Abstract
Purpose of Review
Total joint arthroplasty is regarded as a highly successful procedure. Patient outcomes and implant longevity, however, are related to proper alignment and position of the prostehses. In an attempt to reduce outliers and improve accuracy and precision of component position, navigation and robotics have been introduced. These technologies, however, come at a price. The goals of this review are to evaluate these technologies in total joint arthroplasty and determine if they add value.
Recent Findings
Recent studies have demonstrated that navigation and robotics in total joint arthroplasty can decrease outliers while improving accuracy in component positioning. While some studies have demonstrated improved patient reported outcomes, not all studies have shown this to be true. Most studies cite increased cost of equipment and longer operating room times as the major downsides of the technologies at present. Long-term studies are just becoming available and are promising, as some studies have shown decreased revision rates when navigation is used. Finally, there are relatively few studies evaluating the direct cost and value of these technologies.
Summary
Navigation and robotics have been shown to improve component position in total joint arthroplasty, which can improve patient outcomes and implant longevity. These technologies offer a promising future for total joint arthroplasty.
Keywords: Technology, Arthroplasty, Value, Navigation, Robotics, Cost
Introduction
Total hip and knee arthroplasty are highly successful operations that offer significant pain relief and restore function in most cases [1, 2, 3]. Studies have shown that proper alignment in both hip and knee replacement can lead to improved postoperative outcomes including postoperative function, postoperative pain, and improved longevity of the implant [4•, 5•, 6].
Improving outcomes after total joint replacement have always been a goal for orthopedic surgeons. In the pursuit of improving outcomes after surgery and longevity of the implants, surgeons have begun to use technology in hopes of achieving these goals.
Computer-navigated and robotic-assisted total joint replacement has been introduced over the last two decades. As with any new technology, these systems come with a learning curve and a potential increased cost to the surgical procedure. Increased initial cost can be offset by reduction in hospital stay, complications, and future revision surgeries. The goals of this review are to provide a brief overview of these technologies while evaluating the literature surrounding these technologies and finally to review studies related to value of technology in total joint arthroplasty.
Total Knee Arthroplasty
Total knee arthroplasty has enjoyed long-standing success in pain relief and improvement of function. These two outcomes, along with longevity of the implant, however, have been shown to be related to correct positioning of the components [4•, 6–8]. Navigation has been introduced in total knee arthroplasty in an attempt to improve component position and restore the mechanical axis.
Computer-Assisted Total Knee Replacement
Computer-assisted surgical (CAS) navigation was introduced in the 1990s. CAS total knee replacement comes in three distinct types. These include imageless (including accelerometer based), preoperative image based, and intraoperative image based. Depending on the types of navigation, the computer integrates the information from landmarks on the images and/or landmarks taken at the beginning of the surgery with data acquired during the surgery to determine the frontal and sagittal plane to properly position the cutting guides.
Imageless CAS
Two main types of imageless systems exist: accelerometer based and optical navigation systems. The accelerometer-based (Fig. 1) navigation uses a hand-held accelerometer that attaches to the bone to establish the axis of the limb and then attaches to the cutting guides to guide its position. Optically based imageless navigation systems (Fig. 2) use optical localization between probes and secured pins that define the surface of the bone or bony landmarks and an optical sensor attached to the computer. Imageless systems have the benefit of avoiding radiation with the preoperative computerized tomography (CT) and avoid the cost of preoperative CT or magnetic resonance imaging (MRI). The accelerometer-based system has another added benefit over older imageless and image-based navigation systems as they avoid securing pins to the patient outside the immediate surgical field. Though rare, pin sites have been shown to be susceptible to infection or fracture at the pin site [9]. New technologies are moving towards pinless navigation to avoid these complications [10, 11].
Fig. 1.
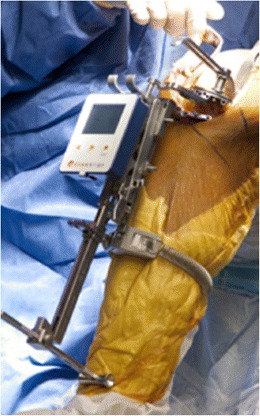
KneeAlign accelerometer-based knee navigation (OrthoAlign Aliso Viejo, CA) (Courtsey of Seth Jerabek)
Fig. 2.
Imageless navigation for total knee arthroplasty (Exactech GPS Primary Knee Gainesville, FL) (Courtesy of Brad Waddell)
Image-Based CAS
Image-based navigation systems use either a pre-operative CT or MRI scan to form a 3D model in the computer of the patient’s specific anatomy. Then, prior to beginning the surgery, the surgeon uses optical navigation to register the patient which is then matched to the 3D model that has been created. The image-based approach gives the surgeon the ability to plan implant positioning before surgery. Further, the surgeon has the ability to intra-operatively alter the position of the components because there is no constraint to the specific plan.
Outcomes
As previously mentioned, the ultimate goal of adding computer assistance to total knee replacement is to add value by improving patient-reported outcomes and reducing surgical costs by reducing complications and the need for revision surgery. In their randomized control trial of patients undergoing simultaneous bilateral total knee arthroplasties, Zhang and colleagues found that the CAS total knee group had significantly better coronal and sagittal alignment compared with the conventional total knee group. In the CAS group, there were no outliers greater than 3° from the mechanical axis in the coronal plane, whereas there were 9 in the conventional group. The two groups did not differ in terms of rotation of the femur, and at early follow-up (6 months), there was no difference in outcomes based on Hospital for Special Surgery (HSS) Scores. Important for the discussion of cost, the CAS group had an average operative time of 90 versus 58 min in the conventional group. In another study which utilized an accelerometer-based navigation technique, Nam and co-authors used postoperative radiographs to determine the accuracy of the system in placing the tibial component. They found this navigation technique to accurately place the tibial component within 2° of the coronal (90°) and sagittal goal (3° slope) 97.6 and 96.2% of the time, respectively [12]. Short-term outcomes in total knee replacement have also been shown to be improved with CAS. In their meta-analysis, Rebal et al. found that CAS was more likely to place the components within 3° of the ideal mechanical alignment compared with conventional total knee arthroplasty (87.1 vs 73.7%, p < 0.01). Further, they found navigated knees to have increased Knee Society Scores (KSS) at both 3-month (68.5 vs 58.1, p = 0.03) and 12–32-month follow-up (53.1 vs 45.8, p < 0.01). Similar to other studies, their analysis also demonstrated that CAS had significantly longer operative times over conventional surgery (101.6 vs 83.3, p < 0.01). Longer term follow-up is important in determining if CAS is in fact adding value to knee replacement. Cip and co-authors demonstrated an improvement in Insall knee Score and HSS knee score at minimum 5-year follow-up. They did not, however, find a difference in Western Ontario and McMaster Universities Arthritis Index (WOMAC) scores [13•]. In a separate study, at an average of 46 months (range 30–96 months), Blakeney and colleagues found a trend towards improved Oxford Knee Scores (OKS) in the computer-navigated group and a significantly improved OKS when the mechanical axis was within 3° of neutral (p = 0.045) [14] (more often seen in the navigated group).
A recent study assessed outcomes at 10-year follow-up. This study, performed by Baumbach and collegaues, retrospectively reviewed a consecutive series of 217 cases and were able to report on 46 conventional and 50 CAS total knee replacements at 10 years [15]. They found that the CAS group had a 22-min longer average surgery (p < 0.0001) and on average were about 5 years younger than conventional patients (p < 0.00001). Further, they found that the navigated group had a mechanical axis within the acceptable 3° of neutral in 78% of the cases, whereas conventional was in the acceptable range in 58% of cases. At 10 years, 19 revisions had been performed, with 8 for aseptic loosening. There were 7 in the conventional group and 1 in the CAS group, equating to 87% survival for the conventional group and 98% in the CAS group (p = 0.03). Using the HSS and KSS scores for postoperative outcomes, they found no difference between the two groups at 10 years (p > 0.19). The authors do comment that those patients undergoing CAS surgery had a lower “pain” category than the average population. They conclude that CAS offers a higher chance of optimal component position and a significantly lower revision rate at 10 years.
In another mid- to long-term study, de Steiger used the Australian registry to assess revision rate in total knee arthroplasty with and without the use of CAS [16]. They evaluated the registry from January 1, 2003 until December 21, 2012 and found that the rate of CAS surgery increased from 2.4 to 22.8%. They found that the overall revision rate among conventional total knees during that time period was 5.2% compared with 4.6% in the navigated group (p = 0.15). This difference became even greater when comparing knees performed in those patients under 65. In patients under 65, there was a statistically smaller revision rate in the CAS group, 6.3 versus 7.8% (p = 0.011). Further, when specifically evaluating revision for aseptic loosening in those patients less than 65, again, CAS had a significantly lower revision rate (1.6 vs 2.6%, p = 0.001). Finally, they evaluated the rates of major revision and found that in the entire age cohort, CAS decreased the rate of major revision over conventional surgery (2.1 vs 2.7%, p < 0.001). They found no difference in the rates of minor revision between the entire cohort with regards to navigation.
Another potential benefit of CAS in knee arthroplasty is mitigating the need to violate the femoral canal, which can decrease blood loss. In their study, Licini and Meneghini demonstrated that total knee arthroplasties performed with computer navigation had less hourly hemovac drain output (p = 0.02), smaller hemoglobin change (p = 0.001), and a lower estimated blood loss (p = 0.001) [17]. They proposed that avoiding violating the femoral canal as a potential source of less blood loss. In a meta-analysis by Moskal and colleagues, they reviewed studies with a total of 7151 knee arthroplasties and found that blood loss was significantly lower in the navigated group, along with improved component alignment and higher clinical ratings [18].
Not all studies demonstrate benefit with CAS in knee replacement surgery. In 2013, Burnett and Barrack performed a systematic review of navigated versus conventional total knee replacement asking the question: “Does the literature contain evidence of better long-term function and lower revision rates with navigated TKA compared with conventional TKA?” [19]. They conclude that navigation does improve coronal plane alignment, but found little evidence of improvement with regards to other variables. They found longer surgical times and unique complications (such as pin site fracture and infection) associated with CAS. At the time of their study, they concluded that current studies did not support CAS over conventional TKA.
Robotic-Assisted Total Knee Arthroplasty
Another technology introduced in knee replacement surgery is robot-assisted surgery. At present, four robotic systems are available for knee replacement.
The Robodoc/TSolution One Surgical System (Curexo Technology, Freemont, CA and Think Surgical Inc.) is available for total knee arthroplasty. The system uses a preoperative CT scan and a milling reamer to prepare the tibia and femur in TKA. While not in widespread use in the USA, Robodoc has been shown to improve accuracy compared with conventional total knee arthroplasty [20, 21].
iBlock is a robotic cutting guide from OMNIlife Science (East Taunton, MA). This system is a motorized bone mounted cutting guide that uses an intraoperatively created 3D model of the patient’s bone from data taken by the surgeon. The robotic-motorized cutting block is mounted to the patient’s femur, and after the sizing and bone cuts are determined from the computer model, the guide moves to allow the surgeon to make the specific cuts. This system is used in conjunction with the Nanoblock, which is a separate adjustable tibial cutting guide. Few studies exist using this system; however, the system has been shown to be extremely precise and accurate in a saw bone model [22]. In the single clinical study available, it allowed a single surgeon to stay within 3° of neutral with regards to bone cuts in the first 100 cases [23].
The Navio surgical system (Smith and Nephew Memphis, TN) (Fig. 3) is somewhat of a robotic system, but is more accurately labeled a hand-held, imageless burring system that utilizes an intraoperatively created 3D model from the patient’s anatomy. The system then allows the surgeon to plan the bone resection and implant sizes prior to beginning the bone resection with the burr tool. The system tracks the patient’s limb and the hand-held burring tool, stopping or retracting the burr to keep the surgeon within the defined limits of the implant resection. Navio began with limitations to only UKA but has now been expanded to total knee applications.
Fig. 3.

Navio Knee System (Courtesy of Brad Waddell)
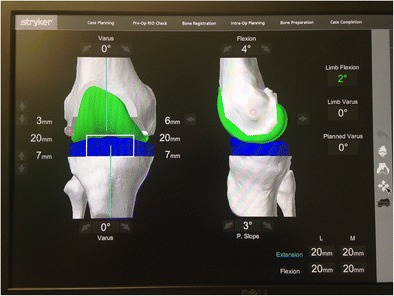
The final robotic system for TKA is the Robotic Arm Interactive System (Rio; Mako Stryker, Mahwah, NJ) (Fig. 4). Only just recently released for total knee application, this system uses a robotic-controlled arm with saw blade attached to make the bone cuts in TKA. The patient undergoes a preoperative CT scan from which a 3D model is created. This allows the surgeon to preoperatively template both the bone cuts and the implant size and position to be used in the surgery. While still early in its release, it has been shown to be more accurate than conventional TKA [24] and more friendly to soft tissues [25]. Future clinical studies are needed to determine the value added by all of these robotic TKA systems.
Fig. 4.
Mako RIO Robotic-Assisted Knee Replacement (Courtesy Brad Waddell)
Unicompartmental Knee Arthroplasty
While not a principle topic of this article, it is necessary to mention the role navigation and robotic assistance has played in improving outcomes and component alignment with unicompartmental knee arthroplasty (UKA). UKA is on the rise and currently comprises about 8% of all knee arthroplasties and the rate of use is increasing [26].
In a prospective, randomized controlled study from the United Kingdom, Bell and colleagues compared 58 conventional UKAs with 62 UKAs performed with robotic assistance (MAKO Surgical at the time of study, currently Stryker Corp. Mahwah, NJ). They found that accuracy of component positioning was significantly improved with the robotic arm assistance (p < 0.01) [27]. In another study using the robotic arm, Pearle et al. followed 909 UKAs for 2.5 years, finding a revision rate of only 1.2%, and in those not revised, they have 92% satisfied or very satisfied [28]. Using the Navio system described above in a cadaveric study, Lonner et al. demonstrated less than 2° of error when implanting unicondylar prostheses with the Navio Precision Freehand Sculpting tool (Blue Belt Technologies at the time of study, currently Smith and Nephew, Memphis TN) [29].
Total Hip Arthroplasty
Imageless Navigation
Non-robotic computer navigation systems can be categorized based on whether they require pre-operative imaging. Imageless navigation relies only on intra-operative registration of bony landmarks to create a virtual 3D model of the patient’s anatomy and determine the patient’s relative positioning. Whereas image-based navigation systems can generate patient-specific 3D reconstructions of the patient’s actual anatomy, imageless systems can only map landmarks identified by the surgeon onto a generic pelvis model. These systems use specific intraoperative landmarks to allow the computer to provide values for version and inclination, offset, and leg length. This can be one of the limitations of this technique, as proper registration is key to success. Imageless navigation requires less capital investment than in robotic equipment, spares the patient radiation exposure and expense associated with pre-operative imaging, and requires only minimal set-up for each surgical case. Although surgical time decreases with experience, imageless navigation typically lengthens the total surgical time by about 12–18 min, due to additional registration steps.
OrthAlign (OrthAlign Inc., Aliso Viejo, CA) is a disposable palm-sized accelerometer-based device that is compatible with all implant systems for both hip and knee arthroplasty [30]. The device consists of a disposable computer display unit and a reference sensor, to be used for acetabular preparation. Rather than pointing to multiple reference points on the limb to define the femoral reference plane, the surgeon moves the limb in specific patterns. The computer then calculates the mechanical axis of the limb based on measurements from the accelerometer. The device is then mounted on cutting jigs, and it provides real-time feedback for the surgeon to perform bony resection [30].
Another imageless system is the HipXpert (Surgical Planning Associates, Medford, MA) that enables a simple mechanical device to dock to the pelvis in a patient-specific manner and thereby guide cup orientation. Cup anteversion and inclination were significantly more accurate in cups placed using the HipXpert system than in those placed with traditional CT-based navigation [31].
Research has confirmed that imageless navigation systems are generally precise and reliable. In one study, imageless navigation yielded precise and reproducible cup positioning within 5° for both inclination and abduction, compared to 12° for inclination and 13° for abduction among cups placed by experienced surgeons without navigation [32]. Similarly, other authors have shown that over 97% of acetabular components placed with imageless navigation were within the safe zone of ±10° for both inclination and anteversion [33].
A primary benefit of computer navigation for THA appears to be a reduction in the number of cups placed far outside the acceptable safe zone. A prospective RCT comparing conventional non-navigated THA to the ORTHOsoft imageless navigation system (Zimmer, Warsaw, IN) demonstrated no difference in cup abduction angles, but final cup anteversion deviated significantly less from the planned angle of 15° in the navigated group [34•].
Imageless navigation can also facilitate limb length restoration. In a randomized comparison of imageless navigation and fluoroscopy (without navigation), there were no significant differences between groups in leg length restoration and femoral offset, but the navigated group had fewer outliers more than 5 mm outside the target zone accounting for both leg length and femoral offset [35]. Other groups have also demonstrated the ability to restore limb length to within 6 mm of the contralateral limb in over 95% of cases [36], although there is currently no clear evidence that navigation restores limb length better than conventional THA.
Renkawitz et al. conducted an RCT comparing conventional THA to a femur-first technique using the Brainlab imageless navigation system, which presented the surgeon with a 3D representation of the recommended cup position to maximize bony coverage and impingement-free motion. Both groups had over 87% bony surface contact with the cup, but more patients in the navigation group achieved maximal impingement-free range of motion (84%, 48/66 vs 65%, 43/69). Harris hip scores were significantly higher in the navigated group at 6 weeks, but the difference was clinically unimportant, and by 6 months and 1 year, there were no differences between groups [37]. Patient satisfaction, clinical outcomes, and manual ROM testing were equivalent in both groups at 1 year. Retrospective comparisons of imageless navigation and conventional THA found no differences in Haris hip scores, periprosthetic bone mineral density, range of motion, or polyethylene wear at 5–7 years postop [38].
Image-Based Navigation
Image-based navigation uses pre-operative CT, MRI, or fluoroscopy to facilitate surgical planning and execution. CT-guided navigation is the most common form of image-based navigation. Pre-op planning for non-robotic CT-based systems is essentially the same as for CT-based robotic systems. Intraoperatively, the surgeon registers bony landmarks and instruments and receives computer feedback about instrument and implant positioning. However, in contrast to robotic THA, the surgeon executes the entire procedure without any constraint from the robot. This gives the surgeon more freedom to alter the preoperative plan based on intraoperative findings, but also allows the surgeon to err or place the components outside the recommended zone.
Fluoroscopic navigation is similar to imageless navigation because neither uses preoperative imaging. Instead, the surgeon registers each landmark intraoperatively using fluoroscopy. Although fluoroscopic navigation may seem more accurate because it includes patient-specific imaging, the registration process is cumbersome and it does not appear to offer any advantages over imageless navigation [39].
CT-based navigation systems provide more accurate measurements of cup alignment than conventional THA, resulting in fewer cup positioning outliers [40, 41, 42•]. One retrospective review of 180 navigated THAs and 120 manual THAs demonstrated a significantly lower rate of cup placement outside the Lewinnek zone (0 vs 26%) and significantly fewer postoperative dislocations (0 vs 8%) in the navigated group, although there was no significant difference in 13-year implant survival [43]. A systematic review of publications including 400 patients revealed no significant difference in mean cup inclination or anteversion between the conventional and navigated groups, but variability in cup position and the risk of placing the cup outside the safe zone were significantly reduced in the navigation group [44].
Robotic Navigation
Robotic systems assist the surgeon in executing the surgery by transferring the pre-operative imaging data and templating to a robotic surgical assistant with an articulating arm that attaches to the surgical instrument or implant, such as the acetabular reamer or cup [45] (Fig. 5).
Fig. 5.
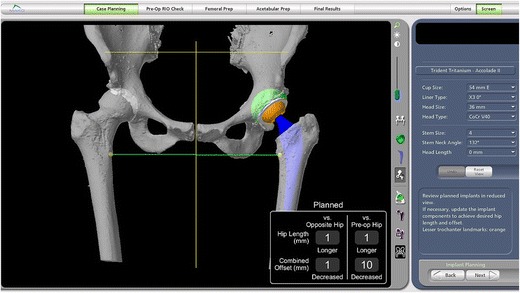
Mako RIO Robotic-Assisted Total Hip Replacement (Courtesy of Brad Waddell MD)
Robotic assistants can be classified into “fully active” and “semi-active” systems, depending on the degree to which they permit the surgeon to retain some control over the task. Drs. William Bargar and Howard Paul created the first robotic device for total joint arthroplasty, the ROBODOC system, in 1985 [46]. It is a CT-based computer-aided robotic milling device originally designed to facilitate femoral component preparation and implantation. Fully active robotic systems like ROBODOC can perform specific tasks or entire procedures autonomously. For example, in this system, the preoperative plan is created using the ORTHODOC software, which creates a 3D virtual model of the patient’s anatomy based on preoperative CT. This plan is then transferred to the ROBODOC surgical assistant, which completes all reaming, broaching, and positioning of the final femoral implant. The surgeon oversees the robot and can activate an emergency stop button, but does not directly control the robot.
Alternatively, “semi-active” systems offer “active constraint,” in which the surgeon has ultimate control over the surgical process, but receives auditory or tactile feedback from the robot to constrain the surgeon to a boundary defined by the computer based on the 3D imaging preoperative plan. For example, a semi-active robot such as the MAKO Robotic Arm Interactive Orthopedic (RIO) System (Stryker, Mahwah, NJ) will permit the surgeon to slightly adjust the angle at which the cup is reamed, but will not permit the surgeon to push the reamer more than a few degrees beyond the planned cup position. By combining 3D femoral and acetabular planning, the surgeon can plan modifications to the femoral stem size, offset and positioning to account for changes to the acetabular center of rotation. The software measures changes in limb length and combined offset relative to both the preoperative ipsilateral limb and the contralateral limb. Because robots do not fatigue and can easily overcome torque from larger reamers, robots permit single-stage acetabular reaming, which can decrease surgical time.
Surgical time is consistently increased in robotic THA, although the reported increase in surgical time varies widely between authors [47–49]. However, with single-stage reaming and familiarity, robotic hip surgery can become time neutral.
Robotic systems require substantial upfront financial investment for the robot and software, as well as recurring costs associated with each surgical case. At one Japanese institution, each robotic surgery incurred $1500 additional cost for disposable equipment such as drapes and bone cutters [47]. Robotic THA is also subject to the disadvantages of CT-based systems, including increased cost and radiation exposure associated with the scan and longer time devoted to pre-operative planning.
Outcomes
Studies demonstrate some improvements in femoral component radiographic parameters associated with the fully active ROBODOC system. The initial randomized multicenter feasibility study for ROBODOC using a posterior approach demonstrated statistically significant improvements in fit, fill and alignment compared to non-robotic THA, and no ROBODOC patient sustained an intra-operative fractur [50, 51]. A Japanese RCT comparing ROBODOC and conventional THA found significantly less proximal femoral stress shielding in the robotic group at 2 and 5 years [47]. This finding has been corroborated by a study of women 24 months after robotic or conventional THA, in which proximal medial femoral spot welding was more prevalent (48%, 15/31 vs 11%, 3/27) and stress shielding was less prevalent (17%, 5/31 vs 31%, 8/27) among ROBODOC patients [52]. Nakamura et al. found no significant difference in average limb length inequality, but the ROBODOC group had significantly less variance in limb length inequality than the conventional THA group [47]. More patients with robotic THAs developed heterotopic ossification (27 vs 16%), although the difference was not significant.
A primary benefit of navigation for THA is a reduction in acetabular component positioning outliers. Acetabular cups implanted outside the “safe zone” identified by Lewinnek, which is 5–25° of anteversion and 30–50° of abduction, are at increased risk for instability and dislocation [5•]. Malpositioned cups, particularly those implanted in excessive abduction, have also been linked to accelerated polyethylene wear [53, 54]. Yet, multiple large retrospective studies have demonstrated that with conventional free-hand techniques, only 60–85% of cups are implanted within this acceptable window [55–57]. In a cadaver study, acetabular components were placed more accurately with the Mako RIO system than by conventional manual reaming [58]. These results are corroborated by clinical case series. Domb et al. found that 100% (50/50) of robotic THA cups were within the Lewinnek safe zone, compared to only 80% (40/50) of conventionally placed cups [59]. Similarly, Elson et al. report that 95% of cups were placed within 3.5° of the intended position [45]. Although few studies have directly compared computer-navigated non-robotic THA to robotic THA, one retrospective review of nearly 2000 THAs showed that both navigated and robotically placed cups were significantly more likely to be within the safe zone than conventional THA, and neither navigated nor robotic THA was superior to the other [60].
Femoral component size and positioning also contribute to the combined anteversion and leg length, which in turn affect hip stability, gait mechanics, and patient satisfaction. Robotic THA has been shown to achieve limb length equality [47].
Despite evidence supporting improved accuracy and fewer outliers with robotic THA, it is not yet clear whether these radiographic benefits translate into improved clinical outcomes. Although some authors have reported better clinical scores among patients with robotic THA at short-term follow-up, these improvements do not persist over longer follow-up periods. Harris hip scores in the original ROBODOC trial were no different between groups at 2 years [48] or at 5–7 years post-op [38].
Nakamura et al. reported a 2-point greater improvement (out of 100 points) in Japanese Orthopedic Association (JOA) clinical scores for the robotic group at 2 and 3 years, JOA scores did not differ at 5 years [47]. In a prospective randomized controlled trial (RCT) comparing ROBODOC and conventional THA through an anterolateral approach, Honl et al. reported better Mayo clinical and Harris hip scores at 12 months, but no difference by 24 months [49]. In the same study, robotic THA was associated with higher dislocation (18%, 11/61) and revision (15%, 3/80) rates, which the authors attribute to abductor damage during robotic milling [49]. Others found that no functional gait impairment when the abductor musculature was protected during ROBODOC surgeries. Other potential technical complications include bone motion during registration or cutting, which requires reregistration, non-displaced femoral shaft fractures requiring cerclage wiring, and milling defects in the acetabulum and greater trochanter [61, 62].
Cost/Value Total Joint Arthroplasty
The introduction of computer navigation and robotic-assisted arthroplasty has been shown to improve precision, improve patient satisfaction scores and lower blood loss, while lowering certain complication rates. Their widespread acceptance, however, will ultimately depend on the cost-effectiveness and value added to joint replacement. Few studies have directly addressed this topic in relation to joint replacement. Moschetti and colleagues performed a Markov decision analysis to determine the costs, outcomes, and incremental cost-effectiveness of robot-assisted UKA [63]. They created a Markov decision model using a low-demand patient population at an age of 65 years. Their model found that case volume has the greatest influence on the cost-effectiveness of the robotic assistance, with a minimum of 94 cases per year (and a revision rate below 1.2%) to become cost-effective over conventional UKA. Further, at this setting, robotic UKA had a slightly better outcome with 0.06 quality-adjusted life-years per case. In another Markov decision model, Novak and colleagues estimated the cost-effectiveness of computer-assisted navigation in total knee arthroplasty using known costs of the technology and procedures and the known outcomes from review of the published literature [64]. They found that computer assistance can be cost-effective if the cost of the technology remained below $629 per case. This decision analysis used current executed revision rates at 15 years and currently published rates of achieving coronal plane alignment within 3° of neutral. Using a Markov model as well, Slover and co-authors found that computer assistance becomes less cost-effective as the volume of cases decreases and as the cost of the technology increases [65]. Further, they found that revision rate must decrease for the technology to be cost-effective. In higher volume centers, the 20-year revision rate would have to decrease by 2% to maintain cost-effectiveness and in lower volume centers (25 arthroplasties per year), the revision rate would have to decrease by 13% to become cost-effective. To the point of reducing revision rates, de Steiger and colleagues did show a reduction in revision rate in the Australian joint registry, as mentioned previously [16].
Not all studies show that technologies in arthroplasty are cost-effective. Burnett and Barrack discuss the cost and complications associated with total knee artrhoplasty computer navigation [19]. They note the increased surgical time, potential need for costly preoperative imaging, the cost of the technology and disposables, and the potential complications unique only to these technologies (pin site infection and fracture).
Summary
In regards to the value added to arthroplasty, there is currently no definitive answer as to how much, or if any, value is added to joint arthroplasty with these newer technologies. Many studies have shown improved outcomes, improved accuracy, and lower revision rates using these technologies. However, the question remains if the extra cost associated with these technologies will keep them viable.
There are many potential benefits to CAS for the future. Beal et al. describe unknown variables that we may be able to elucidate as we use these technologies to solve current problems. Future areas of interest can include teaching, research, rotational alignment of the femur, and surgical documentation opportunities [66].
Better precision and accuracy should relate to longer survival and better outcomes. As technology becomes more mainstream, hopefully, it will become easier and faster to use and at a more neutral cost. It is the authors’ opinion that these technologies are a vital part of the future of orthopaedics.
Compliance with Ethical Standards
Conflict of Interest
Seth Jerabek reports consultancy fees from Stryker, outside of the submitted work. The other authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Quality and Cost Control in TJA
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
- 1.Robertsson O, Dunbar M, Pehrsson T, Knutson K, Lidgren L. Patient satisfaction after knee arthroplasty: a report on 27,372 knees operated on between 1981 and 1995 in Sweden. Acta Orthop Scand. 2000;71:262–267. doi: 10.1080/000164700317411852. [DOI] [PubMed] [Google Scholar]
- 2.Warth LC, Callaghan JJ, Liu SS, Klaassen AL, Goetz DD, Johnston RC. Thirty-five-year results after Charnley total hip arthroplasty in patients less than fifty years old. A concise follow-up of previous reports. J Bone Joint Surg Am. 2014;96:1814–1819. doi: 10.2106/JBJS.M.01573. [DOI] [PubMed] [Google Scholar]
- 3.Knight SR, Aujla R, Biswas SP. Total hip arthroplasty - over 100 years of operative history. Orthop Rev. 2011;3:e16. doi: 10.4081/or.2011.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berend ME, Ritter MA, Meding JB, Faris PM, Keating EM, Redelman R, et al. Tibial component failure mechanisms in total knee arthroplasty. Clin Orthop. 2004;428:26–34. doi: 10.1097/01.blo.0000148578.22729.0e. [DOI] [PubMed] [Google Scholar]
- 5.Lewinnek GE, Lewis JL, Tarr R, Compere CL, Zimmerman JR. Dislocations after total hip-replacement arthroplasties. J Bone Joint Surg Am. 1978;60:217–220. doi: 10.2106/00004623-197860020-00014. [DOI] [PubMed] [Google Scholar]
- 6.Wasielewski RC, Galante JO, Leighty RM, Natarajan RN, Rosenberg AG. Wear patterns on retrieved polyethylene tibial inserts and their relationship to technical considerations during total knee arthroplasty. Clin Orthop. 1994;299:31–43. [PubMed] [Google Scholar]
- 7.Jeffery RS, Morris RW, Denham RA. Coronal alignment after total knee replacement. J Bone Joint Surg Br. 1991;73:709–714. doi: 10.1302/0301-620X.73B5.1894655. [DOI] [PubMed] [Google Scholar]
- 8.Barrack RL, Schrader T, Bertot AJ, Wolfe MW, Myers L. Component rotation and anterior knee pain after total knee arthroplasty. Clin Orthop. 2001;392:46–55. doi: 10.1097/00003086-200111000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Brown MJ, Matthews JR, Bayers-Thering MT, Phillips MJ, Krackow KA. Low incidence of postoperative complications with navigated Total knee arthroplasty. J Arthroplast. 2017; doi:10.1016/j.arth.2017.01.045. [DOI] [PubMed]
- 10.Knee Navigation Application for Arthroplasty Knee3 from Brainlab. Brainlab n.d. https://www.brainlab.com/en/surgery-products/orthopedic-surgery-products/knee-navigation-application/. Accessed 1 April 2017.
- 11.Computer-Assisted Surgery — Exactech, Inc. n.d. https://www.exac.com/products/knee/advanced-surgical-instrumentation. Accessed 1 April 2017.
- 12.Nam D, Jerabek SA, Haughom B, Cross MB, Reinhardt KR, Mayman DJ. Radiographic analysis of a hand-held surgical navigation system for tibial resection in total knee arthroplasty. J Arthroplast. 2011;26:1527–1533. doi: 10.1016/j.arth.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 13.Cip J, Widemschek M, Luegmair M, Sheinkop MB, Benesch T, Martin A. Conventional versus computer-assisted technique for total knee arthroplasty: a minimum of 5-year follow-up of 200 patients in a prospective randomized comparative trial. J Arthroplast. 2014;29:1795–1802. doi: 10.1016/j.arth.2014.04.037. [DOI] [PubMed] [Google Scholar]
- 14.Blakeney WG, Khan RJK, Palmer JL. Functional outcomes following total knee arthroplasty: a randomised trial comparing computer-assisted surgery with conventional techniques. Knee. 2014;21:364–368. doi: 10.1016/j.knee.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Baumbach JA, Willburger R, Haaker R, Dittrich M, Kohler S. 10-year survival of navigated versus conventional TKAs: a retrospective study. Orthopedics. 2016;39:S72–S76. doi: 10.3928/01477447-20160509-21. [DOI] [PubMed] [Google Scholar]
- 16.de Steiger RN, Liu Y-L, Graves SE. Computer navigation for total knee arthroplasty reduces revision rate for patients less than sixty-five years of age. J Bone Joint Surg Am. 2015;97:635–642. doi: 10.2106/JBJS.M.01496. [DOI] [PubMed] [Google Scholar]
- 17.Licini DJ, Meneghini RM. Modern abbreviated computer navigation of the femur reduces blood loss in total knee arthroplasty. J Arthroplast. 2015;30:1729–1732. doi: 10.1016/j.arth.2015.04.020. [DOI] [PubMed] [Google Scholar]
- 18.Moskal JT, Capps SG, Mann JW, Scanelli JA. Navigated versus conventional total knee arthroplasty. J Knee Surg. 2014;27:235–248. doi: 10.1055/s-0033-1360659. [DOI] [PubMed] [Google Scholar]
- 19.Burnett RSJ, Barrack RL. Computer-assisted total knee arthroplasty is currently of no proven clinical benefit: a systematic review. Clin Orthop. 2013;471:264–276. doi: 10.1007/s11999-012-2528-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song EK, Agrawal PR, Kim SK, Seo HY, Seon JK. A randomized controlled clinical and radiological trial about outcomes of navigation-assisted TKA compared to conventional TKA: long-term follow-up. Knee Surg Sports Traumatol Arthrosc Off J ESSKA. 2016;24:3381–3386. doi: 10.1007/s00167-016-3996-2. [DOI] [PubMed] [Google Scholar]
- 21.Song E-K, Seon J-K, Park S-J, Jung WB, Park H-W, Lee GW. Simultaneous bilateral total knee arthroplasty with robotic and conventional techniques: a prospective, randomized study. Knee Surg Sports Traumatol Arthrosc Off J ESSKA. 2011;19:1069–1076. doi: 10.1007/s00167-011-1400-9. [DOI] [PubMed] [Google Scholar]
- 22.Ponder CE, Plaskos C, Cheal EJ. Press-fit Total knee arthroplasty with a robotic-cutting guide: proof of concept and initial clinical experience. Bone Jt J. 2013;95–B:61. [Google Scholar]
- 23.Koenig JA, Suero EM, Plaskos C. Surgical accuracy and efficiency of computer-navigated Tka with a robotic cutting guide – report on the first 100 cases. Orthop Proc. 2012;94–B:103. [Google Scholar]
- 24.Hampp E, Scholl L, Prieto M, Chang T, Abbasi A, Stoker M, et al. Robotic-arm assisted total knee arthroplasty demonstrated greater accuracy to plan compared to manual technique. 2017. [DOI] [PubMed]
- 25.Hampp E, Stoker M, Scholl L, Otto J, Mont M. Robotic-arm assisted total knee arthroplasty demonstrated soft tissue protection. 2017. [PubMed]
- 26.Riddle DL, Jiranek WA, McGlynn FJ. Yearly incidence of unicompartmental knee arthroplasty in the United States. J Arthroplast. 2008;23:408–412. doi: 10.1016/j.arth.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 27.Bell SW, Anthony I, Jones B, MacLean A, Rowe P, Blyth M. Improved accuracy of component positioning with robotic-assisted Unicompartmental knee arthroplasty: data from a prospective, randomized controlled study. J Bone Joint Surg Am. 2016;98:627–635. doi: 10.2106/JBJS.15.00664. [DOI] [PubMed] [Google Scholar]
- 28.Pearle AD, van der List JP, Lee L, Coon TM, Borus TA, Roche MW. Survivorship and patient satisfaction of robotic-assisted medial unicompartmental knee arthroplasty at a minimum two-year follow-up. Knee. 2017;24:419–428. doi: 10.1016/j.knee.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lonner JH, Smith JR, Picard F, Hamlin B, Rowe PJ, Riches PE. High degree of accuracy of a novel image-free handheld robot for unicondylar knee arthroplasty in a cadaveric study. Clin Orthop. 2015;473:206–212. doi: 10.1007/s11999-014-3764-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nam D, Weeks KD, Reinhardt KR, Nawabi DH, Cross MB, Mayman DJ. Accelerometer-based, portable navigation vs imageless, large-console computer-assisted navigation in total knee arthroplasty: a comparison of radiographic results. J Arthroplast. 2013;28:255–261. doi: 10.1016/j.arth.2012.04.023. [DOI] [PubMed] [Google Scholar]
- 31.Steppacher SD, Kowal JH, Murphy SB. Improving cup positioning using a mechanical navigation instrument. Clin Orthop. 2011;469:423–428. doi: 10.1007/s11999-010-1553-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dorr LD, Malik A, Wan Z, Long WT, Harris M. Precision and bias of imageless computer navigation and surgeon estimates for acetabular component position. Clin Orthop. 2007;465:92–99. doi: 10.1097/BLO.0b013e3181560c51. [DOI] [PubMed] [Google Scholar]
- 33.Davis ET, Schubert M, Wegner M, Haimerl M. A new method of registration in navigated hip arthroplasty without the need to register the anterior pelvic plane. J Arthroplast. 2015;30:55–60. doi: 10.1016/j.arth.2014.08.026. [DOI] [PubMed] [Google Scholar]
- 34.Lass R, Kubista B, Olischar B, Frantal S, Windhager R, Giurea A. Total hip arthroplasty using imageless computer-assisted hip navigation: a prospective randomized study. J Arthroplast. 2014;29:786–791. doi: 10.1016/j.arth.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 35.Weber M, Woerner M, Springorum R, Sendtner E, Hapfelmeier A, Grifka J, et al. Fluoroscopy and imageless navigation enable an equivalent reconstruction of leg length and global and femoral offset in THA. Clin Orthop. 2014;472:3150–3158. doi: 10.1007/s11999-014-3740-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ellapparadja P, Mahajan V, Deakin AH, Deep K. Reproduction of hip offset and leg length in navigated Total hip arthroplasty: how accurate are we? J Arthroplast. 2015;30:1002–1007. doi: 10.1016/j.arth.2015.01.027. [DOI] [PubMed] [Google Scholar]
- 37.Renkawitz T, Weber M, Springorum H-R, Sendtner E, Woerner M, Ulm K, et al. Impingement-free range of movement, acetabular component cover and early clinical results comparing “femur-first” navigation and “conventional” minimally invasive total hip arthroplasty: a randomised controlled trial. Bone Jt J. 2015;97–B:890–898. doi: 10.1302/0301-620X.97B7.34729. [DOI] [PubMed] [Google Scholar]
- 38.Keshmiri A, Schröter C, Weber M, Craiovan B, Grifka J, Renkawitz T. No difference in clinical outcome, bone density and polyethylene wear 5-7 years after standard navigated vs. conventional cementfree total hip arthroplasty. Arch Orthop Trauma Surg. 2015;135:723–730. doi: 10.1007/s00402-015-2201-2. [DOI] [PubMed] [Google Scholar]
- 39.Stiehl JB, Heck DA, Jaramaz B, Amiot L-P. Comparison of fluoroscopic and imageless registration in surgical navigation of the acetabular component. Comput Aided Surg Off J Int Soc Comput Aided Surg. 2007;12:116–124. doi: 10.3109/10929080701292939. [DOI] [PubMed] [Google Scholar]
- 40.Kalteis T, Handel M, Bäthis H, Perlick L, Tingart M, Grifka J. Imageless navigation for insertion of the acetabular component in total hip arthroplasty: is it as accurate as CT-based navigation? J Bone Joint Surg Br. 2006;88:163–167. doi: 10.1302/0301-620X.88B2.17163. [DOI] [PubMed] [Google Scholar]
- 41.Digioia AM, Jaramaz B, Plakseychuk AY, Moody JE, Nikou C, Labarca RS, et al. Comparison of a mechanical acetabular alignment guide with computer placement of the socket. J Arthroplast. 2002;17:359–364. doi: 10.1054/arth.2002.30411. [DOI] [PubMed] [Google Scholar]
- 42.Parratte S, Argenson J-NA. Validation and usefulness of a computer-assisted cup-positioning system in total hip arthroplasty. A prospective, randomized, controlled study. J Bone Joint Surg Am. 2007;89:494–499. doi: 10.2106/JBJS.F.00529. [DOI] [PubMed] [Google Scholar]
- 43.Sugano N, Takao M, Sakai T, Nishii T, Miki H. Does CT-based navigation improve the long-term survival in ceramic-on-ceramic THA? Clin Orthop. 2012;470:3054–3059. doi: 10.1007/s11999-012-2378-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beckmann J, Stengel D, Tingart M, Götz J, Grifka J, Lüring C. Navigated cup implantation in hip arthroplasty. Acta Orthop. 2009;80:538–544. doi: 10.3109/17453670903350073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Elson L, Dounchis J, Illgen R, Marchand RC, Padgett DE, Bragdon CR, et al. Precision of acetabular cup placement in robotic integrated total hip arthroplasty. Hip Int J Clin Exp Res Hip Pathol Ther. 2015;25:531–536. doi: 10.5301/hipint.5000289. [DOI] [PubMed] [Google Scholar]
- 46.Bargar WL. Robots in orthopaedic surgery: past, present, and future. Clin Orthop. 2007;463:31–36. [PubMed] [Google Scholar]
- 47.Nakamura N, Sugano N, Nishii T, Kakimoto A, Miki H. A comparison between robotic-assisted and manual implantation of Cementless Total hip arthroplasty. Clin Orthop. 2010;468:1072–1081. doi: 10.1007/s11999-009-1158-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bargar WL, Bauer A, Börner M. Primary and revision total hip replacement using the Robodoc system. Clin Orthop. 1998:82–91. [DOI] [PubMed]
- 49.Honl M, Dierk O, Gauck C, Carrero V, Lampe F, Dries S, et al. Comparison of robotic-assisted and manual implantation of a primary total hip replacement. A prospective study. J Bone Joint Surg Am. 2003;85–A:1470–1478. doi: 10.2106/00004623-200308000-00007. [DOI] [PubMed] [Google Scholar]
- 50.Spencer EH. The ROBODOC clinical trial: a robotic assistant for total hip arthroplasty. Orthop Nurs. 1996;15:9–14. doi: 10.1097/00006416-199601000-00003. [DOI] [PubMed] [Google Scholar]
- 51.Nishihara S, Sugano N, Nishii T, Miki H, Nakamura N, Yoshikawa H. Comparison between hand rasping and robotic milling for stem implantation in cementless total hip arthroplasty. J Arthroplast. 2006;21:957–966. doi: 10.1016/j.arth.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 52.Hananouchi T, Sugano N, Nishii T, Nakamura N, Miki H, Kakimoto A, et al. Effect of robotic milling on periprosthetic bone remodeling. J Orthop Res Off Publ Orthop Res Soc. 2007;25:1062–1069. doi: 10.1002/jor.20376. [DOI] [PubMed] [Google Scholar]
- 53.Gallo J, Havranek V, Zapletalova J. Risk factors for accelerated polyethylene wear and osteolysis in ABG I total hip arthroplasty. Int Orthop. 2010;34:19–26. doi: 10.1007/s00264-009-0731-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leslie IJ, Williams S, Isaac G, Ingham E, Fisher J. High cup angle and microseparation increase the wear of hip surface replacements. Clin Orthop. 2009;467:2259–2265. doi: 10.1007/s11999-009-0830-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Callanan MC, Jarrett B, Bragdon CR, Zurakowski D, Rubash HE, Freiberg AA, et al. The John Charnley award: risk factors for cup malpositioning: quality improvement through a joint registry at a tertiary hospital. Clin Orthop. 2011;469:319–329. doi: 10.1007/s11999-010-1487-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bosker BH, Verheyen CCPM, Horstmann WG, Tulp NJA. Poor accuracy of freehand cup positioning during total hip arthroplasty. Arch Orthop Trauma Surg. 2007;127:375–379. doi: 10.1007/s00402-007-0294-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Leichtle U, Gosselke N, Wirth CJ, Rudert M. Radiologic evaluation of cup placement variation in conventional total hip arthroplasty. ROFO Fortschr Geb Rontgenstr Nuklearmed. 2007;179:46–52. doi: 10.1055/s-2006-927085. [DOI] [PubMed] [Google Scholar]
- 58.Nawabi DH, Conditt MA, Ranawat AS, Dunbar NJ, Jones J, Banks S, et al. Haptically guided robotic technology in total hip arthroplasty: a cadaveric investigation. Proc Inst Mech Eng [H] 2013;227:302–309. doi: 10.1177/0954411912468540. [DOI] [PubMed] [Google Scholar]
- 59.Domb BG, El Bitar YF, Sadik AY, Stake CE, Botser IB. Comparison of robotic-assisted and conventional acetabular cup placement in THA: a matched-pair controlled study. Clin Orthop. 2014;472:329–336. doi: 10.1007/s11999-013-3253-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Domb BG, Redmond JM, Louis SS, Alden KJ, Daley RJ, LaReau JM, et al. Accuracy of component positioning in 1980 Total hip arthroplasties: a comparative analysis by surgical technique and mode of guidance. J Arthroplast. 2015;30:2208–2218. doi: 10.1016/j.arth.2015.06.059. [DOI] [PubMed] [Google Scholar]
- 61.Schulz AP, Seide K, Queitsch C, von Haugwitz A, Meiners J, Kienast B, et al. Results of total hip replacement using the Robodoc surgical assistant system: clinical outcome and evaluation of complications for 97 procedures. Int J Med Robot Comput Assist Surg MRCAS. 2007;3:301–306. doi: 10.1002/rcs.161. [DOI] [PubMed] [Google Scholar]
- 62.Chun YS, Kim KI, Cho YJ, Kim YH, Yoo MC, Rhyu KH. Causes and patterns of aborting a robot-assisted arthroplasty. J Arthroplast. 2011;26:621–625. doi: 10.1016/j.arth.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 63.Moschetti WE, Konopka JF, Rubash HE, Genuario JW. Can robot-assisted Unicompartmental knee arthroplasty be cost-effective? A Markov decision analysis. J Arthroplast. 2016;31:759–765. doi: 10.1016/j.arth.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 64.Novak EJ, Silverstein MD, Bozic KJ. The cost-effectiveness of computer-assisted navigation in total knee arthroplasty. J Bone Joint Surg Am. 2007;89:2389–2397. doi: 10.2106/JBJS.F.01109. [DOI] [PubMed] [Google Scholar]
- 65.Slover JD, Tosteson ANA, Bozic KJ, Rubash HE, Malchau H. Impact of hospital volume on the economic value of computer navigation for total knee replacement. J Bone Joint Surg Am. 2008;90:1492–1500. doi: 10.2106/JBJS.G.00888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Beal MD, Delagramaticas D, Fitz D. Improving outcomes in total knee arthroplasty-do navigation or customized implants have a role? J Orthop Surg. 2016;11:60. doi: 10.1186/s13018-016-0396-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


