Abstract
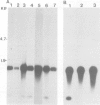
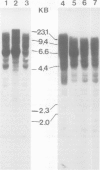
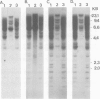
A human X chromosome-derived gene sequence which recognises an abundant, 1.2-kb mRNA in several cell types was previously isolated during a study to identify expressed sequences from an X chromosome recombinant library. Further characterisation of this clone, acronym OA1, has shown that it maps to the short arm of the X, at Xp21 to Xp22. A 777-bp fragment of the clone which hybridizes to the 1.2-kb mRNA has been sequenced, and the inferred amino acid sequence shows 80% homology with the published protein sequence for human muscle glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The fragment shows even higher homology (87%) with pig muscle GAPDH. The OA1 clone selects an mRNA which translates in vitro into a polypeptide of 36 K, the subunit size of GAPDH. However, the X-sequence is most probably a pseudogene whose structure is consistent with it having arisen by reverse transcription of a GAPDH or GAPDH-related mRNA followed by insertion into the X chromosome. The GAPDH-related portion of OA1 hybridizes to several DNA fragments in human and mouse DNA, and six fibroblast cDNA clones which cross-hybridize to OA1 identify the same genomic fragments as OA1. This series of clones identifies a new, conserved GAPDH-related multigene family.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. W., Bronson D. L., Benham F., Strickland S., Knowles B. B. A comparative study of eight cell lines derived from human testicular teratocarcinoma. Int J Cancer. 1980 Sep 15;26(3):269–280. doi: 10.1002/ijc.2910260304. [DOI] [PubMed] [Google Scholar]
- Beaudet A. L., Su T. S., O'Brien W. E., D'Eustachio P., Barker P. E., Ruddle F. H. Dispersion of argininosuccinate-synthetase-like human genes to multiple autosomes and the X chromosome. Cell. 1982 Aug;30(1):287–293. doi: 10.1016/0092-8674(82)90034-4. [DOI] [PubMed] [Google Scholar]
- Bruns G. A., Gerald P. S. Human glyceraldehyde-3-phosphate dehydrogenase in man-rodent somatic cell hybrids. Science. 1976 Apr 2;192(4234):54–56. doi: 10.1126/science.176725. [DOI] [PubMed] [Google Scholar]
- Chen M. J., Shimada T., Moulton A. D., Harrison M., Nienhuis A. W. Intronless human dihydrofolate reductase genes are derived from processed RNA molecules. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7435–7439. doi: 10.1073/pnas.79.23.7435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cory R. P., Wold F. Isolation and characterization of enolase from rainbow trout (Salmo gairdnerii gairdnerii). Biochemistry. 1966 Oct;5(10):3131–3137. doi: 10.1021/bi00874a008. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jelinek W. R., Molloy G. R. Biogenesis of mRNA: genetic regulation in mammalian cells. Science. 1973 Sep 28;181(4106):1215–1221. doi: 10.1126/science.181.4106.1215. [DOI] [PubMed] [Google Scholar]
- Deininger P. L., Jolly D. J., Rubin C. M., Friedmann T., Schmid C. W. Base sequence studies of 300 nucleotide renatured repeated human DNA clones. J Mol Biol. 1981 Sep 5;151(1):17–33. doi: 10.1016/0022-2836(81)90219-9. [DOI] [PubMed] [Google Scholar]
- Edwards Y. H., Clark P., Harris H. Isozymes of glyceraldehyde-3-phosphate dehydrogenase in man and other mammals. Ann Hum Genet. 1976 Jul;40(1):67–77. doi: 10.1111/j.1469-1809.1976.tb00165.x. [DOI] [PubMed] [Google Scholar]
- Goodfellow P., Banting G., Levy R., Povey S., McMichael A. A human X-linked antigen defined by a monoclonal antibody. Somatic Cell Genet. 1980 Nov;6(6):777–787. doi: 10.1007/BF01538976. [DOI] [PubMed] [Google Scholar]
- Grossbard L., Schimke R. T. Multiple hexokinases of rat tissues. Purification and comparison of soluble forms. J Biol Chem. 1966 Aug 10;241(15):3546–3560. [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J. I., Perham R. N. Glyceraldehyde 3-phosphate dehydrogenase from pig muscle. Nature. 1968 Sep 7;219(5158):1025–1028. doi: 10.1038/2191025a0. [DOI] [PubMed] [Google Scholar]
- Holland M. J., Holland J. P., Jackson K. A. Cloning of yeast genes coding for glycolytic enzymes. Methods Enzymol. 1979;68:408–419. doi: 10.1016/0076-6879(79)68030-8. [DOI] [PubMed] [Google Scholar]
- Hollis G. F., Hieter P. A., McBride O. W., Swan D., Leder P. Processed genes: a dispersed human immunoglobulin gene bearing evidence of RNA-type processing. Nature. 1982 Mar 25;296(5855):321–325. doi: 10.1038/296321a0. [DOI] [PubMed] [Google Scholar]
- Hope R. M., Goodfellow P. N., Solomon E., Bodmer W. F. Identification of MIC5, a human X-linked gene controlling expression of a cell surface antigen: definition by a monoclonal antibody raised against a human X mouse somatic cell hybrid. Cytogenet Cell Genet. 1982;33(3):204–212. doi: 10.1159/000131756. [DOI] [PubMed] [Google Scholar]
- Jakob H., Boon T., Gaillard J., Nicolas J., Jacob F. Tératocarcinome de la spuris: isolement, culture et propriétés de cellules a potentialités multiples. Ann Microbiol (Paris) 1973 Oct;124(3):269–282. [PubMed] [Google Scholar]
- Jeffreys A. J., Flavell R. A. A physical map of the DNA regions flanking the rabbit beta-globin gene. Cell. 1977 Oct;12(2):429–439. doi: 10.1016/0092-8674(77)90119-2. [DOI] [PubMed] [Google Scholar]
- Klebe R. J., Chen T., Ruddle F. H. Controlled production of proliferating somatic cell hybrids. J Cell Biol. 1970 Apr;45(1):74–82. doi: 10.1083/jcb.45.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lemischka I., Sharp P. A. The sequences of an expressed rat alpha-tubulin gene and a pseudogene with an inserted repetitive element. Nature. 1982 Nov 25;300(5890):330–335. doi: 10.1038/300330a0. [DOI] [PubMed] [Google Scholar]
- MacLeod A. R., Talbot K. A processed gene defining a gene family encoding a human non-muscle tropomyosin. J Mol Biol. 1983 Jul 5;167(3):523–537. doi: 10.1016/s0022-2836(83)80096-5. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Minowada J., Onuma T., Moore G. E. Rosette-forming human lymphoid cell lines. I. Establishment and evidence for origin of thymus-derived lymphocytes. J Natl Cancer Inst. 1972 Sep;49(3):891–895. [PubMed] [Google Scholar]
- Musti A. M., Zehner Z., Bostian K. A., Paterson B. M., Kramer R. A. Transcriptional mapping of two yeast genes coding for glyceraldehyde 3-phosphate dehydrogenase isolated by sequence homology with the chicken gene. Gene. 1983 Nov;25(1):133–143. doi: 10.1016/0378-1119(83)90175-0. [DOI] [PubMed] [Google Scholar]
- Nabholz M., Miggiano V., Bodmer W. Genetic analysis with human--mouse somatic cell hybrids. Nature. 1969 Jul 26;223(5204):358–363. doi: 10.1038/223358a0. [DOI] [PubMed] [Google Scholar]
- Nowak K., Wolny M., Banaś T. The complete amino acid sequence of human muscle glyceraldehyde 3-phosphate dehydrogenase. FEBS Lett. 1981 Nov 16;134(2):143–146. doi: 10.1016/0014-5793(81)80587-x. [DOI] [PubMed] [Google Scholar]
- O'Brien S. J., Nash W. G., Goodwin J. L., Lowy D. R., Chang E. H. Dispersion of the ras family of transforming genes to four different chromosomes in man. Nature. 1983 Apr 28;302(5911):839–842. doi: 10.1038/302839a0. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Penhoet E., Rajkumar T., Rutter W. J. Multiple forms of fructose diphosphate aldolase in mammalian tissues. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1275–1282. doi: 10.1073/pnas.56.4.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploegh H. L., Orr H. T., Strominger J. L. Molecular cloning of a human histocompatibility antigen cDNA fragment. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6081–6085. doi: 10.1073/pnas.77.10.6081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povey S., Gardiner S. E., Watson B., Mowbray S., Harris H., Arthur E., Steel C. M., Blenkinsop C., Evans H. J. Genetic studies on human lymphoblastoid lines: isozyme analysis on cell lines from forty-one different individuals and on mutants produced following exposure to a chemical mutagen. Ann Hum Genet. 1973 Jan;36(3):247–266. doi: 10.1111/j.1469-1809.1973.tb00588.x. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid C. W., Jelinek W. R. The Alu family of dispersed repetitive sequences. Science. 1982 Jun 4;216(4550):1065–1070. doi: 10.1126/science.6281889. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilbur W. J., Lipman D. J. Rapid similarity searches of nucleic acid and protein data banks. Proc Natl Acad Sci U S A. 1983 Feb;80(3):726–730. doi: 10.1073/pnas.80.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde C. D., Crowther C. E., Cripe T. P., Gwo-Shu Lee M., Cowan N. J. Evidence that a human beta-tubulin pseudogene is derived from its corresponding mRNA. Nature. 1982 May 6;297(5861):83–84. doi: 10.1038/297083a0. [DOI] [PubMed] [Google Scholar]
- Woods D., Crampton J., Clarke B., Williamson R. The construction of a recombinant cDNA library representative of the poly(A)+ mRNA population from normal human lymphocytes. Nucleic Acids Res. 1980 Nov 25;8(22):5157–5168. doi: 10.1093/nar/8.22.5157. [DOI] [PMC free article] [PubMed] [Google Scholar]