Abstract
Purpose of Review
The purpose of this review is to provide an update to the most objective, evidence-based path through a non-operative course of rehabilitation after anterior cruciate ligament injury for those hoping to return to pivoting and cutting sports.
Recent Findings
Anterior cruciate ligament (ACL) injuries are prevalent in pivoting and cutting athletes with many of these patients electing to pursue surgical reconstruction in hopes of returning to prior levels of function. Despite many athletes pursing ACL reconstruction, some may elect to pursue a non-operative course of care. Success with this treatment plan should be defined as the ability to return to sport without subsequent giving way episodes.
Summary
Identification of those most likely to successfully return to sport with a non-operative course begins with completion of an evidence-based screening tool. If the patient has no concomitant injury and successfully passes the screening, they may proceed to a systematic, evidence-based progression through rehabilitation. Finally, the patient must complete a return to sport program and meet appropriate objective criteria, prior to return to sport.
Keywords: ACL deficient, Non-operative management, Perturbation training, Screening, Return to sport
Introduction
Annually, over 200,000 anterior cruciate ligament (ACL) injuries occur in the USA [1] with the majority of these patients electing to undergo ACL reconstruction (ACLR). Historically, ACL reconstruction was executed to attempt to restore normal joint arthrokinematics, improve the patient’s potential to return to sport, and decrease the likelihood of post-traumatic osteoarthritis. However, recent evidence suggests higher than previously reported second ACL injury rates [2, 3], lower return to sport rates [4], and a high incidence of OA, despite ACL reconstruction [5]. In addition, a recent systematic review indicates there is weak evidence to suggest superiority of ACL reconstruction over conservative management [6••]. Therefore, some patients elect to not undergo ACL reconstruction, with a percentage of them successfully able to return to some level of function with non-operative management. Although less prevalent in the USA, non-operative management of ACL injury may be an appropriate treatment option for a percentage of patients after ACL rupture.
The concept of differential patient presentation after ACL injury was first discussed by Noyes et al. in 1983 [7]. These authors introduced the “rule of thirds,” which hypothesized as many as 1/3 of patients with ACL deficiency could function well with some level of pivoting and cutting activity without having functional instability or “giving way.” As such, this group had the potential to function without an ACL reconstruction to restore mechanical stability. The authors further theorized the remaining 2/3 of patients with ACL deficiency would either function well with only Activity of Daily Living Scale (ADLS) necessitating some level of activity modification or they would be unable to functional at all with ACL deficiency due to instability. These patients would likely be indicated for surgical reconstruction. Essentially, these authors identified that a continuum of functional instability may exist in patients after ACL injury, ranging from grossly unstable to functionally stable with no giving way. Although identifying the possibility that some patients may be able to function without ACL reconstruction, this original work from Noyes et al. [7] failed to present a method to identify patients likely to need surgical reconstruction prior to an attempt to return to activity.
Screening to Identify Potential Copers
The theory of potential copers has advanced through a series of studies out of the University of Delaware over the last two decades [8]. This work proposed a comprehensive screening tool to identify patients likely to be able to successfully return to pivoting and cutting activity without an ACL reconstruction. These patients were subsequently labeled as “potential copers,” due to their potential to successfully resume activity while remaining ACL deficient. Unfortunately, no single assessment tool has been successful at identifying this subset of the ACL-deficient patient population who has potential to succeed with a non-operative course. This necessitated a more dynamic screening tool [9••]. The screening tool proposed by Fitzgerald et al. [8] included four one-legged hop tests previously identified by Noyes et al. (single leg hop for distance, single leg triple hop, single leg triple cross over hop, and the 6-m timed hop test [10]), the incidence of knee giving way, a self-report functional survey (Knee outcome survey-Activity of Daily Living Scale—KOS-ADLS [11]), and a self-report global knee function rating [8]. Patients who presented without concomitant injuries who achieved a minimum score of 80% limb symmetry on all hop testing, >80% on the KOS-ADLS, >60 on the self-report of knee function, and ≤1 subjective report of knee giving way were considered “potential copers” [8]. If patients with ACL deficiency successfully passed the screening tool, they were given the opportunity to pursue a non-operative plan of care after ACL injury. Failure to achieve all of these factors resulted in the patient being identified as a “non-coper” and surgery was recommended.
Outcomes
Hurd et al. [12••] published a 10-year outcome study on patients undergoing this screening to participate in non-operative care. These authors reported 54% of patients with an ACL injury were not eligible to participate in the screening due to concomitant injury, such as meniscal injury, chondral damage, other ligamentous injuries, or other factors [12••]. Of the remaining 46% of patients with ACL deficiency, less than half (20% of the population) were labeled as potential copers and even fewer successfully completed rehabilitation and were able to return to activity without surgical reconstruction [12••]. Therefore, the current evidence suggests a small percentage of patients can successfully return to pivoting and cutting sports, in the absence of an intact ACL. If a patient decides to pursue non-operative management in hopes of returning to pivoting and cutting sports, he/she should either participate in the successful completion of a rigorous screening tool, such as the one developed by Fitzgerald et al. [8], or have a discussion about activity modification away from pivoting and cutting activities [13••].
Non-operative Treatment: Activity Modification
One option for patients electing to pursue non-operative treatment following ACL injury is a commitment to activity modification. Prior focus in this population has been on patients who plan to return to level I/II pivoting and cutting activities [14]. These types of activities typically include sports such as football, basketball, soccer, and skiing, which require a high level of dynamic stability to successfully participate [13••]. Patients who live a more sedentary lifestyle have less physically demanding occupations, or who chose to modify activity to only participate in primarily straight-line sporting activities such as jogging and cycling have a greater likelihood of succeeding with a non-operative course. In addition, patients with no concomitant injuries to the knee, further compromising joint stability and joint health, are typically the best candidates to succeed with this treatment option. If patients elect to modify their activity, their post-operative rehabilitation is focused on addressing post-acute injury impairments, maximizing strength, and insuring the patient is able to participate in their desired activities without episodes of functional instability or giving way.
Non-operative Treatment: “Potential Copers” Returning to Pivoting and Cutting Sports
Patients who sustain an isolated ACL injury, successfully pass the screening tool, and wish to return to pivoting and cutting sports must participate in a systematic rehabilitation progression to address impairments, insure functional stability, and determine readiness to safely return to sport. This requires participation in a phased progression initially focused on residual impairments and stability and ultimately linking to a successful, dynamic return to sport progression.
Acute Phase
The acute phase of non-operative rehabilitation after ACL injury in patients identified as potential copers is focused on targeting residual acute symptoms and impairments from the injury. Patients with ACL injury typically present with a significant acute hemarthrosis, loss of motion, acute weakness in the involved extremity, and reflex inhibition of the quadriceps femoris musculature. Utilization of therapeutic exercise and appropriate modalities during the acute phase are essential to address these impairments.
Aggressive management of the acute hemarthrosis is essential to facilitate normal quadriceps contraction. Reflex inhibition of the quadriceps musculature with secondary atrophy is a known complication with knee joint effusion [15]. Cryotherapy and compression are utilized at this time to help manage the acute effusion. If the patient is limited in their ability to actively contract the quadriceps musculature, neuromuscular electric stimulation (NMES) may be recommended at this phase of treatment to help facilitate a normal quadriceps contraction [9••]. (Fig. 1) Recommended parameters to optimize the use of NMES to improve quadriceps strength and activation after ACL injury have been reported in the literature [16, 17].
Fig. 1.
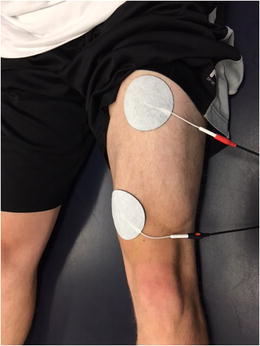
Neuromuscular electric stimulation (NMES) is an appropriate intervention to enhance quadriceps muscle activation and strengthening after ACL injury
Therapeutic interventions at this phase are often focused on restoration of full range of motion and key foundational strength deficits which must be addressed prior to implementing more dynamic interventions. The most common of these is quadriceps musculature weakness. Residual weakness in the quadriceps muscle that persists through the advanced stages of rehabilitation (which focuses on dynamic closed kinetic chain tasks, plyometric and proprioceptive activities) may result in the development of abnormal compensatory movement patterns or residual instability. Therefore, initiating dynamic quadriceps muscle activation and strengthening interventions at this time is appropriate. Typically, a combination of both open kinetic chain (OKC) activities to address residual isolated quadriceps weakness as well as closed kinetic chain (CKC) exercises to strengthen the quadriceps muscle while dynamically incorporating lower extremity movement is appropriate. The utilization of both OKC and CKC activities is critical as OKC quadriceps strengthening may be more effective at addressing the isolated weakness [18] (Fig. 2), while CKC strengthening may help incorporate developing quadriceps strength into dynamic movements (Figs. 3 and 4). OKC knee extension in an ACL-deficient knee should be limited to an arc of motion from 100° of flexion to 30° of flexion. This limited range, protecting against full extension, will reduce anterior shearing seen at the end range seen in the absence of a knee with an intact ACL [19].
Fig. 2.
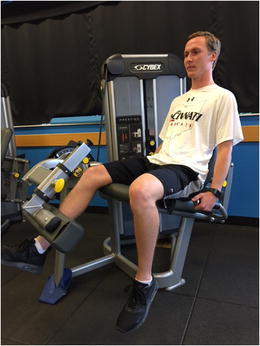
Open kinetic chain quadriceps strengthening on a leg extension machine executed in a protected range of motion of 100° of flexion to 30° of flexion. Permission obtained from subject
Fig. 3.
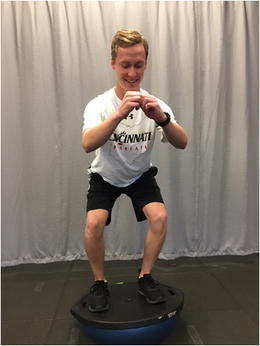
Closed kinetic chain quadriceps strengthening: mini-squatting on an unstable surface. Permission obtained from subject
Fig. 4.

Closed kinetic chain quadriceps strengthening: single leg step down. Permission obtained from subject
Other important foundational strength deficits to begin to address in the acute phase of rehabilitation include hamstring, hip, and core weakness. Hamstrings serve as agonists to the ACL as they resist anterior translation of the tibia when contracting. Restoring an appropriate quadriceps to hamstrings strength ratio and avoiding a quadriceps-dominant leg [20] will assist in providing additional dynamic stability to the knee. Deficits in hip and core strength are believed to be associated with decreased trunk control. Alterations in trunk control are believed to be risk factors for ACL injury [21], subsequently placing the knee at risk for further injury. Beginning to address potential impairments in these areas acutely may help facilitate more normal movement patterns over time.
Neuromuscular Training Phase
Progression to the neuromuscular training phase of rehabilitation occurs when the patient has achieved full range of motion, resolution of effusion, and sufficient lower extremity strength to participate in more dynamic weight-bearing exercises. During this phase, the patient continues to focus on maximizing lower extremity and core strength, but now can progress to participate in additional advanced balance, proprioception, cardiovascular conditioning, and neuromuscular interventions.
One specific type of neuromuscular training designed specifically to improve knee stability in patients with ACL deficiency is perturbation training [9••, 22, 23]. Developed by investigators at the University of Delaware, perturbation training is designed to challenge the patient with ACL deficiency through a series of balance tasks enhanced with unanticipated perturbations to the unstable surface. Classically described as a 10-session program, the patient begins with stance on a rocker board and then progresses to a roller board (Fig. 5). While standing on the unstable surfaces, progressive perturbations are applied, challenging the patient to maintain balance, enhancing dynamic knee stability. Ideally, the task is designed to assist in the development of individualized patterns of muscle contraction, rather than global co-contraction, to facilitate dynamic knee stability [9••]. Multiple studies investigating the effects of perturbation training in the ACL-deficient population have reported improved knee kinematics [24], improved gait mechanics [25], and a reduction in episodes of giving way [26]. Although the greatest body of evidence has been published using the University of Delaware perturbation training program with ACL-deficient athletes, other neuromuscular training programs may also be effective, however they have yet to be investigated in a rigorous fashion.
Fig. 5.
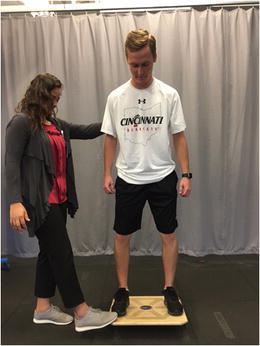
Example of perturbation training on a rocker board with perturbation applied by the physical therapist. Permission obtained from subjects
Prior to progression to the return to sport phase of rehabilitation, the patient must demonstrate a successful completion of the neuromuscular training phase of rehabilitation with no episodes of giving way. In addition, the patient must present with sufficient quadriceps and hamstring strength, as demonstrated by isokinetic strength symmetry of >90% compared to the contralateral limb. Once these factors are achieved, the patient is ready to progress to the final phase of rehabilitation.
Return to Sport Phase
The final phase of rehabilitation prior to returning to pivoting and cutting sports is focused on a sports-specific re-integration into the desired level of activity. Patients returning to dynamic pivoting and cutting, who are ACL deficient, are recommended to utilize a functional performance brace to enhance stability. Although the mechanism by which the brace enhances stability is not clear, some data suggests marginal reduction in anterior tibial translation and enhanced proprioception, both of which may contribute to a feeling of stability by the patient [13••, 27]. Patients who plan to integrate into dynamic sports should be gradually introduced to these activities.
A dynamic incorporation of agility and sports-specific training should focus on the introduction of high-speed change of direction tasks seen in sport [28]. Agility tasks should begin in a straight line at sub-maximal speed and then be progressed with increased intensity of movement and more dynamic directions. Ultimately, full speed maneuvers in three planes of movement should be executed and mastered. These activities can then be progressed into sport-specific tasks based on the individual needs of the patient. For example, soccer players can incorporate ball handling, agility, and shooting drills into their return to sport program until mastery is achieved. Finally, the progression of load experienced during the injured state should be gradually advanced during this phase to insure a safe return to sport. Recent evidence from Blanch and Gabbett suggests that athletes returning too quickly from an injury are at increased risk for future injury [29]. Appropriately progressing the intensity of the rehabilitation program as well as a complimentary cardiovascular conditioning program is critical to reduce future injury risk.
Return to Sport Decision Making
Prior to medical release to return to sport, each athlete should successfully complete a return to sport assessment. This assessment must objectively analyze lower extremity strength, functional movement patterns, and psychological readiness to return to sport. Isokinetic assessment of quadriceps and hamstring strength as well as functional hop testing introduced in the initial screening process should reveal limb symmetry of >90% [30]. There is no general consensus in the literature regarding the optimal tool to assess movement symmetry; however, tools such as the tuck jump assessment and single leg squat assessment may be used to screen for movement asymmetries with dynamic tasks [31]. Finally, recent evidence has evaluated the psychological readiness to return to sport after ACL reconstruction [32]. Although not validated in an ACL-deficient population, some screening of a patient’s psychological readiness to return to sport may be appropriate in this population.
Conclusion
Although ACL reconstruction is the most prevalent treatment for ACL deficiency, a subset of the population may benefit from a non-operative course of care. Patients who plan to modify activity as well as those who plan to continue to participate in pivoting and cutting sports may be candidates for non-operative care if they are able to successfully complete a rigorous screening process. Careful and objective identification of those patients most likely to succeed with non-operative care after ACL injury is the first step. Once identified, completion of a systematic progression of rehabilitation focused on resolution of impairments, participation in neuromuscular training, and successful execution of a return to sport program greatly enhance the likelihood of successful non-operative management in this population.
Compliance with Ethical Standards
Conflict of Interest
The author declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors. Additional informed consent was obtained from all individual participants for whom identifying information is included in this article.
Footnotes
This article is part of the Topical Collection on ACL Rehab
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
- 1.Gottlob CA, Baker CL., Jr Anterior cruciate ligament reconstruction: socioeconomic issues and cost effectiveness. Am J Orthop (Belle Mead NJ) 2000;29:472–476. [PubMed] [Google Scholar]
- 2.Wright RW, Magnussen RA, Dunn WR, Spindler KP. Ipsilateral graft and contralateral ACL rupture at five years or more following ACL reconstruction: a systematic review. J Bone Joint Surg Am. 2011;93:1159–1165. doi: 10.2106/JBJS.J.00898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paterno MV, Rauh MJ, Schmitt LC, Ford KR, Hewett TE. Incidence of second ACL injuries 2 years after primary ACL reconstruction and return to sport. Am J Sports Med. 2014;42:1567–1573. doi: 10.1177/0363546514530088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ardern CL, Webster KE, Taylor NF, Feller JA. Return to sport following anterior cruciate ligament reconstruction surgery: a systematic review and meta-analysis of the state of play. Br J Sports Med. 2011;45:596–606. doi: 10.1136/bjsm.2010.076364. [DOI] [PubMed] [Google Scholar]
- 5.Lohmander LS, Ostenberg A, Englund M, Roos H. High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis Rheum. 2004;50:3145–3152. doi: 10.1002/art.20589. [DOI] [PubMed] [Google Scholar]
- 6.•• Smith TO, Postle K, Penny F, McNamara I, Mann CJ. Is reconstruction the best management strategy for anterior cruciate ligament rupture? A systematic review and meta-analysis comparing anterior cruciate ligament reconstruction versus non-operative treatment. Knee. 2014;21:462–70. Systematic review and meta analysis comparing operative and non-operative management of ACL injury. [DOI] [PubMed]
- 7.Noyes FR, Butler DL, Paulos LE, Grood ES. Intra-articular cruciate reconstruction. I: Perspectives on graft strength, vascularization, and immediate motion after replacement. Clin Orthop Relat Res. 1983;71–7. [PubMed]
- 8.Fitzgerald GK, Axe MJ, Snyder-Mackler L. A decision-making scheme for returning patients to high-level activity with nonoperative treatment after anterior cruciate ligament rupture. Knee Surg Sports Traumatol Arthrosc. 2000;8:76–82. doi: 10.1007/s001670050190. [DOI] [PubMed] [Google Scholar]
- 9.•• Hurd W, Axe M, Snyder-Mackler L. Management of the athlete with acute anterior cruciate ligament deficiency. Sports health. 2009;1:39–46. This is a review article highlighting evidence based management of the athlete with ACL deficiency. [DOI] [PMC free article] [PubMed]
- 10.Barber SD, Noyes FR, Mangine RE, McCloskey JW, Hartman W. Quantitative assessment of functional limitations in normal and anterior cruciate ligament-deficient knees. Clin Orthop. 1990;204–14. [PubMed]
- 11.Irrgang JJ, Snyder-Mackler L, Wainner RS, Fu FH, Harner CD. Development of a patient-reported measure of function of the knee. J Bone Joint Surg Am. 1998;80:1132–1145. doi: 10.2106/00004623-199808000-00006. [DOI] [PubMed] [Google Scholar]
- 12.•• Hurd WJ, Axe MJ, Snyder-Mackler L. A 10-year prospective trial of a patient management algorithm and screening examination for highly active individuals with anterior cruciate ligament injury: Part 1, outcomes. Am J Sports Med. 2008;36:40–7. This is a 10 year outcome study of patients who have pursued non-operative management of ACL injury. [DOI] [PMC free article] [PubMed]
- 13.•• Bogunovic L, Matava MJ. Operative and nonoperative treatment options for ACL tears in the adult patient: a conceptual review. Phys Sportsmed. 2013;41:33–40. This is a clinical update of both operative and non-operative management of ACl injury in the athletic population. [DOI] [PubMed]
- 14.Daniel DM, Stone ML, Dobson BE, Fithian DC, Rossman DJ, Kaufman KR. Fate of the ACL-injured patient. A prospective outcome study. Am J Sports Med. 1994;22:632–644. doi: 10.1177/036354659402200511. [DOI] [PubMed] [Google Scholar]
- 15.Kennedy JC, Alexander IJ, Hayes KC. Nerve supply of the human knee and its functional importance. Am J Sports Med. 1982;10:329–335. doi: 10.1177/036354658201000601. [DOI] [PubMed] [Google Scholar]
- 16.Snyder-Mackler L, Delitto A, Bailey SL, Stralka SW. Strength of the quadriceps femoris muscle and functional recovery after reconstruction of the anterior cruciate ligament. A prospective, randomized clinical trial of electrical stimulation. J Bone Joint Surg Am. 1995;77:1166–1173. doi: 10.2106/00004623-199508000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Snyder-Mackler L, Ladin Z, Schepsis AA, Young JC. Electrical stimulation of the thigh muscles after reconstruction of the anterior cruciate ligament. Effects of electrically elicited contraction of the quadriceps femoris and hamstring muscles on gait and on strength of the thigh muscles. J Bone Joint Surg Am. 1991;73:1025–1036. doi: 10.2106/00004623-199173070-00010. [DOI] [PubMed] [Google Scholar]
- 18.Tagesson S, Oberg B, Good L, Kvist J. A comprehensive rehabilitation program with quadriceps strengthening in closed versus open kinetic chain exercise in patients with anterior cruciate ligament deficiency: a randomized clinical trial evaluating dynamic tibial translation and muscle function. Am J Sports Med. 2008;36:298–307. doi: 10.1177/0363546507307867. [DOI] [PubMed] [Google Scholar]
- 19.Beynnon BD, Fleming BC, Johnson RJ, Nichols CE, Renstrom PA, Pope MH. Anterior cruciate ligament strain behavior during rehabilitation exercises in vivo. Am J Sports Med. 1995;23:24–34. doi: 10.1177/036354659502300105. [DOI] [PubMed] [Google Scholar]
- 20.Hewett TE, Paterno MV, Myer GD. Strategies for enhancing proprioception and neuromuscular control of the knee. Clin Orthop Relat Res. 2002:76–94. [DOI] [PubMed]
- 21.Hewett TE, Torg JS, Boden BP. Video analysis of trunk and knee motion during non-contact anterior cruciate ligament injury in female athletes: lateral trunk and knee abduction motion are combined components of the injury mechanism. Br J Sports Med. 2009;43:417–422. doi: 10.1136/bjsm.2009.059162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chmielewski TL, Hurd WJ, Rudolph KS, Axe MJ, Snyder-Mackler L. Perturbation training improves knee kinematics and reduces muscle co-contraction after complete unilateral anterior cruciate ligament rupture. Phys Ther. 2005;85:740–749. [PubMed] [Google Scholar]
- 23.Chmielewski TL, Rudolph KS, Snyder-Mackler L. Development of dynamic knee stability after acute ACL injury. J Electromyogr Kinesiol. 2002;12:267–274. doi: 10.1016/S1050-6411(02)00013-5. [DOI] [PubMed] [Google Scholar]
- 24.Di Stasi SL, Hartigan EH, Snyder-Mackler L. Unilateral stance strategies of athletes with ACL deficiency. J Appl Biomech. 2012;28:374–386. doi: 10.1123/jab.28.4.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Di Stasi SL, Snyder-Mackler L. The effects of neuromuscular training on the gait patterns of ACL-deficient men and women. Clin Biomech (Bristol, Avon) 2012;27:360–365. doi: 10.1016/j.clinbiomech.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fitzgerald GK, Axe MJ, Snyder-Mackler L. The efficacy of perturbation training in nonoperative anterior cruciate ligament rehabilitation programs for physical active individuals. Phys Ther. 2000;80:128–140. [PubMed] [Google Scholar]
- 27.Beynnon BD, Fleming BC, Churchill DL, Brown D. The effect of anterior cruciate ligament deficiency and functional bracing on translation of the tibia relative to the femur during nonweightbearing and weightbearing. Am J Sports Med. 2003;31:99–105. doi: 10.1177/03635465030310012801. [DOI] [PubMed] [Google Scholar]
- 28.Fitzgerald GK, Axe MJ, Snyder-Mackler L. Proposed practice guidelines for nonoperative anterior cruciate ligament rehabilitation of physically active individuals. J Orthop Sports Phys Ther. 2000;30:194–203. doi: 10.2519/jospt.2000.30.4.194. [DOI] [PubMed] [Google Scholar]
- 29.Blanch P, Gabbett TJ. Has the athlete trained enough to return to play safely? The acute:chronic workload ratio permits clinicians to quantify a player’s risk of subsequent injury. Br J Sports Med. 2016;50:471–475. doi: 10.1136/bjsports-2015-095445. [DOI] [PubMed] [Google Scholar]
- 30.Schmitt LC, Paterno MV, Ford KR, Myer GD, Hewett TE. Strength asymmetry and landing mechanics at return to sport after anterior cruciate ligament reconstruction. Med Sci Sports Exerc. 2015;47:1426–1434. doi: 10.1249/MSS.0000000000000560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Myer GD, Ford KR, Hewett TE. Tuck jump assessment for reducing anterior cruciate ligament injury risk. Athl Ther Today. 2008;13:39–44. doi: 10.1123/att.13.5.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Webster KE, Feller JA, Lambros C. Development and preliminary validation of a scale to measure the psychological impact of returning to sport following anterior cruciate ligament reconstruction surgery. Physical therapy in sport: official journal of the Association of Chartered Physiotherapists in Sports Medicine. 2008;9:9–15. doi: 10.1016/j.ptsp.2007.09.003. [DOI] [PubMed] [Google Scholar]


