Abstract
Periodontitis is a chronic inflammatory disease associated with overactivation of the complement system. Recent preclinical studies suggest that host-modulation therapies may contribute to effective treatment of human periodontitis, which may lead to loss of teeth and function if untreated. We previously showed that locally administered AMY-101 (Cp40), a peptidic inhibitor of the central complement component C3, can inhibit naturally occurring periodontitis in non-human primates (NHPs) when given once a week. This study was undertaken to determine the local safety of increasing doses of the drug as well as its efficacy when given at a reduced frequency or after systemic administration. Our findings have determined a local dose of AMY-101 (0.1 mg/site) that is free of local irritation and effective when given once every 3 weeks. Moreover, a daily subcutaneous dose of AMY-101 (4 mg/kg bodyweight) was protective against NHP periodontitis, suggesting that patients treated for systemic disorders (e.g., paroxysmal nocturnal hemoglobinuria) can additionally benefit in terms of improved periodontal condition. In summary, AMY-101 appears to be a promising candidate drug for the adjunctive treatment of human periodontitis, a notion that merits investigation in human clinical trials.
Keywords: complement, compstatin, periodontitis, inflammation, AMY-101, non-human primates
Introduction
Periodontitis is a chronic inflammatory disease that affects the integrity of the periodontium (i.e., the tooth-supporting tissues including the gingiva, periodontal ligament, and alveolar bone).1 The destructive inflammation driving the disease is induced by dysbiotic microbial communities that colonize subgingival tooth sites within the so-called periodontal pockets.2 If untreated, periodontitis can lead to tooth loss and impaired mastication and esthetics3 and may affect the quality of life.4 Almost half of adults are affected by some form of periodontal disease (ranging from mild to severe), whereas approximately 10% of the global adult population is afflicted by severe periodontitis.5, 6, 7 Current standard-of-care periodontal therapy aims to control the pathogenic microbial biofilm through subgingival mechanical debridement (scaling and root planing [SRP]). However, SRP is only partially effective for the majority of patients and a significant minority of patients do not respond favorably to SRP (“refractory periodontitis patients”).8 Therefore, periodontitis continues to be a significant health and economic burden.3, 9, 10
The periodontitis-associated microbial communities not only induce but also exploit inflammation as a means to obtain nutrients for growth and persistence.11, 12 Nutrients derived from inflammatory tissue breakdown and bleeding include degraded collagen peptides and heme-containing compounds and favor the selective expansion of proteinase-rich species with iron acquisition capacity. The resulting feed-forward loop between dysbiosis and inflammation suggests that the control of inflammation could both ameliorate inflammatory tissue destruction and inhibit the outgrowth of the dysbiotic microbiota in periodontitis. This notion has been confirmed experimentally in animal models of the disease13, 14, 15, 16 and provides a strong rationale for adjunctive host-modulation therapies in the treatment of periodontitis.
The complement system is a sophisticated network of interacting fluid-phase and cell surface-associated molecules that trigger and regulate signaling pathways involved in immune surveillance and homeostasis.17 However, complement dysregulation or overactivation drives a number of inflammatory disorders.18 Clinical studies have associated periodontitis with an increased presence of complement activation products in the gingival tissue and the gingival crevicular fluid (GCF), an inflammatory serum exudate that bathes the periodontal pockets.19, 20, 21, 22, 23, 24, 25 Induction of experimental gingival inflammation in human volunteers (through abstinence from oral hygiene) causes progressive complement activation, as determined by conversion of the complement component C3.26 C3 constitutes the central point in the complement cascade where all triggering mechanisms converge.17 Consistent with the aforementioned experimental gingivitis study,26 complement activation in the GCF of periodontitis patients decreases after successful periodontal treatment (i.e., that resolved clinical inflammation).27 C3 was shown to be among the most promising candidate genes involved in periodontitis, according to a study that used an integrative gene prioritization method and databases from genome-wide association studies and microarray experiments.28 Together, these important clinical studies have shown that there is a correlative, yet not necessarily cause-and-effect, relationship between complement and periodontitis. We recently provided direct evidence for a causative role of complement in periodontal disease pathogenesis. Specifically, we showed that a locally administered inhibitor of C3 could prevent periodontal inflammation and bone loss in a model of ligature-induced periodontitis in young non-human primates (NHPs).29 In a follow-up study, we additionally showed that local C3 inhibition is also effective in a therapeutic setting. Specifically, local C3 inhibition blocked pre-existing, naturally occurring chronic periodontal inflammation in aged NHPs, in the absence of additional treatments, such as SRP.30 These NHP studies have thus identified a promising anti-inflammatory therapy that merits investigation for the treatment of human periodontitis.
The inhibitor we used in the aforementioned NHP studies29, 30 is Cp40, an improved analog of compstatin that is also known as AMY-101 (Amyndas Pharmaceuticals). Compstatin and new-generation analogs are small peptidic compounds that block C3 activation exclusively in humans and NHPs.31, 32, 33 Mechanistically, these C3 inhibitors bind C3 and block its binding to and cleavage by the C3 convertase, thereby inhibiting the generation of downstream effector molecules regardless of the initiation mechanism of complement activation.31, 32
In our study involving pre-existing natural periodontitis,30 AMY-101 was injected locally into the gingiva (0.1 mg/site; 50 μL of 2 mg/mL solution). The drug was administered either three times per week or once a week for 6 weeks followed by a 6-week follow-up period without treatment. Here we report additional NHP periodontitis studies, which were undertaken to answer questions relevant to the development of AMY-101-based treatment for human periodontitis: (1) Is the established therapeutic dose (0.1 mg/site) free of local irritation in the gingiva? (2) What is the maximum dose of AMY-101 that would still be safe in terms of local irritation? (3) Is the protective effect of AMY-101 maintained when the drug is administered less frequently than once a week? (4) Can AMY-101 protect against NHP periodontitis when administered systemically? Our data reported here indicate that a therapeutic dose of locally administered AMY-10130 is free of local irritation and has long-lasting protective effects even when given as infrequently as once per 3 weeks. Moreover, our data suggest that systemic AMY-101 is likely to benefit the periodontal condition of patients treated for systemic disorders associated with complement activation. The findings reported here, therefore, pave the way for an AMY-101-based adjunctive treatment of human periodontitis.
Results
Locally Administered AMY-101 (0.1 mg/Site) Does Not Cause Irritation in Healthy Gingiva
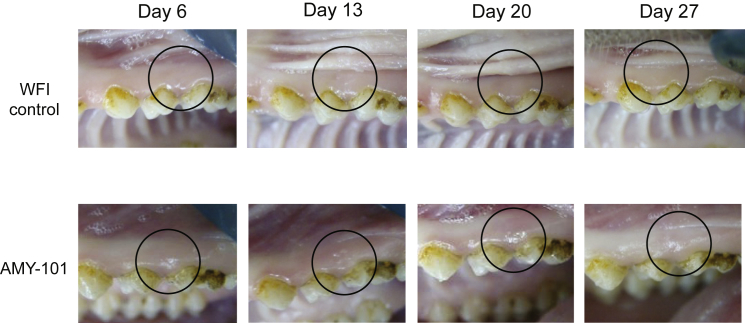
To determine possible local gingival irritation after administration of the peptidic C3 inhibitor AMY-101 in NHPs, a therapeutic dose of AMY-101 (50 μL of 2 mg/mL solution corresponding to 0.1 mg/site)30 was injected in healthy gingiva of posterior teeth in five animals. Each animal received a total of four injections, one per quadrant; two injections were with AMY-101 and the other two injections involved water for injection (WFI) containing 5% dextrose (control). In each animal, AMY-101 was administered on both maxillary and mandibular quadrants (2 sites total), whereas the control solution was injected on the two contralateral sites. AMY-101 and control solution were injected a total of three times, at days 0, 7, and 14, followed by a 2-week observation period without further injections. Intraoral photographs were taken at baseline (day 0) and every 2–3 days to document the gingival condition around injection sites. Careful daily clinical examination revealed no signs of irritation after injection of AMY-101 or control solution throughout the observation period (Figure 1). Blood samples were collected at day −1 and day 15 and were processed for hematology and biochemistry analysis. All measurements were within the normal range for all animals (not shown).
Figure 1.
AMY-101, at a Therapeutic Dose, Causes No Signs of Irritation after Injection
AMY-101 (2 mg/mL; 0.1 mg/site) or WFI containing 5% dextrose (control) was administered locally into interdental papillae at days 0, 7, and 14. Intraoral photos were taken to examine periodontal conditions. Shown are representative pictures of injection sites at days 6, 13, 20, and 27. A black circle demarcates the point where AMY-101 or control reagent was injected.
Dose-Escalation Study for Local Injection of AMY-101
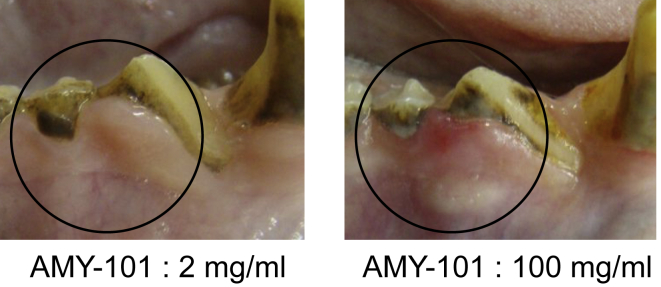
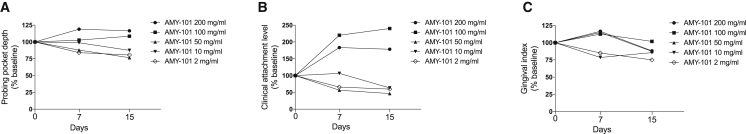
This study was designed to determine possible local gingival irritation after local injection of increasing concentrations of AMY-101 in NHPs with naturally occurring periodontitis. Escalating doses of AMY-101 tested were 2, 10, 50, 100, and 200 mg/mL in a total volume of 50 μL, thus corresponding to 0.1, 0.5, 2.5, 5, and 10 mg/site, respectively. The injected sites involved posterior teeth on both sides of the maxilla (palatal interdental papillae) and mandible (buccal interdental papillae). Five animals were used and all injections were given in a single session followed by a 2-week observation period. The animals were examined daily for the possible presence of local gingival irritation in response to AMY-101 injections. Doses equal to or higher than 10 mg/mL caused mild to moderate inflammation, which was observed more often with the highest doses (100 and 200 mg/mL) (Figure 2). No irritation was observed with the 2-mg/mL dose at any treated site, consistent with the data discussed above. Clinical examinations to determine periodontal disease activity and intraoral photography were performed at baseline and after 1 and 2 weeks. In terms of efficacy, doses up to 50 mg/mL caused a reduction in periodontal clinical parameters (probing pocket depth [PPD], clinical attachment level [CAL], and gingival index [GI]) (Figure 3). In contrast, the highest doses (100 and 200 mg/mL) caused deterioration in the same clinical parameters (Figure 3). Therefore, among the different AMY-101 concentrations tested, the 2-mg/mL dose appears to be an optimal dose fulfilling both safety and protection requirements.
Figure 2.
High Concentration of AMY-101 Causes Gingival Inflammation
AMY-101 was injected locally at 2 mg/mL (0.1 mg/site) or 100 mg/mL (5 mg/site) into interdental papillae. Shown are intraoral photos taken after 9 days. A black circle demarcates the point where AMY-101 or control reagent was injected.
Figure 3.
High Concentration of AMY-101 Induces Deterioration of Periodontal Condition
(A–C) Escalating doses of AMY-101 (2, 10, 50, 100, and 200 mg/mL) were administered locally into interdental papillae once at day 0. Clinical examinations for (A) PPD, (B) CAL, and (C) GI were performed at baseline (day 0), day 7, and day 15.
AMY-101 Confers Protection, Even when Administered Once Every 3 Weeks
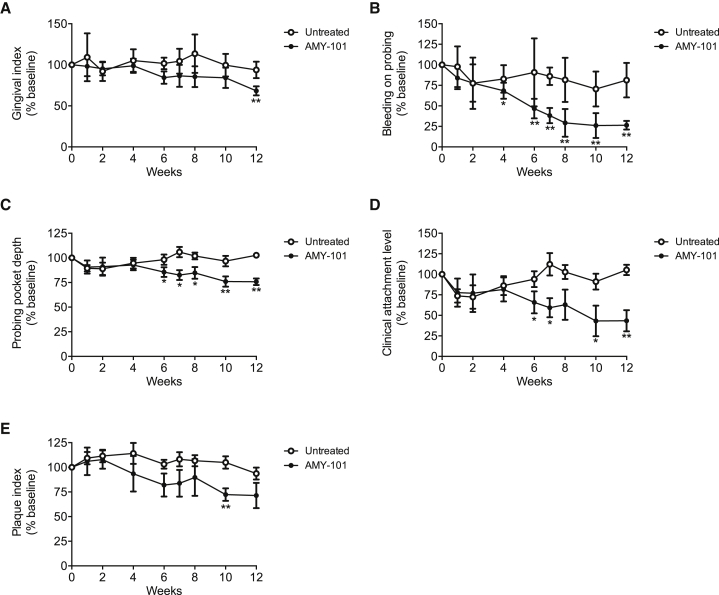
We have previously shown that weekly intragingival injections of AMY-101 can improve the periodontal condition of NHPs with natural chronic periodontitis.30 A less frequent but nevertheless successful administration would facilitate the application of AMY-101 for human use. To explore this possibility, we tested whether AMY-101 can be efficacious also when administered less frequently. To this end, a 2-mg/mL solution of AMY-101 was administered once every 2 weeks in 5 animals or once every 3 weeks in another 5 animals. Specifically, AMY-101 was injected locally into the gingiva of anterior and posterior teeth on both sides of the maxilla (17 sites total; palatal papilla between the teeth [15 sites], and distal gingiva of third molars [2 sites]). Clinical examinations were performed at baseline and 1, 2, 4, 6, 7, 8, 10, and 12 weeks throughout the study to determine the progression of the disease and the potential beneficial effects of AMY-101. Clinical readings made before AMY-101 injection served as baseline controls. The mandible was not treated but was monitored by clinical periodontal examination throughout the study for comparative purposes. The study consisted of 6 weeks of AMY-101 treatments (treatment period), followed by 6 weeks without AMY-101 treatment (follow-up period).
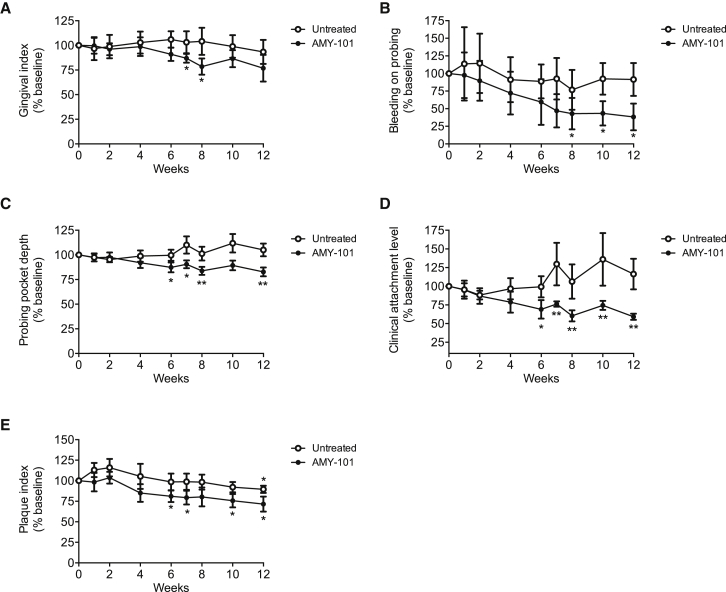
Regardless of the frequency of administration, AMY-101 caused a significant reduction in clinical indices that measure periodontal inflammation (GI and bleeding on probing [BOP]) or tissue destruction (PPD and CAL) (Figures 4 and 5). Interestingly, differences between baseline and subsequent readings reached statistical significance at or after 6 weeks (i.e., at the time point when the treatments with AMY-101 were discontinued). Many of the differences observed at 6 weeks remained statistically significant even at 12 weeks (BOP, PPD, and CAL) (Figures 4B–4D and 5B–5D). The aforementioned clinical indices were also monitored in the untreated jaw (mandible) during the same 12-week interval. In contrast to the improved clinical condition in the AMY-101-treated maxillae, the clinical indices in the mandibles did not show significant differences in the course of the study as compared to their baseline values (Figures 4 and 5). In conclusion, AMY-101 can induce a long-lasting clinical anti-inflammatory effect.
Figure 4.
AMY-101 Decreases Inflammatory Clinical Parameters of Naturally Occurring Chronic Periodontitis in NHPs after Local Administration Once per Every 2 Weeks
(A–E) AMY-101 was injected (once per every 2 weeks for 6 weeks) into the interdental papillae and the distal gingiva of the third molars of the maxilla (“AMY-101”), whereas the mandible was not treated (“Untreated”). Each animal was clinically examined at the indicated time points and the following clinical parameters were recorded: (A) gingival index, (B) bleeding on probing, (C) probing pocket depth, (D) clinical attachment level, and (E) plaque index. The data are expressed relative to the baseline values (at week 0), set as 100. Results are means ± SD (n = 5 animals). *p < 0.05 and **p < 0.01 compared to baseline (one-way repeated-measures ANOVA and Bonferroni’s multiple-comparisons test).
Figure 5.
AMY-101 Decreases Inflammatory Clinical Parameters of Naturally Occurring Chronic Periodontitis in NHPs after Local Administration Once per Every 3 Weeks
(A–E) AMY-101 was injected (once per every 3 weeks for 6 weeks) into the interdental papillae and the distal gingiva of the third molars of the maxilla (“AMY-101”), whereas the mandible was not treated (“Untreated”). Each animal was clinically examined at the indicated time points and the following clinical parameters were recorded: (A) gingival index, (B) bleeding on probing, (C) probing pocket depth, (D) clinical attachment level, and (E) plaque index. The data are expressed relative to the baseline values (at week 0), set as 100. Results are means ± SD (n = 5 animals). *p < 0.05 and **p < 0.01 compared to baseline (one-way repeated-measures ANOVA and Bonferroni’s multiple-comparisons test).
Systemic Administration of AMY-101 Can Improve the Periodontal Condition of NHPs
Given that AMY-101 is also being considered for systemic disorders and periodontitis is a highly prevalent disease,34 we tested whether AMY-101 can be effective when administered systemically. AMY-101 was administered in 10 animals via subcutaneous injection at a concentration of 4 mg/kg bodyweight, once per 24 hr for a total of 28 days. To determine the progression of the disease and the potential beneficial effects of AMY-101, clinical examinations were performed at baseline (week 0) and throughout the study (at the 1-, 2-, 3-, 4-, and 11-week time points). Additionally, biopsies were taken from the gingiva and bone at baseline, 4 weeks, and 11 weeks.
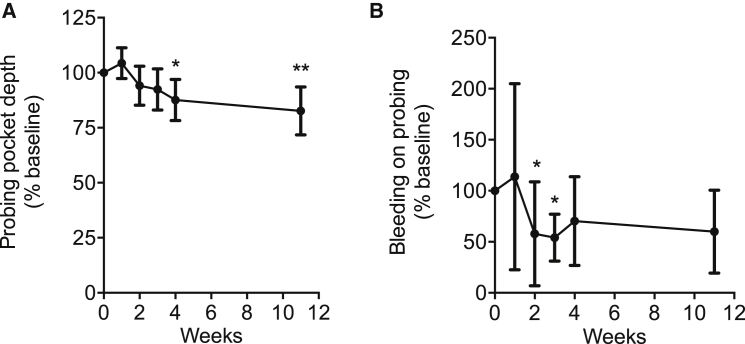
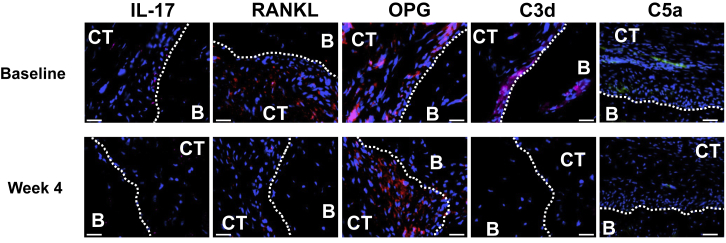
Systemically administered AMY-101 caused a significant and long-lasting reduction in PPD, an index that measures tissue destruction (Figure 6A). The protective effect was first observed at week 4. Strikingly, the protective effect persisted without decline for at least another 7 weeks (week 11) (Figure 6A), even though the drug was discontinued after week 4. Improvement of BOP, which assesses periodontal inflammation, was also observed; differences relative to the baseline reached statistical significance at weeks 2 and 3 (Figure 6B). Histological observations at 4 weeks showed that AMY-101 caused decreased expression of pro-inflammatory and pro-osteoclastogenic cytokines (interleukin [IL]-17 and receptor activator of nuclear factor-κB ligand [RANKL]) and elevated expression of osteoprotegerin (OPG; a natural inhibitor of RANKL) in the connective tissue adjacent to the alveolar bone, as compared to their baseline expression (Figure 7). Moreover, AMY-101 treatment caused a decrease in the complement cleavage fragments C3d and C5a, further confirming its ability to inhibit complement activation. In conclusion, systemic AMY-101 improves the periodontal condition of NHPs, which is stably maintained for at least 7 weeks after drug withdrawal.
Figure 6.
Systemic Administration of AMY-101 Improves the Periodontal Condition of NHPs
(A and B) AMY-101 was given systemically by subcutaneous injection every day for 28 days. Clinical examination of (A) probing pocket depth and (B) bleeding on probing was performed at the indicated time points until week 11. The data are expressed relative to the baseline values (at week 0), set as 100. Results are means ± SD (n = 10 animals). *p < 0.05 and **p < 0.01 compared to baseline (one-way repeated-measures ANOVA and Bonferroni’s multiple-comparisons test except for BOP, where the Friedman test was used followed by the Dunn’s multiple-comparisons test due to non-normality of the data).
Figure 7.
Expression of Inflammatory and Osteoclastogenesis-Related Molecules in Periodontal Biopsy Specimens from AMY-101-Treated NHPs
Periodontal biopsy specimens from NHPs treated with AMY-101 were processed for fluorescent microscopy. After antibody incubation, nuclei staining was performed with 4′,6-diamidino-2-phenylindole (DAPI; blue). The specimens were taken before (week 0) and after (week 4) systemic treatment with AMY-101 (administered every 24 hr). Shown are representative fluorescent images stained for the indicated molecules. B, bone; CT, connective tissue. Scale bar, 25 μm.
Discussion
Among various possible complement targets, C3 is strategically located at a central “hub” that relays upstream initiation signals to activate downstream effectors that stimulate and amplify host immune and inflammatory responses.35 C3 blockade is therefore an appropriate choice in disorders requiring broad complement inhibition (i.e., involving different complement pathways). Moreover, since C3b is required for activation of the alternative pathway, C3 inhibition should also be appropriate for inflammatory disorders driven predominantly by the alternative pathway. In this regard, early clinical and laboratory studies have shown that the alternative pathway was activated in human periodontitis and is thought to be more likely to play a major role in the disease than the classical pathway.24, 36, 37 Although the significance of the individual pathways of complement activation in periodontitis can be more definitely determined in intervention studies with pathway-specific inhibitors, the above-discussed considerations provide a strong rationale for pursuing a C3-targeted therapy for the treatment of human periodontitis.
The compstatin family of complement inhibitors are to this point the only reported small-size clinical drug candidates that act directly on C3.32 The original compstatin, a 13-residue cyclic peptide, binds both native C3 and its cleavage fragments C3b, iC3b, and C3c.38 The resolution of its crystal structure in complex with C3c revealed that compstatin sterically hinders the interaction of C3 with the C3 convertases, thus explaining mechanistically how the drug blocks the convertase-dependent cleavage of C3.39, 40 These structural insights facilitated subsequent optimization approaches that led to improved compstatin analogs displaying enhanced inhibitory action, target binding affinity, and pharmacokinetic parameters.32 For instance, the compstatin derivative used in the present report, AMY-101 (Cp40), exhibits subnanomolar affinity for C3 (KD = 0.5 nM) and a plasma half-life that exceeds expectations for most peptidic drugs.32, 33
The exceptional pharmacokinetic properties of AMY-101 are consistent with a “target-driven” model, wherein an initial fast clearance of excess free peptide (i.e., not bound to C3) is followed by slow clearance of C3-bound peptide. Further in line with this model, the measured half-life values of different compstatin analogs are correlated with their C3-binding affinities.33 In practical terms, the tight binding of AMY-101 to C3 appears to delay its clearance. Therefore, the high abundance of C3 in the diseased periodontal tissue24, 26, 27 is likely to delay the clearance of intragingivally administered AMY-101 or of AMY-101 reaching the periodontal tissue upon systemic injection. These notions are consistent with the herein observed sustained protective effects of AMY-101 after local or systemic administration. Indeed, although AMY-101 was administered locally as infrequently as once every 3 weeks and was withdrawn at 6 weeks, the treated animals maintained significantly reduced clinical periodontal/inflammatory indices for at least 6 weeks later. Moreover, the protective effect of systemic AMY-101 persisted for at least 7 weeks after drug withdrawal. However, it should be noted that additional factors might contribute to the observed long-lasting effects. Given the feed-forward loop between inflammation and dysbiosis,11, 12, 41 it is plausible that inflammation inhibition by AMY-101 tips the balance toward host-microbe homeostasis, which might be resilient (at least for some time) to pathological processes that would re-instate active periodontal disease.
Given that periodontitis is associated with increased RANKL and decreased OPG levels,42 the ability of AMY-101 to cause decreased expression of RANKL and elevated expression of OPG is therapeutically important. In this regard, clinical studies have shown that standard mechanical periodontal therapy (SRP) does not influence the RANKL/OPG ratio,43 further suggesting the urgent need to develop adjunctive therapies, such as C3 inhibition, that can potentiate the effectiveness of the current standard of care.
A potential concern regarding the therapeutic use of complement-targeted compounds, including C3 inhibitors, is whether long-term complement inhibition could impair host antimicrobial defenses. Although individuals with primary C3 deficiencies exhibit increased risk of pyogenic infections, their susceptibility is evident in the early years of life but normally subsides in adulthood, probably owing to compensatory defense mechanisms.44 Nevertheless, patients receiving systemic treatment with approved anti-complement drugs (e.g., anti-C5 treatment with eculizumab) are additionally vaccinated against encapsulated bacteria (e.g., meningococci) to further reduce potential risks of infection. Similar risks and preventive measures (vaccination and/or antibiotics) likely apply to C3 inhibitors in the setting of chronic conditions. Of course, if necessary, small-molecule inhibitors of C3 can be phased out more readily than antibodies or other protein-based therapeutics, thereby enabling rapid recovery of complement’s opsonic activity during an infection. As C3 inhibitors enter clinical trials, more definitive clinical experience will be obtained regarding their safety. On the other hand, such safety concerns are not likely to apply to the treatment of periodontitis. As alluded to above, C3 inhibition in periodontitis is more likely to contribute to anti-microbial host defense by limiting the nutrient supply to the dysbiotic microbiota rather than to interfere with immune surveillance. In this respect, C3-deficient mice subjected to experimental periodontitis have reduced periodontal bacterial burden compared to C3-sufficient controls.29
In principle, a potential concern for a locally administered C3 inhibitor such as AMY-101 is whether the drug could impair systemic complement activation during an infection. However, systemic exposure with AMY-101 after local injection into the gingival tissue should be minimal and thus should not affect complement activity in the circulation. Here it should be noted that C3 is the most abundant complement protein in the blood (1.0–1.5 mg/mL) and its inhibition requires much higher doses than those used locally in the gingiva to treat periodontitis. Specifically, in the treatment regimen used here, a total of 1.7 mg AMY-101 was injected (0.1 mg/site for 17 sites). Even if the full intragingival dose were administered systemically rather than locally, this would only amount to 0.2–0.3 mg/kg bodyweight in cynomolgus monkeys or 0.02–0.03 mg/kg bodyweight in humans. On the other hand, an AMY-101 dose of 1–2 mg/kg bodyweight was required in NHPs in order to reliably achieve target-exceeding drug levels after systemic administration.45 In the present study, moreover, we have shown that a therapeutic dose of locally administered AMY-10130 is free of local irritation and is thus suitable for consideration for the treatment of human periodontitis. In fact, among the different doses tested in this study, the 2 mg/mL concentration (0.1 mg/site) of AMY-101 appeared to be an optimal dose, in that it met both safety and protection requirements.
The rationale for testing the efficacy of systemically administered AMY-101 in NHP periodontitis is derived from the fact that the drug is also being considered for the treatment of several systemic conditions, such as paroxysmal nocturnal hemoglobinuria, complications of ABO-incompatible kidney transplantation, C3 glomerulopathy, hemodialysis-related inflammation, and ischemia-reperfusion injury.34 Given the high prevalence of chronic periodontitis in the adult population,5, 6, 7 our data suggest that many systemically treated patients can additionally benefit in terms of improved periodontal condition. The safety and efficacy features of locally administered AMY-101, as indicated by the present and our earlier NHP studies,29, 30 pave the way to clinical trials to determine whether this drug can contribute to the treatment of human periodontitis.
Materials and Methods
NHPs
All animal procedures were performed according to protocols reviewed and approved by the Institutional Animal Care and Use Committees of the University of Pennsylvania and of the Simian Conservation Breeding and Research Center (SICONBREC; Makati City, Philippines), an Association for Assessment and Accreditation of Laboratory Animal Care International-accredited facility where the NHP work was performed. Adult cynomolgus monkeys (Macaca fascicularis) (7–10 years old; 5.0–7.6 kg body weight) were used for the study. The animals were socially housed in stainless steel cages and were used in the experiments after they were acclimatized to the protocol procedures for 4 weeks. Environmental enrichment was provided through daily handling by animal care technicians, environmental enrichment items, and visual contact with other study animals. Each animal was offered a measured amount of an approved feed mixture. Fresh, potable drinking water was available to the animals ad libitum. Clinical periodontal examinations and periodontal tissue biopsies were performed in a manner similar to a human clinical study, except that the animals were anesthetized during the procedures. Blood samples were obtained using vacutainer blood-collecting tubes. All animals enrolled in the studies reported here were systemically healthy and maintained good systemic health during the observation period. No adverse effects were noted during the course of the study.
C3 Inhibitor AMY-101
The 14-residue compstatin analog AMY-101 [(D)Tyr-Ile-[Cys-Val-Trp(Me)-Gln-Asp-Trp-Sar-Ala-His-Arg-Cys]-mIle-NH2, where Sar is sarcosine/N-methyl glycine and mIle is N-methyl isoleucine] was produced as a disulfide-bridged, cyclic peptide by solid-phase peptide synthesis methodology as previously described.33 AMY-101 was injected locally into the gingiva (50 μL volume) at different concentrations (2–200 mg/mL) using a 30G short needle. Alternatively, for systemic administration, AMY-101 was given by subcutaneous injection (4 mg/kg bodyweight) using a 1-mL insulin safety syringe with a 28G × 1/2-inch needle.
Clinical Examination and Observation
Clinical periodontal examinations were performed and the diagnosis was established according to the criteria of the American Academy of Periodontology for human periodontal disease.46 Examinations using a periodontal probe were performed at baseline and throughout the study to monitor the progression of the disease and the effect of AMY-101 treatment. The examinations included determination of PPD (by measuring the distance [in millimeters] from the gingival margin to the base of the pocket), CAL (distance from the cementoenamel junction to the base of the pocket), GI (using a scale of 0–3, according to Löe47), BOP (percentage of positive sites), and plaque index (PI; scale of 0–3 according to Löe47). PPD, CAL, and BOP were measured at six sites: mesio-buccal, mid-buccal, disto-buccal, mesio-lingual, mid-lingual, and disto-lingual aspects of each tooth. GI and PI were assessed at four sites (buccal, lingual, mesial, and distal). GI and BOP are measures of periodontal inflammation, while CAL and PPD assess tissue destruction. The PI is a clinical measure of biofilm accumulation on tooth surfaces. In the irritation study, injection sites were clinically observed daily for signs of inflammation or the formation of an abscess, redness, itching, hematoma, bruising, bleb, or nodules. The degree of gingival inflammation was assessed as healthy, mild (slight change in color, no BOP), moderate (redness, BOP), or severe (marked redness, tendency to spontaneous bleeding).
Immunofluorescence Histochemistry
Gingival biopsy specimens were fixed in 4% paraformaldehyde and embedded in optimum cutting temperature (OCT) compound. Mesio-distal sections were stained using the following primary antibodies (all from Abcam): rabbit polyclonal antibodies to IL-17A, receptor activator of nuclear factor-κB ligand (RANKL) and OPG or rabbit monoclonal antibody to C3d (clone E28-P) or mouse monoclonal antibody to C5a/C5a-desArg (clone 2942). Secondary reagents included Alexa Fluor 594- or Alexa Fluor 647-conjugated goat anti-rabbit immunoglobulin G (IgG) or Alexa Fluor 488-conjugated goat anti-mouse IgG (Life Technologies). The specificity of staining was confirmed by using appropriate isotype controls or non-immune rabbit IgG followed by Alexa Fluor 488-, Alexa Fluor 594-, or Alexa Fluor 647-conjugated anti-IgG. Images were captured using a Nikon Eclipse NiE automated upright fluorescent microscope.
Statistical Analysis
For the comparison of mean values within the groups during the time-course studies, one-way repeated-measures ANOVA with Greenhouse-Geisser correction was performed using GraphPad Prism software (version 6.0h; GraphPad, La Jolla, CA, USA). In case of significant differences, Bonferroni’s multiple-comparisons test was performed. When a non-parametric test was warranted (due to non-normality of data, as in the assessment of BOP in Figure 6), the Friedman test was used followed by the Dunn’s multiple-comparisons test. Data are expressed as means ± SD and p < 0.05 was taken as the level of significance.Author Contributions
J.D.L. and G.H. conceived and supervised the project. T.K. and G.H. wrote the paper. T.K. analyzed the clinical data and performed immunofluorescence histochemistry. R.A.B. and J.V.T performed the animal experiments and clinical evaluation, and R.R.G.R. supervised the animal research. E.S.R., E.H., C.A.G.G., and D.Y. provided advice and contributed to the experimental design. All authors read and edited the paper.
Conflicts of Interest
G.H. and J.D.L. have a joint patent application that describes the use of complement inhibitors for therapeutic purposes in periodontitis. J.D.L. is the founder of Amyndas Pharmaceuticals, which is developing complement inhibitors for clinical applications. The other authors declare no conflict of interest.
Acknowledgments
This work was supported by grants from the NIH (AI068730 and AI030040 to J.D.L. and DE015254, DE021685, and DE024716 to G.H.) and the European Commission (FP7-DIREKT 602699 to J.D.L.).
Contributor Information
John D. Lambris, Email: lambris@upenn.edu.
George Hajishengallis, Email: geoh@upenn.edu.
References
- 1.Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 2015;15:30–44. doi: 10.1038/nri3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lamont R.J., Hajishengallis G. Polymicrobial synergy and dysbiosis in inflammatory disease. Trends Mol. Med. 2015;21:172–183. doi: 10.1016/j.molmed.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chapple I.L. Time to take periodontitis seriously. BMJ. 2014;348:g2645. doi: 10.1136/bmj.g2645. [DOI] [PubMed] [Google Scholar]
- 4.Brauchle F., Noack M., Reich E. Impact of periodontal disease and periodontal therapy on oral health-related quality of life. Int. Dent. J. 2013;63:306–311. doi: 10.1111/idj.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eke P.I., Dye B.A., Wei L., Slade G.D., Thornton-Evans G.O., Borgnakke W.S., Taylor G.W., Page R.C., Beck J.D., Genco R.J. Update on prevalence of periodontitis in adults in the United States: NHANES 2009 to 2012. J. Periodontol. 2015;86:611–622. doi: 10.1902/jop.2015.140520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Demmer R.T., Papapanou P.N. Epidemiologic patterns of chronic and aggressive periodontitis. Periodontol. 2000. 2010;53:28–44. doi: 10.1111/j.1600-0757.2009.00326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frencken J.E., Sharma P., Stenhouse L., Green D., Laverty D., Dietrich T. Global epidemiology of dental caries and severe periodontitis - a comprehensive review. J. Clin. Periodontol. 2017;44(Suppl 18):S94–S105. doi: 10.1111/jcpe.12677. [DOI] [PubMed] [Google Scholar]
- 8.Tonetti M.S., Chapple I.L., Working Group 3 of Seventh European Workshop on Periodontology Biological approaches to the development of novel periodontal therapies--consensus of the Seventh European Workshop on Periodontology. J. Clin. Periodontol. 2011;38(Suppl 11):114–118. doi: 10.1111/j.1600-051X.2010.01675.x. [DOI] [PubMed] [Google Scholar]
- 9.Kassebaum N.J., Bernabé E., Dahiya M., Bhandari B., Murray C.J., Marcenes W. Global burden of severe periodontitis in 1990-2010: a systematic review and meta-regression. J. Dent. Res. 2014;93:1045–1053. doi: 10.1177/0022034514552491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beikler T., Flemmig T.F. Oral biofilm-associated diseases: trends and implications for quality of life, systemic health and expenditures. Periodontol. 2000. 2011;55:87–103. doi: 10.1111/j.1600-0757.2010.00360.x. [DOI] [PubMed] [Google Scholar]
- 11.Hajishengallis G. The inflammophilic character of the periodontitis-associated microbiota. Mol. Oral Microbiol. 2014;29:248–257. doi: 10.1111/omi.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diaz P.I., Hoare A., Hong B.Y. Subgingival microbiome shifts and community dynamics in periodontal diseases. J. Calif. Dent. Assoc. 2016;44:421–435. [PubMed] [Google Scholar]
- 13.Eskan M.A., Jotwani R., Abe T., Chmelar J., Lim J.H., Liang S., Ciero P.A., Krauss J.L., Li F., Rauner M. The leukocyte integrin antagonist Del-1 inhibits IL-17-mediated inflammatory bone loss. Nat. Immunol. 2012;13:465–473. doi: 10.1038/ni.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee C.T., Teles R., Kantarci A., Chen T., McCafferty J., Starr J.R., Brito L.C., Paster B.J., Van Dyke T.E. Resolvin E1 reverses experimental periodontitis and dysbiosis. J Immunol. 2016;197:2796–2806. doi: 10.4049/jimmunol.1600859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moutsopoulos N.M., Konkel J., Sarmadi M., Eskan M.A., Wild T., Dutzan N., Abusleme L., Zenobia C., Hosur K.B., Abe T. Defective neutrophil recruitment in leukocyte adhesion deficiency type I disease causes local IL-17-driven inflammatory bone loss. Sci. Transl. Med. 2014;6:229ra40. doi: 10.1126/scitranslmed.3007696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hasturk H., Kantarci A., Goguet-Surmenian E., Blackwood A., Andry C., Serhan C.N., Van Dyke T.E. Resolvin E1 regulates inflammation at the cellular and tissue level and restores tissue homeostasis in vivo. J. Immunol. 2007;179:7021–7029. doi: 10.4049/jimmunol.179.10.7021. [DOI] [PubMed] [Google Scholar]
- 17.Ricklin D., Hajishengallis G., Yang K., Lambris J.D. Complement: a key system for immune surveillance and homeostasis. Nat. Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ricklin D., Reis E.S., Lambris J.D. Complement in disease: a defence system turning offensive. Nat. Rev. Nephrol. 2016;12:383–401. doi: 10.1038/nrneph.2016.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Courts F.J., Boackle R.J., Fudenberg H.H., Silverman M.S. Detection of functional complement components in gingival crevicular fluid from humans with periodontal diseases. J. Dent. Res. 1977;56:327–331. doi: 10.1177/00220345770560032001. [DOI] [PubMed] [Google Scholar]
- 20.Attström R., Laurel A.B., Lahsson U., Sjöholm A. Complement factors in gingival crevice material from healthy and inflamed gingiva in humans. J. Periodontal Res. 1975;10:19–27. doi: 10.1111/j.1600-0765.1975.tb00003.x. [DOI] [PubMed] [Google Scholar]
- 21.Toto P.D., Lin L., Gargiulo A. Identification of C3a, IgG, IgM in inflamed human gingiva. J. Dent. Res. 1978;57:696. doi: 10.1177/00220345780570050501. [DOI] [PubMed] [Google Scholar]
- 22.Nikolopoulou-Papaconstantinou A.A., Johannessen A.C., Kristoffersen T. Deposits of immunoglobulins, complement, and immune complexes in inflamed human gingiva. Acta Odontol. Scand. 1987;45:187–193. doi: 10.3109/00016358709098858. [DOI] [PubMed] [Google Scholar]
- 23.Rautemaa R., Meri S. Protection of gingival epithelium against complement-mediated damage by strong expression of the membrane attack complex inhibitor protectin (CD59) J. Dent. Res. 1996;75:568–574. doi: 10.1177/00220345960750010901. [DOI] [PubMed] [Google Scholar]
- 24.Schenkein H.A., Genco R.J. Gingival fluid and serum in periodontal diseases. II. Evidence for cleavage of complement components C3, C3 proactivator (factor B) and C4 in gingival fluid. J. Periodontol. 1977;48:778–784. doi: 10.1902/jop.1977.48.12.778. [DOI] [PubMed] [Google Scholar]
- 25.Lally E.T., McArthur W.P., Baehni P.C. Biosynthesis of complement components in chronically inflamed gingiva. J. Periodontal Res. 1982;17:257–262. doi: 10.1111/j.1600-0765.1982.tb01152.x. [DOI] [PubMed] [Google Scholar]
- 26.Patters M.R., Niekrash C.E., Lang N.P. Assessment of complement cleavage in gingival fluid during experimental gingivitis in man. J. Clin. Periodontol. 1989;16:33–37. doi: 10.1111/j.1600-051x.1989.tb01609.x. [DOI] [PubMed] [Google Scholar]
- 27.Niekrash C.E., Patters M.R. Simultaneous assessment of complement components C3, C4, and B and their cleavage products in human gingival fluid. II. Longitudinal changes during periodontal therapy. J. Periodontal Res. 1985;20:268–275. doi: 10.1111/j.1600-0765.1985.tb00434.x. [DOI] [PubMed] [Google Scholar]
- 28.Zhan Y., Zhang R., Lv H., Song X., Xu X., Chai L., Lv W., Shang Z., Jiang Y., Zhang R. Prioritization of candidate genes for periodontitis using multiple computational tools. J. Periodontol. 2014;85:1059–1069. doi: 10.1902/jop.2014.130523. [DOI] [PubMed] [Google Scholar]
- 29.Maekawa T., Abe T., Hajishengallis E., Hosur K.B., DeAngelis R.A., Ricklin D., Lambris J.D., Hajishengallis G. Genetic and intervention studies implicating complement C3 as a major target for the treatment of periodontitis. J. Immunol. 2014;192:6020–6027. doi: 10.4049/jimmunol.1400569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maekawa T., Briones R.A., Resuello R.R., Tuplano J.V., Hajishengallis E., Kajikawa T., Koutsogiannaki S., Garcia C.A., Ricklin D., Lambris J.D., Hajishengallis G. Inhibition of pre-existing natural periodontitis in non-human primates by a locally administered peptide inhibitor of complement C3. J. Clin. Periodontol. 2016;43:238–249. doi: 10.1111/jcpe.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ricklin D., Lambris J.D. Complement in immune and inflammatory disorders: therapeutic interventions. J. Immunol. 2013;190:3839–3847. doi: 10.4049/jimmunol.1203200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mastellos D.C., Yancopoulou D., Kokkinos P., Huber-Lang M., Hajishengallis G., Biglarnia A.R., Lupu F., Nilsson B., Risitano A.M., Ricklin D., Lambris J.D. Compstatin: a C3-targeted complement inhibitor reaching its prime for bedside intervention. Eur. J. Clin. Invest. 2015;45:423–440. doi: 10.1111/eci.12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qu H., Ricklin D., Bai H., Chen H., Reis E.S., Maciejewski M., Tzekou A., DeAngelis R.A., Resuello R.R., Lupu F. New analogs of the clinical complement inhibitor compstatin with subnanomolar affinity and enhanced pharmacokinetic properties. Immunobiology. 2013;218:496–505. doi: 10.1016/j.imbio.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mastellos D.C., Ricklin D., Hajishengallis E., Hajishengallis G., Lambris J.D. Complement therapeutics in inflammatory diseases: promising drug candidates for C3-targeted intervention. Mol. Oral Microbiol. 2016;31:3–17. doi: 10.1111/omi.12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ricklin D., Lambris J.D. Therapeutic control of complement activation at the level of the central component C3. Immunobiology. 2016;221:740–746. doi: 10.1016/j.imbio.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Niekrash C.E., Patters M.R. Assessment of complement cleavage in gingival fluid in humans with and without periodontal disease. J. Periodontal Res. 1986;21:233–242. doi: 10.1111/j.1600-0765.1986.tb01455.x. [DOI] [PubMed] [Google Scholar]
- 37.Allison A.C., Schorlemmer H.U. Activation of complement by the alternative pathway as a factor in the pathogenesis of periodontal disease. Lancet. 1976;2:1001–1004. doi: 10.1016/s0140-6736(76)90837-0. [DOI] [PubMed] [Google Scholar]
- 38.Sahu A., Kay B.K., Lambris J.D. Inhibition of human complement by a C3-binding peptide isolated from a phage-displayed random peptide library. J. Immunol. 1996;157:884–891. [PubMed] [Google Scholar]
- 39.Janssen B.J., Halff E.F., Lambris J.D., Gros P. Structure of compstatin in complex with complement component C3c reveals a new mechanism of complement inhibition. J. Biol. Chem. 2007;282:29241–29247. doi: 10.1074/jbc.M704587200. [DOI] [PubMed] [Google Scholar]
- 40.Ricklin D., Lambris J.D. Compstatin: a complement inhibitor on its way to clinical application. Adv. Exp. Med. Biol. 2008;632:273–292. doi: 10.1007/978-0-387-78952-1_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bartold P.M., Van Dyke T.E. Periodontitis: a host-mediated disruption of microbial homeostasis. Unlearning learned concepts. Periodontol. 2000. 2013;62:203–217. doi: 10.1111/j.1600-0757.2012.00450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Belibasakis G.N., Bostanci N. The RANKL-OPG system in clinical periodontology. J. Clin. Periodontol. 2012;39:239–248. doi: 10.1111/j.1600-051X.2011.01810.x. [DOI] [PubMed] [Google Scholar]
- 43.Bostanci N., Saygan B., Emingil G., Atilla G., Belibasakis G.N. Effect of periodontal treatment on receptor activator of NF-κB ligand and osteoprotegerin levels and relative ratio in gingival crevicular fluid. J. Clin. Periodontol. 2011;38:428–433. doi: 10.1111/j.1600-051X.2011.01701.x. [DOI] [PubMed] [Google Scholar]
- 44.Reis E.S., Falcão D.A., Isaac L. Clinical aspects and molecular basis of primary deficiencies of complement component C3 and its regulatory proteins factor I and factor H. Scand. J. Immunol. 2006;63:155–168. doi: 10.1111/j.1365-3083.2006.01729.x. [DOI] [PubMed] [Google Scholar]
- 45.Risitano A.M., Ricklin D., Huang Y., Reis E.S., Chen H., Ricci P., Lin Z., Pascariello C., Raia M., Sica M. Peptide inhibitors of C3 activation as a novel strategy of complement inhibition for the treatment of paroxysmal nocturnal hemoglobinuria. Blood. 2014;123:2094–2101. doi: 10.1182/blood-2013-11-536573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Armitage G.C. Development of a classification system for periodontal diseases and conditions. Ann. Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 47.Löe H. The gingival index, the plaque index and the retention index systems. J. Periodontol. 1967;38(Suppl):610–616. doi: 10.1902/jop.1967.38.6.610. [DOI] [PubMed] [Google Scholar]