist and levodopa treatment duration and (f) amount of daily and lifetime dopamine agonist and levodopa intake. Possible correlations between PET and clinical data were carried out using Pearson r and Spearman ρ depending on the distribution of each variable; the analyses were repeated including age as covariate. Hochberg-multiple comparison correction was applied for each set of correlations using PPLot (version 1.0) in Matlab (Turkheimer et al., 2001). All data are presented as mean ± SD and the level of α was set for all comparisons at p < 0.05, corrected.
3. Results
PD patients had a mean disease duration of 10.7 years and mean 6.5 years of dopaminergic drug treatment (Table 1). PD patients (n = 68) showed a mean 16.7% decrease in caudate [11C]raclopride BPND compared to healthy controls (p < 0.001). In the putamen PD patients showed a mean 3.5% increase in [11C]raclopride BPND compared to healthy controls (Table 2). Lower individual caudate [11C]raclopride BPND values correlated with longer disease duration (Pearson r = − 0.39, p = 0.0012; Fig. 1). No correlations were found between [11C]raclopride BPND and UPDRS or BDI-II scores.
Table 2.
Caudate and putamen [11C]raclopride binding potentials in healthy controls and Parkinson's disease patients.
| Caudate [11C]raclopride BPND | Putamen [11C]raclopride BPND | |
|---|---|---|
| Healthy controls (n = 12; mean ± SD) | 2.27 (± 0.2) | 2.58 (± 0.2) |
| All PD patients (n = 68; mean ± SD; Δ%) | 1.89 (± 0.3)⁎⁎⁎; − 16.7% | 2.67 (± 0.5); + 3.5% |
| Non-dyskinetic PD patients (n = 30; mean ± SD; Δ%) | 2.03 (± 0.3)⁎; − 10.6% | 2.76 (± 0.4); + 7.0% |
| Dyskinetic PD (n = 38; mean ± SD; Δ%) | 1.78 (± 0.4)⁎⁎⁎; − 21.6% | 2.59 (± 0.5); + 0.4% |
| PD patients receiving NO dopamine agonist treatment (n = 22; mean ± SD; Δ%) | 2.03 (± 0.3)⁎; − 10.6% | 2.89 (± 0.4)*; + 12.0% |
| PD patients receiving dopamine agonist treatment (n = 46; mean ± SD; Δ%) | 1.82 (± 0.3)⁎⁎⁎; − 19.8% | 2.56 (± 0.5); − 0.8% |
Δ% = percentage difference compared to healthy controls; BPND = non-displaceable binding potential; PD = Parkinson's disease.
p < 0.05.
p < 0.001.
Fig. 1.
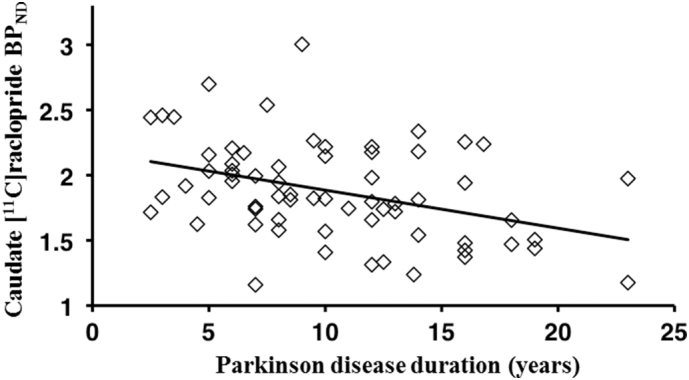
Correlation between Parkinson disease duration and caudate postsynaptic dopamine receptor type-2 availability. Inverse correlation between individual caudate [11C]raclopride BPND values and disease duration in Parkinson's disease patients (n = 68).
3.1. Volumetric analysis
FreeSurfer analysis revealed no volumetric difference in the left and right caudate and putamen between PD patients and healthy controls (p > 0.10).
3.2. Effect of medication on D2R availability
PD patients on dopamine agonist treatment had no differences in terms of age, cognitive status (MMSE), depression levels (BDI-II), disease stage and burden compared to PD patients who had not received treatment with dopamine agonists (p > 0.05). We found a significant interaction between dopamine agonist treatment and [11C]raclopride BPND in ROIs (p < 0.001).
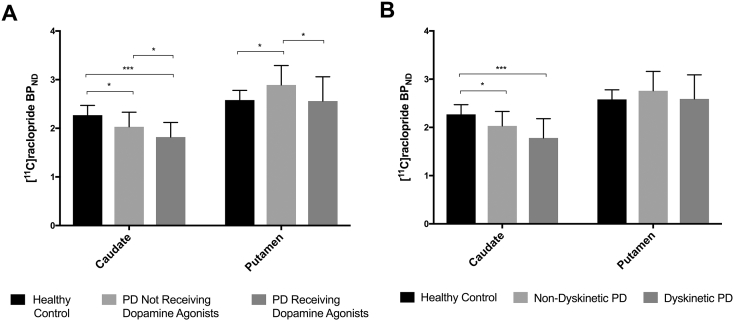
Compared to healthy controls, PD patients who had not received treatment with dopamine agonists (n = 22) showed a mean 10.6% decreased caudate [11C]raclopride BPND (p < 0.05) and a mean 12% increased putamen [11C]raclopride BPND (p < 0.05) (Table 2; Fig. 2A). PD patients on dopamine agonist treatment (n = 46) showed a mean 19.8% decrease in caudate [11C]raclopride BPND (p < 0.001) and a mean 0.8% decrease in putamen [11C]raclopride BPND compared to healthy controls (Table 2; Fig. 2A). In comparisons to PD patients who never received treatment with dopamine agonists, PD patients on dopamine agonist treatment had 9.2% reduced D2R availability in the caudate (p < 0.05) and 12.8% decreased in the putamen (p < 0.05) (Fig. 2A). There were no differences in the relation between BPND and specific dopamine agonists (ropinerole or pramiprexole).
Fig. 2.
Effect of dopamine agonist treatment (A) and presence of dyskinesia (B) on dopamine receptor type-2 receptor availability in the caudate and putamen of patients with Parkinson's disease. *p < 0.05; **p < 0.001.
Higher amounts of lifetime dopamine agonist therapy correlated with lower individual [11C]raclopride BPND values in the caudate (Spearman ρ = − 0.33; p < 0.05) and in the putamen (Spearman ρ = − 0.33; p < 0.05) of PD patients. These results remained significant after using age as a covariate (caudate ρ = − 0.32, p < 0.05; putamen ρ = 0.31, p < 0.05). Levodopa treatment duration, levodopa daily and lifetime amount were not associated with [11C]raclopride BPND values in the caudate and in the putamen.
3.3. Effect of dyskinesias on D2R availability
We found a significant interaction between the presence of dyskinesias and [11C]raclopride BPND in ROIs (p < 0.001). PD patients with dyskinesias (n = 38) showed a mean 21.6% decreased caudate [11C]raclopride BPND (p < 0.001) and a mean 0.4% increased [11C]raclopride BPND in the putamen compared to healthy controls (Fig. 2B). PD patients without dyskinesias (n = 30) showed a mean 10.6% decreased caudate [11C]raclopride BPND (p < 0.05) and a mean 7% increased [11C]raclopride BPND in the putamen compared to healthy controls (Table 2; Fig. 2B).
PD patients with dyskinesias had longer disease duration (p < 0.001), were at a more advanced disease stage (H&Y OFF: p < 0.001), and had received larger amounts of dopaminergic therapy (LEDtotal: p < 0.001) due to higher levodopa doses (Daily LEDl-DOPA: p < 0.001) compared to PD patients with no dyskinesias. Dopamine agonist intake was no different between PD patients with and without dyskinesias (LEDDA: p > 0.10). We found no correlations between individual caudate or putamen [11C]raclopride BPND values and AIMS scores. These results were confirmed after controlling for age.
4. Discussion
Our [11C]raclopride PET findings demonstrate that as PD advances striatal D2R availability is altered and is influenced by chronic exposure to dopamine agonist treatment. We found a mean 16.7% loss of D2R availability in the caudate and a mean 3.5% increase of D2R availability in the putamen in the collective cohort of PD patients studied. In PD patients who had never received treatment with dopamine agonists, D2R availability in the caudate was reduced by 10.8% and in the putamen was increased by 12%. In contrast, matched PD patients on dopamine agonist treatment had 9.2% reduced D2R availability in the caudate and 12.8% reduction in the putamen compared to PD patients who had not received treatment with dopamine agonists. Higher amounts of dopamine agonist therapy received over PD patient's lifetime correlated with reduced D2R availability in both caudate and putamen.
Post mortem studies have reported normal (Pierot et al., 1988), reduced (Rinne et al., 1991) or increased (Bokobza et al., 1984) D2R binding in the striatum of PD patients treated with dopaminergic drugs. Previous [11C]raclopride PET studies have suggested that reductions in caudate D2R availability in advanced PD stages may reflect either disease progression or an effect of chronic exposure to dopaminergic therapy (Antonini et al., 1994, Turjanski et al., 1997, Brooks et al., 1992, Dentresangle et al., 1999). Our [11C]raclopride PET findings suggest that with longer disease duration, D2R availability is reduced in the caudate, but not in the putamen. Chronic exposure to dopamine agonist treatment suppresses D2R availability in both the caudate and putamen. Dopamine agonists directly target D2Rs mimicking the effects of endogenous dopamine. Therefore, down-regulation could be the result of direct competition between [11C]raclopride and exogenous sources of dopamine, or due to internalization of D2R upon dopamine binding leading to reduced availability of [11C]raclopride binding sites. According to our findings chronic treatment with levodopa does not influence striatal D2R availability in PD. It has been shown that chronic exposure to levodopa mainly affects striatal D1R availability (Turjanski et al., 1997).
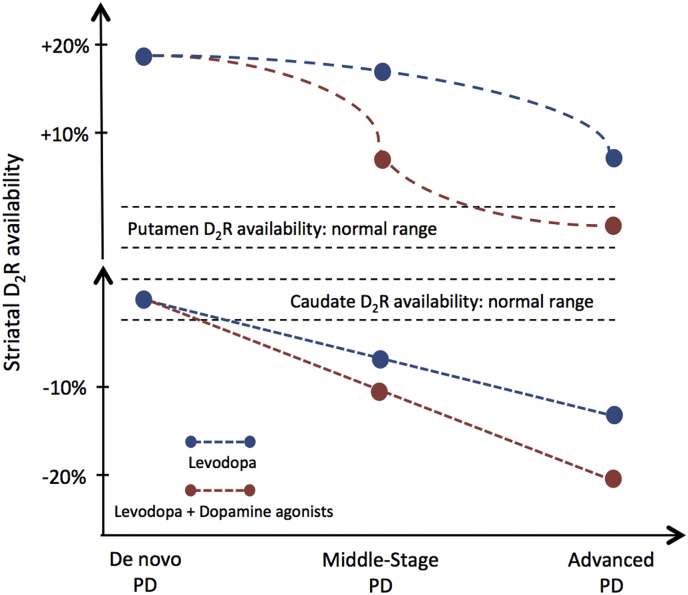
Previous [11C]raclopride PET studies in de novo PD patients have shown up to 20% increases in D2R availability in the putamen while the D2R availability in the caudate was normal (Turjanski et al., 1997, Leenders and Antonini, 1992, Antonini et al., 1994). Further [11C]raclopride PET studies have shown that as PD progresses, D2R availability normalizes in the putamen, but D2Rs in the caudate are reduced by around 20% (Turjanski et al., 1997, Brooks et al., 1990, Brooks et al., 1992, Dentresangle et al., 1999, Antonini et al., 1994). Our [11C]raclopride PET findings are compatible with the previous [11C]raclopride PET studies, showing that D2R availability in the striatum is affected by advancing disease stage and suppressed by chronic exposure to dopamine agonist treatment. Dopamine agonist intake may also explain the normalization of putaminal [11C]raclopride availability and further reductions of caudate [11C]raclopride availability in advanced PD that has been reported in the previous studies (Turjanski et al., 1997, Brooks et al., 1990, Brooks et al., 1992, Dentresangle et al., 1999, Antonini et al., 1994). Dopamine agonists exert their pharmacological effect by directly activating post-synaptic D2Rs. Putaminal D2R availability returns to within normal levels in PD patients receiving dopamine agonist treatment, counteracting compensatory D2R up-regulation observed in the putamen in the absence of dopaminergic treatment. Therefore, early treatment with dopamine agonists could have beneficial effects to slow loss of dopamine terminal function in the putamen. Moreover, another [11C]raclopride PET study has shown that withdrawal of dopaminergic drugs in previously treated advanced PD patients, undergoing deep brain stimulation of the subthalamic nucleus, resulted in up-regulation of striatal D2Rs (Thobois et al., 2004). The authors of this study did not investigate the effect of dopaminergic treatment but our findings suggest this would have been due to withdrawal of dopamine agonist treatment. Fig. 3 illustrates the summary of our findings taken together with the findings from the previous [11C]raclopride PET studies regarding striatal D2R availability in advanced PD patients treated with and without dopamine agonists.
Fig. 3.
Graphical representation of caudate and putamen dopamine receptors type-2 (D2R) availability in patients with Parkinson's disease. The illustration summarizes our findings together with the findings from the previous [11C]raclopride PET studies (Brooks et al., 1990, Brooks et al., 1992, Leenders and Antonini, 1992, Rinne et al., 1993, Antonini et al., 1994, Antonini et al., 1997, Turjanski et al., 1997, Dentresangle et al., 1999) and shows a linear caudate reduction and a non-linear reduction of D2R availability in the putamen as PD progresses. Also, the suppressing effect of chronic dopamine agonist treatment in striatal D2R availability in patients with PD. PD = Parkinson's disease; D2R = dopamine receptor type-2.
Our findings show that longer PD duration correlated with decreased D2R availability in the caudate but not in the putamen. These findings suggest that although caudate and putamen D2R availability is affected with advancing disease, the loss of D2R availability is linear in the caudate and non-linear or linear with a wide variance in the putamen (Fig. 3). A future longitudinal study, with stratified treatment arms, is required to confirm the true kinetics of putamen D2R reductions. The gradual loss of D2R availability in the caudate could impact the output signaling pathways from the caudate to the frontal cortex, which are critical for executive functions. A previous [11C]raclopride PET study has shown that significant attenuation of dopamine release in the caudate was associated with executive deficits in patients with PD (Sawamoto et al., 2008a, Sawamoto et al., 2008b). Similar associations between loss of caudate D2R availability and cognitive dysfunction have also been reported in Huntington's disease (Pagano et al., 2016). Therefore, reduced D2R availability in the caudate, likely worsened with dopamine agonist treatment, could have implications for cognitive impairments and executive function deficits in PD patients. Further longitudinal studies, in larger cohorts of PD patients, are required to fully understand the impact of dopamine agonist treatment, and altered D2R availability, on cognitive symptomatology in advancing PD.
Our [11C]raclopride findings also show that PD patients with dyskinesias had greater striatal reductions (11% in the caudate and 6.6% in the putamen) in D2R availability compared to PD patients without dyskinesias. PD patients with dyskinesias, however, were more disabled and had more advanced disease. Also, we found no correlations between striatal D2R availability and dyskinesias scores and therefore, our findings suggest that the difference in striatal D2R availability between dyskinetic and non-dyskinetic PD reflects the more advanced disease in the dyskinetic PD group rather than a direct relationship between the development of dyskinesias and D2R availability. These findings are compatible with a previous [11C]raclopride PET study which demonstrated no association between striatal D2R availability and dyskinesias in PD (Turjanski et al., 1997). Although it has been postulated that alterations in striatal D2Rs may be involved in the development of dyskinesias in PD (Boyce et al., 1990, Graham et al., 1993), it is more likely that loss of dopamine terminals and priming with levodopa play a prominent role. Other non-dopaminergic systems such as the glutamatergic and the serotonergic may play a prominent role in the development of dyskinesias in PD (Niccolini et al., 2015, Politis et al., 2014). Lines of evidence suggest that relatively preserved presynaptic serotonergic terminals in the basal ganglia mishandle synaptic levels of dopamine and contribute in the occurrence of dyskinesias in PD (Politis et al., 2014, Smith et al., 2015).
In conclusion, our findings indicate that D2R availability in the caudate and in the putamen are differentially affected with advancing PD. Chronic exposure to treatment with dopamine agonists, but not levodopa, suppresses striatal D2R availability, which may have relevance to caudate output signaling to frontal lobes and the occurrence of executive deficits, but not dyskinesias. Availability of D2Rs is not confined only to the striatum and previous PET studies have shown alterations in extra-striatal regions that may be implicated in the development of non-motor symptoms in PD (Kaasinen et al., 2000, Kaasinen et al., 2003, Politis et al., 2008). Hopefully, future studies will help understand the relevance of extra-striatal D2R system in the development of non-motor symptoms and the associations with exposure to dopaminergic treatment in patients with PD.
Acknowledgments
Acknowledgment
We thank all the participants who took part in this study.
Study funding
This work was supported by: (a) Neurodegeneration Imaging Group Research Fund, King's College London; (b) Medical Research Council, United Kingdom (Clinical Sciences Center, Neurology group core grant, 2008–2014); (c) Michael J Fox Foundation; (d) Parkinson's UK.
Author disclosures
M. Politis: received research support from Parkinson's UK, Lilly and Edmond J. Safra Foundation, Michael J Fox Foundation (MJFF) for Parkinson's disease, and NIHR BRC. H. Wilson: reports no disclosures. K. Wu: reports no disclosures. D.J. Brooks: Consultant to GE Healthcare, Plexxikon, and GenePod. P. Piccini: reports no disclosures.
References
- Antonini A., Schwarz J., Oertel W.H., Beer H.F., Madeja U.D., Leenders K.L. [11C]raclopride and positron emission tomography in previously untreated patients with Parkinson's disease: influence of l-dopa and lisuride therapy on striatal dopamine D2-receptors. Neurology. 1994;44:1325–1329. doi: 10.1212/wnl.44.7.1325. [DOI] [PubMed] [Google Scholar]
- Antonini A., Schwarz J., Oertel W.H., Pogarell O., Leenders K.L. Long-term changes of striatal dopamine D2 receptors in patients with Parkinson's disease: a study with positron emission tomography and [11C]raclopride. Mov. Disord. 1997;12:33–38. doi: 10.1002/mds.870120107. [DOI] [PubMed] [Google Scholar]
- Bokobza B., Ruberg M., Scatton B., Javoy-Agid F., Agid Y. [3H]spiperone binding, dopamine and HVA concentrations in Parkinson's disease and supranuclear palsy. Eur. J. Pharmacol. 1984;99:167–175. doi: 10.1016/0014-2999(84)90238-3. [DOI] [PubMed] [Google Scholar]
- Boyce S., Rupniak N.M., Steventon M.J., Iversen S.D. Nigrostriatal damage is required for induction of dyskinesias by l-DOPA in squirrel monkeys. Clin. Neuropharmacol. 1990;13:448–458. doi: 10.1097/00002826-199010000-00006. [DOI] [PubMed] [Google Scholar]
- Brix G., Zaers J., Adam L.E. Performance evaluation of a whole-body PET scanner using the NEMA protocol. National Electrical Manufacturers Association. J. Nucl. Med. 1997;38:1614–1623. [PubMed] [Google Scholar]
- Brooks D.J., Salmon E.P., Mathias C.J. The relationship between locomotor disability, autonomic dysfunction, and the integrity of the striatal dopaminergic system in patients with multiple system atrophy, pure autonomic failure, and Parkinson's disease, studied with PET. Brain. 1990;113:1539–1552. doi: 10.1093/brain/113.5.1539. [DOI] [PubMed] [Google Scholar]
- Brooks D.J., Ibanez V., Sawle G.V. Striatal D2 receptor status in patients with Parkinson's disease, striatonigral degeneration, and progressive supranuclear palsy, measured with 11C-raclopride and positron emission tomography. Ann. Neurol. 1992;31:184–192. doi: 10.1002/ana.410310209. [DOI] [PubMed] [Google Scholar]
- Buckner R.L., Head D., Parker J., Fotenos A.F., Marcus D., Morris J.C. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. NeuroImage. 2004;23:724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Dentresangle C., Veyre L., Le Bars D. Striatal D2 dopamine receptor status in Parkinson's disease: an [18F]dopa and [11C]raclopride PET study. Mov. Disord. 1999;14:1025–1030. doi: 10.1002/1531-8257(199911)14:6<1025::aid-mds1020>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Duvernoy H.M. Springer-Verlag Wien; New York: 1999. The Human Brain: Surface, Blood Supply, and Three-dimensional Sectional Anatomy. [Google Scholar]
- Fischl B., Salat D.H., Busa E., Albert M., Dieterich M., Haselgrove C. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Graham W.C., Sambrook M.A., Crossman A.R. Differential effect of chronic dopaminergic treatment on dopamine D1 and D2 receptors in the monkey brain in MPTP-induced parkinsonism. Brain Res. 1993;602:290–303. doi: 10.1016/0006-8993(93)90694-i. [DOI] [PubMed] [Google Scholar]
- Gunn R.N., Lammertsma A.A., Hume S.P., Cunningham V.J. Parametric imaging of ligand-receptor binding in PET using a simplified reference region model. NeuroImage. 1997;6:279–287. doi: 10.1006/nimg.1997.0303. [DOI] [PubMed] [Google Scholar]
- Herz D.M., Haagensen B.N., Christensen M.S. Abnormal dopaminergic modulation of striato-cortical networks underlies levodopa-induced dyskinesias in humans. Brain. 2015;138:1658–1666. doi: 10.1093/brain/awv096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaasinen V., Nagren K., Hietala J. Extrastriatal dopamine D2 and D3 receptors in early and advanced Parkinson's disease. Neurology. 2000;54:1482–1487. doi: 10.1212/wnl.54.7.1482. [DOI] [PubMed] [Google Scholar]
- Kaasinen V., Aalto S., K NA, Hietala J, Sonninen P, Rinne JO. Extrastriatal dopamine D(2) receptors in Parkinson's disease: a longitudinal study. J. Neural Transm. 2003;110:591–601. doi: 10.1007/s00702-003-0816-x. [DOI] [PubMed] [Google Scholar]
- Laruelle M. Imaging synaptic neurotransmission with in vivo binding competition techniques: a critical review. J. Cereb. Blood Flow Metab. 2000;20:423–451. doi: 10.1097/00004647-200003000-00001. [DOI] [PubMed] [Google Scholar]
- Leenders K.L., Antonini A. Striatal dopamine D2 receptors in “de novo” parkinsonian patients measured using PET and [11C]raclopride. Neurology. 1992;42:295–296. doi: 10.1212/wnl.44.11.2101. (Abstract) [DOI] [PubMed] [Google Scholar]
- Montgomery A.J., Thielemans K., Mehta M.A., Turkheimer F., Mustafovic S., Grasby P.M. Correction of head movement on PET studies: comparison of methods. J. Nucl. Med. 2006;47:1936–1944. [PubMed] [Google Scholar]
- Niccolini F., Rocchi L., Politis M. Molecular imaging of levodopa-induced dyskinesias. Cell. Mol. Life Sci. 2015;72:2107–2117. doi: 10.1007/s00018-015-1854-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano G., Niccolini F., Politis M. Current status of PET imaging in Huntington's disease. Eur. J. Nucl. Med. Mol. Imaging. 2016;22 doi: 10.1007/s00259-016-3324-6. (Epub) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavese N., Evans A.H., Tai Y.F. Clinical correlates of levodopa-induced dopamine release in Parkinson disease: a PET study. Neurology. 2006;67:1612–1617. doi: 10.1212/01.wnl.0000242888.30755.5d. [DOI] [PubMed] [Google Scholar]
- Pierot L., Desnos C., Blin J. D1 and D2-type dopamine receptors in patients with Parkinson's disease and progressive supranuclear palsy. J. Neurol. Sci. 1988;86:291–306. doi: 10.1016/0022-510x(88)90106-2. [DOI] [PubMed] [Google Scholar]
- Politis M., Piccini P., Pavese N., Koh S.B., Brooks D.J. Evidence of dopamine dysfunction in the hypothalamus of patients with Parkinson's disease: an in vivo 11C-raclopride PET study. Exp. Neurol. 2008;214:112–116. doi: 10.1016/j.expneurol.2008.07.021. [DOI] [PubMed] [Google Scholar]
- Politis M., Wu K., Loane C., Kiferle L., Molloy S., Brooks D.J., Piccini P. Staging of serotonergic dysfunction in Parkinson's disease: an in vivo 11C-DASB PET study. Neurobiol. Dis. 2010;40:216–221. doi: 10.1016/j.nbd.2010.05.028. [DOI] [PubMed] [Google Scholar]
- Politis M., Wu K., Loane C. Serotonergic mechanisms responsible for levodopa-induced dyskinesias in Parkinson's disease patients. J. Clin. Invest. 2014;124:1340–1349. doi: 10.1172/JCI71640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinne J.O., Laihinen A., Lonnberg P., Marjamaki P., Rinne U.K. A post-mortem study on striatal dopamine receptors in Parkinson's disease. Brain Res. 1991;556:117–122. doi: 10.1016/0006-8993(91)90554-9. [DOI] [PubMed] [Google Scholar]
- Rinne J.O., Laihinen A., Rinne U.K., Nagren K., Bergman J., Ruotsalainen U. PET study on striatal dopamine D2 receptor changes during the progression of early Parkinson's disease. Mov. Disord. 1993;8:134–138. doi: 10.1002/mds.870080203. [DOI] [PubMed] [Google Scholar]
- Samii A., Nutt J.G., Ransom B.R. Parkinson's disease. Lancet. 2004;363:1783–1793. doi: 10.1016/S0140-6736(04)16305-8. [DOI] [PubMed] [Google Scholar]
- Sawamoto N., Piccini P., Hotton G., Pavese N., Thielemans K., Brooks D.J. Cognitive deficits and striato-frontal dopamine release in Parkinson's disease. Brain. 2008;131:1294–1302. doi: 10.1093/brain/awn054. [DOI] [PubMed] [Google Scholar]
- Sawamoto N., Piccini P., Hotton G., Pavese N., Thielemans K., Brooks D.J. Cognitive deficits and striato-frontal dopamine release in Parkinson's disease. Brain. 2008;13:1294–1302. doi: 10.1093/brain/awn054. [DOI] [PubMed] [Google Scholar]
- Sawle G.V., Playford E.D., Brooks D.J., Quinn N., Frackowiak R.S. Asymmetrical presynaptic and postsynaptic changes in the striatal dopamine projection in Dopa naïve parkinsonism. Diagnostic implications of the D2 receptor status. Brain. 1993;116:853–867. doi: 10.1093/brain/116.4.853. [DOI] [PubMed] [Google Scholar]
- Smith R., Wu K., Hart T. The role of pallidal serotonergic function in Parkinson's disease dyskinesias: a positron emission tomography study. Neurobiol. Aging. 2015;36:1736–1742. doi: 10.1016/j.neurobiolaging.2014.12.037. [DOI] [PubMed] [Google Scholar]
- Studholme C., Hill D.L., Hawkes D.J. Automated three-dimensional registration of magnetic resonance and positron emission tomography brain images by multiresolution optimization of voxel similarity measures. Med. Phys. 1997;24:25–35. doi: 10.1118/1.598130. [DOI] [PubMed] [Google Scholar]
- Talairach J., Tournous P. Thieme; New York: 1988. Co-planar Sterotaxic Atlas of the Human Brain. [Google Scholar]
- Thobois S., Vingerhoets F., Fraix V. Role of dopaminergic treatment in dopamine receptor down-regulation in advanced Parkinson disease: a positron emission tomographic study. Arch. Neurol. 2004;61:1705–1709. doi: 10.1001/archneur.61.11.1705. [DOI] [PubMed] [Google Scholar]
- Turjanski N., Lees A.J., Brooks D.J. In vivo studies on striatal dopamine D1 and D2 site binding in l-dopa-treated Parkinson's disease patients with and without dyskinesias. Neurology. 1997;49:717–723. doi: 10.1212/wnl.49.3.717. [DOI] [PubMed] [Google Scholar]
- Turkheimer F.E., Brett M., Visvikis D., Cunningham V.J. Multiresolution analysis of emission tomography images in the wavelet domain. J. Cereb. Blood Flow Metab. 1999;19:1189–1208. doi: 10.1097/00004647-199911000-00003. [DOI] [PubMed] [Google Scholar]
- Turkheimer F.E., Smith C.B., Schmidt K. Estimation of the number of “true” null hypotheses in multivariate analysis of neuroimaging data. NeuroImage. 2001;13:920–930. doi: 10.1006/nimg.2001.0764. [DOI] [PubMed] [Google Scholar]