Abstract
Purpose
The purpose of this study was to compare the characteristics of single- and dual-species in vitro oral biofilms made by static and dynamic methods.
Methods
Hydroxyapatite (HA) disks, 12.7 mm in diameter and 3 mm thick, were coated with processed saliva for 4 hours. The disks were divided into a static method group and a dynamic method group. The disks treated with a static method were cultured in 12-well plates, and the disks in the dynamic method group were cultured in a Center for Disease Control and Prevention (CDC) biofilm reactor for 72 hours. In the single- and dual-species biofilms, Fusobacterium nucleatum and Porphyromonas gingivalis were used, and the amount of adhering bacteria, proportions of species, and bacterial reduction of chlorhexidine were examined. Bacterial adhesion was examined with scanning electron microscopy (SEM) and confocal laser scanning microscopy (CLSM).
Results
Compared with the biofilms made using the static method, the biofilms made using the dynamic method had significantly lower amounts of adhering and looser bacterial accumulation in SEM and CLSM images. The proportion of P. gingivalis was higher in the dynamic method group than in the static method group; however, the difference was not statistically significant. Furthermore, the biofilm thickness and bacterial reduction by chlorhexidine showed no significant differences between the 2 methods.
Conclusions
When used to reproduce periodontal biofilms composed of F. nucleatum and P. gingivalis, the dynamic method (CDC biofilm reactor) formed looser biofilms containing fewer bacteria than the well plate. However, this difference did not influence the thickness of the biofilms or the activity of chlorhexidine. Therefore, both methods are useful for mimicking periodontitis-associated oral biofilms.
Keywords: Bacterial adhesion, Biofilms, Chlorhexidine, Electron microscope tomography, Periodontitis
Graphical Abstract
INTRODUCTION
Most surfaces of the human body (skin, respiratory, and gastrointestinal mucosa and the oral cavity) provide a unique environment for microorganisms and are colonized by bacteria [1,2]. More specifically, dental plaques in the oral cavity are dynamic microbial biofilms composed of hundreds of bacterial species and extracellular matrices [3]. These biofilms contain commensal and pathogenic microorganisms. Common oral diseases, dental caries, and periodontitis are influenced by imbalances between biofilms and host defenses.
The initial process in periodontal biofilm formation starts with pellicle, derived from saliva, covering the tooth surface within a few minutes after mechanical cleaning. This acquired pellicle is composed of many molecules and plays a major role in the development and maintenance of bacterial communities. Then, a complex microbial community develops in a few days, and its components can be divided into 2 categories [4]. Early colonizers that directly adhere to the pellicles are predominantly streptococci and Actinomyces spp. [5]. These species are mostly Gram-positive and have minor pathogenic effects on periodontal tissue. Late colonizers, such as Fusobacterium nucleatum, Porphyromonas gingivalis, Tannerella forsythia, Treponema denticola, and Aggregatibacter actinomycetemcomitans, tend to be more pathogenic than early colonizers.
Studies on in vivo periodontitis-associated oral biofilms are difficult because the composition of biofilms can be altered by host or site specificity, and many ethical considerations are associated with in vivo microbiological studies in humans [6,7]. Therefore, several studies have tried to develop an in vitro periodontitis-associated oral biofilm model [7,8,9]. One study by Park et al. [8] described 4 species in a periodontitis-associated oral biofilm model. They developed an in vitro biofilm model on glass slips in 12-well plates. Walker and Sedlacek [9] developed an in vitro subgingival biofilm model using 6- or 12-well plates. These studies reported successful biofilm formation; however, the biofilms were formed in static conditions. Due to the lack of media flow, bacteria in these biofilms may not have adhered, but just settled down.
In the oral cavity, biofilms are formed in dynamic conditions by saliva and gingival crevicular fluid flow, among other factors. Bacterial adherence may vary according to the shear forces of fluid flow. Thomas et al. [11] showed that FimH expressed by Escherichia coli attached to erythrocytes more strongly in shear conditions than in static conditions. They suggested that shear conditions increased adhesion-receptor bond formations, which was demonstrated for rolling leukocytes on adhesive surfaces. Ding et al. [12] demonstrated that shear conditions enhanced the adhesion of Streptococcus gordonii; however, this phenomenon was not observed for oral Actinomyces oris and Actinomyces naeslundii.
The Center for Disease Control and Prevention (CDC) biofilm reactor (BioSurface Technologies Corporation, Bozeman, MT, USA) was developed for modeling dynamic conditions. This reactor generates shear conditions with a baffled stir bar that is magnetically driven and continuously pumps a supply of nutrients into and out of the reactor at a constant rate [13]. We expected that this reactor would be able to reproduce an oral environment and mimic periodontitis-associated oral biofilms.
We hypothesized that biofilms formed in shear conditions, such as an oral cavity, would differ from those formed in static conditions. Several studies have generated reproducible in vitro oral biofilm models using dynamic methods [7,14,15]; nevertheless, very few of these studies have provided comparisons between biofilms formed by static and dynamic methods. Therefore, the purpose of this study was to compare the characteristics of periodontal pathogenic biofilms made by the static method (well plate) and the dynamic method (CDC biofilm reactor).
MATERIALS AND METHODS
Bacterial strains and culture conditions
The bacterial strains used for the biofilm formation assay were F. nucleatum ATCC 23726 and P. gingivalis ATCC 33277, which were obtained from the culture collection at the Department of Oral Microbiology, College of Dentistry, Gangneung-Wonju National University. All bacteria were grown in prepared trypticase soy broth (Becton, Dickinson and Company, Sparks, MD, USA) containing 1 mg/mL of yeast extract (Becton, Dickinson and Company), 5 μg/mL of hemin (Sigma Chemical Co., St. Louis, MO, USA), and 1 μg/mL of menadione (Sigma Chemical Co.), under anaerobic conditions (90% N2, 5% CO2, and 5% H2) (Bactron Anaerobic Chamber, Sheldon Manufacturing Inc., Cornelius, OR, USA) at 37°C.
Saliva preparation
Whole, stimulated saliva was obtained over 30 minutes from 4 systemically healthy male volunteers without periodontal disease or any other oral diseases (age range, 26–28 years old). All volunteers were given a thorough explanation of this study and signed an informed consent form. The study design was approved by the Institutional Review Board of Gangneung-Wonju National University Dental Hospital according to the World Medical Association Declaration of Helsinki (IRB No. 2015-012).
To obtain stimulated saliva, volunteers masticated dental paraffin wax and spit out the first sample to remove debris attached to the oral surfaces. Then, stimulated saliva was collected in sterile 50 mL polypropylene tubes and frozen at −20°C until the total amount of collected saliva was 500 mL. All saliva was obtained during stimulation and mixed for homogenization and centrifuged (27,000 ×g) for 30 minutes at 4°C. The supernatant was pasteurized for 30 minutes at 60°C to inactivate endogenous enzymes and re-centrifuged (27,000 ×g) for 30 minutes at 4°C [10].
The resulting supernatant was filtered twice by vacuum filtration (Vacuum Filter System, Corning, NY, USA) with pore sizes of 0.45 and 0.22 μm, respectively, and stored at −20°C. We assessed the remaining bacteria by plating the processed saliva on blood agar plates and incubating them for 72 hours at 37°C in anaerobic conditions (90% N2, 5% CO2, and 5% H2). No bacterial colonies were observed on the plates.
Preparation of biofilms
To generate biofilm formation, hydroxyapatite (HA) disks (BioSurface Technologies Corporation), 12.7 mm in diameter and 3 mm thick, were sterilized by autoclaving (121°C, 1 hour). The sterilized disks were placed in their own well on a 24-well polystyrene cell culture plate (SPL Life Sciences, Pocheon, Korea) and incubated with 500 μL of processed saliva for 4 hours (gently shaken, at room temperature) to form an acquired pellicle [10].
After salivary pellicle formation, disks were divided into the static method group or the dynamic method group. Each group was divided into 3 subgroups: 1) F. nucleatum single-species biofilm, 2) P. gingivalis single-species biofilm, and 3) dual-species biofilm (Table 1). We prepared 12 disks for each group, for a total of 72 disks.
Table 1. Disk characteristics for each group.
| Parameters | Static method (well plate) | Dynamic method (CDC biofilm reactor) | |
|---|---|---|---|
| Single-species biofilm | F. nucleatum | 12 | 12 |
| P. gingivalis | 12 | 12 | |
| Dual-species biofilm | Total | 12 | 12 |
CDC: Center for Disease Control and Prevention.
The disks treated with the static method were transferred to a 12-well cell culture plate. Each species (F. nucleatum and P. gingivalis) was diluted with fresh liquid medium to an optical density at 550 nm (OD550) of 0.15, and then 3 mL of a bacterial solution was added to each well containing the disks. The dual-species biofilms had 1.5 mL of each species solution added to each well. The 12-well culture plates were incubated for 72 hours at 37°C in anaerobic conditions (90% N2, 5% CO2, and 5% H2).
The disks in the dynamic method group were mounted on rods that fit into the CDC biofilm reactor. After transferring the disks into the CDC biofilm reactor, the reactor was filled with 350 mL of the bacterial solution using a bacterial concentration the same optical density (OD) as the static method. The dual-species biofilms had 175 mL of each bacterial suspension added to the reactor. A magnetic stir plate continuously mixed the fluid with a stirring rate of 50 rpm. The CDC biofilm reactor was incubated for 72 hours at 37°C in anaerobic conditions (90% N2, 5% CO2, and 5% H2).
To assess the total number of colony forming units (CFUs), each disk (n=5) was rinsed twice with 1 mL of phosphate-buffered saline (PBS) to remove any unattached bacteria and then was transferred into a sterile glass test tube containing 3 mL of PBS and glass beads. The glass test tube was vortexed vigorously for 1 minute to detach the bacteria. The sample was serially diluted with PBS, and aliquots (50 μL) of the sample were spirally plated (Eddy Jet spiral plater, IUL, S.A., Barcelona, Spain) onto trypticase soy agar (TSA) plates containing 1 mg/mL of yeast extract (Becton, Dickinson and Company), 5 μg/mL of hemin (Sigma Chemical Co.), 1 μg/mL of menadione (Sigma Chemical Co.), 5% sheep blood (Hanil-KOMED, Sungnam, Korea) and 1.5% Bacto Agar (Becton, Dickinson and Company). Total CFUs were counted by an automatic colony counter (Flash & Go colony counter, IUL, S.A.). In the dual-species samples, the species were distinguished by their colony morphology.
Scanning electron microscopy (SEM)
SEM was performed to observe biofilm conditions. An additional disk from each group was prepared to generate the specimen. The disks with biofilm formation were fixed in a 4% glutaraldehyde and paraformaldehyde solution in 0.1 M cacodylate buffer (pH 7.4) for 3 hours. Then, the fixed samples were then washed 3 times with 0.1 M cacodylate buffer for 10 minutes and dehydrated for 30 minutes in a graded series of ethanol. For SEM observation, ethanol was replaced by isoamyl acetate, and after reaching the critical point of drying, the samples were mounted on a stub and coated with gold. The biofilm surfaces were observed with variable-pressure field emission SEM (SUPRA55VP, Carl Zeiss, Oberkochen, Germany).
Confocal laser scanning microscopy (CLSM)
CLSM (FV300, Olympus, Tokyo, Japan) was performed to observe the attached bacteria and determine the thickness of the biofilm. Additional disks from each group were stained with the LIVE/DEAD® BacLight™ Bacterial Viability Kit solution (Molecular Probes, Inc., Eugene, OR, USA) according to the manufacturer's instructions.
An argon laser (485±14 nm) was used as the excitation source for the reagents. Emitted fluorescent light was collected in 2 separate emission filters at 500 nm (SYTO 9; green-fluorescent nucleic acid stain) and 635 nm (propidium iodide; red-fluorescent nucleic acid stain). The collected images were analyzed by an image-processing program (FluoView 5.0, Olympus) to assess the depth of the biofilm matrices.
Bacterial reduction by chlorhexidine
Bacterial reduction by chlorhexidine was tested using 0.1% chlorhexidine digluconate solution (Bukwang Pharmaceutical Co. Ltd., Seoul, Korea), which was used to treat the biofilms from the static method group and the dynamic method group. The disks from each group (n=5) were gently washed with 1 mL of PBS to remove non-adherent bacteria and culture medium. Then, each disk was placed into a well in a 24-well plate containing 1 mL of chlorhexidine solution for 1 minute and rinsed with 1 mL of PBS. The disks were vortexed, and samples were plated on TSA plates, as previously discussed.
Statistical analysis
Data were analyzed using SPSS version 21.0 (IBM Corp., Armonk, NY, USA). One-way analysis of variance was conducted to calculate significant differences between the mean log CFU/mL and the bacterial reduction percentage by chlorhexidine in each group, using the Duncan test for post hoc analysis. Any differences in species proportions among the groups were analyzed using the Student t-test. The level of significance was set at P<0.05.
RESULTS
Number and composition of bacteria in biofilms
The mean values of log CFU/disk are presented in Table 2. In the single-species biofilms, the mean log CFU/disk of biofilms in the dynamic method group was lower than in the static method group, and the difference between the methods was statistically significant for same-species biofilms (P<0.05). In terms of inter-species comparison, the log CFU/disk values of single-species biofilms grown with the same method were not significantly different. In the dual-species group, biofilms from the dynamic method group had lower bacterial counts than those of the static method group, and these differences were statistically significant.
Table 2. Number of bacteria in each biofilm.
| Parameters | Log CFU/disk | ||
|---|---|---|---|
| Static method | Dynamic method | ||
| Single-species biofilm | F. nucleatum | 7.35±0.08a) | 6.56±0.09c) |
| P. gingivalis | 7.48±0.12a) | 6.41±0.05c) | |
| Dual-species biofilm | Total | 6.92±0.40b) | 6.39±0.27c) |
Values are presented as mean±SD.
CFU: colony forming unit, SD: standard deviation.
a-c)Different superscript letters indicate values that are significantly different (P<0.05).
Comparisons between the total bacterial colony counts among single-species biofilms and dual-species biofilms showed no significant difference within the dynamic method group. In the static method group, however, there was a significant difference between the mean log CFU/disk for single-species biofilms vs. dual-species biofilms. Dual-species biofilms had lower bacterial counts than the single-species biofilms.
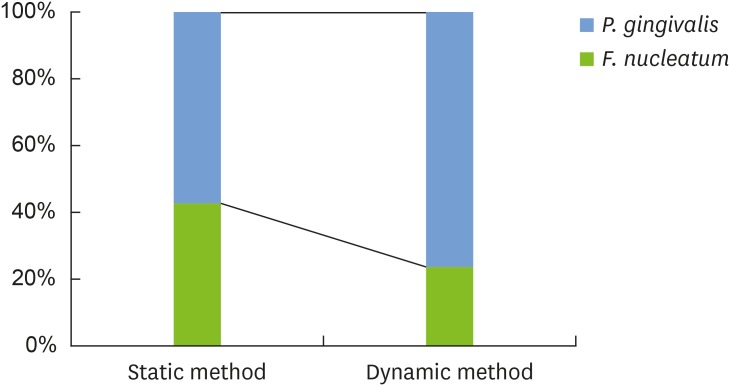
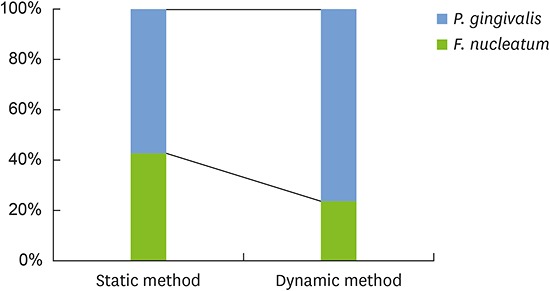
Figure 1 shows the percentage of each species in the dual-species biofilms according to the method used. Biofilms generated using different methods contained different percentages of each species. Biofilms from the static method group had similar mean percentages for F. nucleatum (42.5%±25.7%) and P. gingivalis (57.5%±25.7%). In biofilms from the dynamic method group, P. gingivalis (76.5%±19.4%) comprised a larger proportion than F. nucleatum (23.5%±19.4%). Nevertheless, no statistically significant difference was observed in the composition of each species within the 2 groups of biofilms (P=0.227).
Figure 1.
Bacterial composition of the dual-species biofilms. P. gingivalis comprised a larger proportion than F. nucleatum, and this difference was more pronounced in the dynamic method group than in the static method group. No statistically significant differences were observed in the composition of each species within the 2 groups of biofilms.
SEM
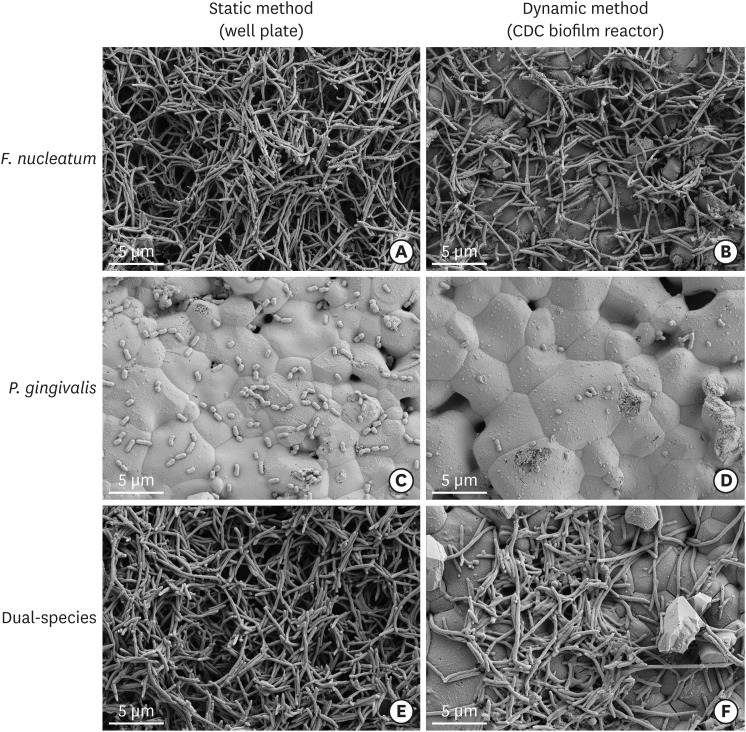
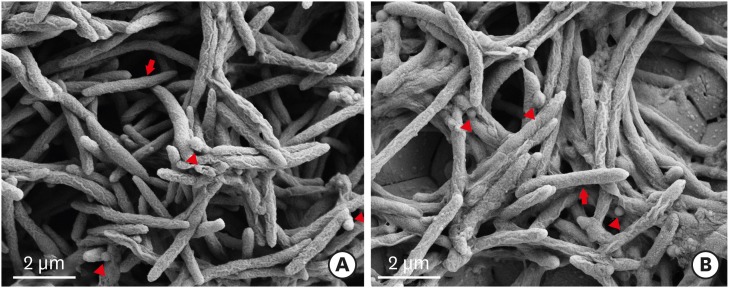
SEM images of the biofilms are shown in Figure 2. In the single-species groups, disks from both the static group and the dynamic group were covered by F. nucleatum (Figure 2A and B), however, P. gingivalis less observed on the disks from both the static group and the dynamic group (Figure 2C and D). In the dual-species groups, the biofilms in the static method group showed a denser accumulation of F. nucleatum than was observed in the dynamic method group (Figure 2E and F). Similar to the F. nucleatum single-species biofilms, images of the P. gingivalis monoculture model showed that more bacteria were attached to the disk surface in static conditions than in dynamic conditions. In contrast to the F. nucleatum biofilms, colonies of P. gingivalis did not cover the surfaces of the HA disks. HA disks from each group were coated with dual-species biofilms. The biofilms grown in the CDC biofilm reactor were less attached to the well plate than the biofilms grown in static conditions. Higher magnification (×30,000) showed that the dual-species biofilms in both static and dynamic conditions had P. gingivalis caught up in F. nucleatum (Figure 3).
Figure 2.
Scanning electron microscopic images of biofilm formation on HA disks (original magnification: ×10,000). In the single-species groups, disks from both the static group and the dynamic group were covered by F. nucleatum, instead of P. gingivalis (A-D). However, in the dual-species groups, the biofilms from the static method group showed a denser accumulation of F. nucleatum than those from the dynamic method group (E, F).
HA: hydroxyapatite, CDC: Center for Disease Control and Prevention.
Figure 3.
Dual-species biofilms with higher magnification (original magnification: ×30,000). (A) Biofilm in well plate (static method). (B) Biofilm in CDC biofilm reactor (dynamic method). The arrow indicate F. nucleatum and the arrowhead indicate P. gingivalis.
CDC: Center for Disease Control and Prevention.
CLSM
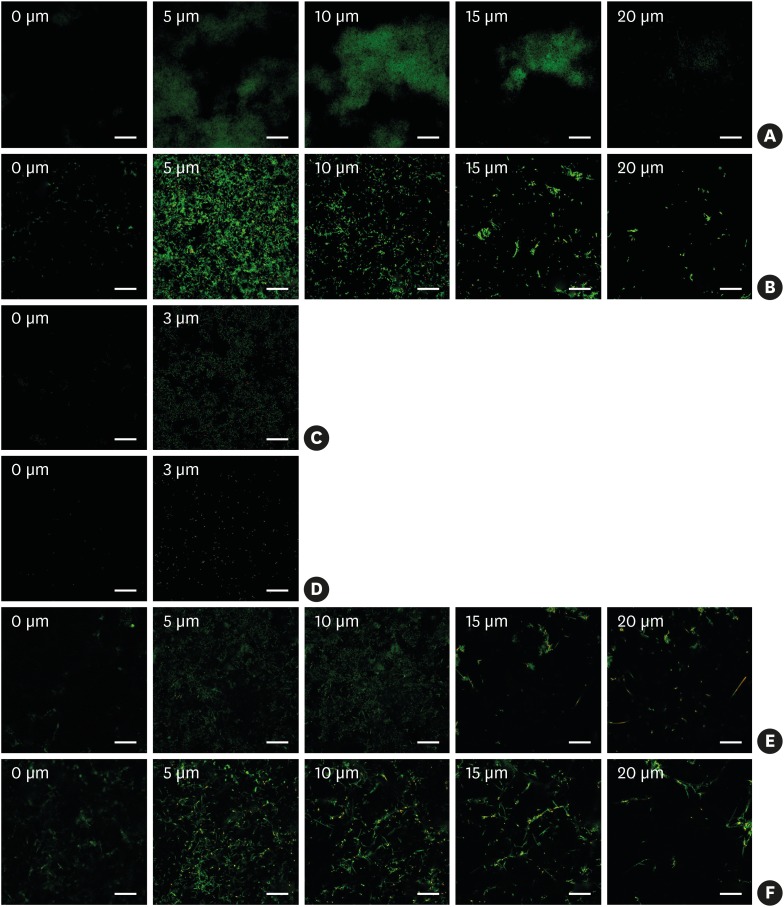
Figure 4 shows a CLSM image series of biofilm depths. The left side of the series represents the biofilm bottom and the right side of each series represents the top of the biofilm. In every species, the thickness was not significantly different between methods, although more bacteria were found in the static method group than in the dynamic method group. The F. nucleatum biofilms and dual-species biofilms were approximately 20 μm thick. Single-species biofilms from P. gingivalis were very thin and formed monolayers.
Figure 4.
Confocal laser scanning microscope images of biofilm formation on HA disks (Scale bar=50 µm). The values in each image represent the distance from the surface of the HA disks. (A) F. nucleatum biofilm in a well plate. (B) F. nucleatum biofilm in the CDC biofilm reactor. (C) P. gingivalis biofilm in a well plate. (D) P. gingivalis biofilm in the CDC biofilm reactor. (E) Dual-species biofilm in a well plate. (F) Dual-species biofilm in the CDC biofilm reactor.
HA: hydroxyapatite, CDC: Center for Disease Control and Prevention.
Amount of bacterial reduction by chlorhexidine
When compared with biofilms grown in well plates, the biofilms grown in the CDC biofilm reactor showed a similar extent of bacterial reduction by chlorhexidine. No significant differences were found between the groups. Chlorhexidine was able to reduce over 90% of the viable bacteria in both biofilms (Table 3).
Table 3. Bacterial reduction by chlorhexidine.
| Parameters | Bacterial reduction (%) | ||
|---|---|---|---|
| Static method | Dynamic method | ||
| Single-species biofilm | F. nucleatum | 95.0±2.7 | 98.9±0.5 |
| P. gingivalis | 96.0±3.2 | 97.0±2.4 | |
| Dual-species biofilm | Total | 92.5±9.1 | 94.7±6.0 |
Values are presented as mean±SD. There were no significant differences between groups (P>0.05).
SD: standard deviation.
DISCUSSION
In this study, we attempted to generate periodontitis-associated oral biofilms using static and dynamic methods, and evaluated the difference between the formation methods. We used F. nucleatum and P. gingivalis to reproduce a biofilm model. As previously mentioned, although there are hundreds of bacterial species in the oral cavity, several specific species have been found to play a crucial role in biofilm formation and to be closely associated with periodontal diseases. Specific to the late pathogenic colonizers, F. nucleatum was included in this experiment because it is located between early and other late colonizers, functioning as a bridge between the organisms [4]. This species is the most predominant Gram-negative anaerobic bacterium in periodontal biofilms from healthy gingiva and it becomes more common with periodontitis progression. P. gingivalis is a major periodontal pathogen and a late colonizer in periodontal biofilms [16]. The cytotoxic products of P. gingivalis damage periodontal tissue directly and indirectly.
Before initiating the experiments, we needed to confirm that the aforementioned bacteria could coaggregate with the other species. Coaggregation is essential for multispecies biofilms, and Okuda et al. [17] claimed that coaggregation was the key factor in the formation of dual-species biofilms of F. nucleatum and Prevotella species. We observed large coaggregates of F. nucleatum and P. gingivalis that left moderate turbidity in the fluid (data not shown). In light of these findings, we used both periodontal pathogens and observed that the 2 methods could develop dual-species biofilms.
However, in regard to total bacterial count, we observed significant differences between the 2 methods. Biofilms grown in the CDC biofilm reactor contained less bacteria than those grown in the well plate; therefore, we suggest that the dynamic condition negatively influenced the adherence of bacteria. Fonseca and Sousa [18] noted that shear strength had a negative effect on the adherence of Pseudomonas aeruginosa in the presence of antibiotics, and Cotter et al. [19] identified a negative correlation between rotational speed and Staphylococcus epidermidis biofilm quantities. Our report agrees with their results, even though their reports were not about periodontal pathogens.
In comparison with single-species biofilms, the bacteria in the dual-species biofilms formed in both methods were not more abundant. These data did not agree with those of Saito et al. [20], who demonstrated that P. gingivalis had synergistic effects on biofilm formation and enhanced biofilm formation by F. nucleatum. The differences between these results might be due to the following reasons. The previous studies cultured biofilms for 2 days, while we cultured our biofilm formation samples for approximately 3 days. In their study, OD was used, but in our study, we assessed bacterial count using the plating method. OD estimates both live and dead cells, whereas the plating method only evaluates viable cells.
The average proportion of P. gingivalis in dual-species biofilms was higher for the dynamic method group than for the static method group. P. gingivalis was found to be the major periodontal pathogen, with elevated levels in periodontitis patients, while F. nucleatum was found to be the abundant pathogen in the supra-gingival plaque and remained relatively stable between various pocket depths [21,22]. Therefore, we should consider using the dynamic method model to represent biofilms in deep periodontal pockets. However, due to the wide variety of proportions, no significant differences were found between the 2 methods. Future studies should be conducted with more specimens.
The SEM and CLSM images of P. gingivalis single-species biofilms showed that they formed single-layer structures, not oral biofilms, regardless of shear forces. Unlike P. gingivalis single-species biofilms, F. nucleatum single-species biofilms and dual-species biofilms were organized into multiple layers of bacteria, and their thicknesses were 20 μm. This may be attributed to the rod shape of F. nucleatum. In summary, the species that affected biofilm thickness was F. nucleatum, not P. gingivalis.
In this study, chlorhexidine was used to test the biofilms. However, no significant difference in viable bacterial reduction was observed between the groups. This result might be related to the presence of thinner biofilms that chlorhexidine could penetrate more easily. Additionally, biofilm maturation might have influenced this result. Similarly to the study of Blanc et al. [7], the maturation of biofilms in dynamic conditions was completed in 4 days, and Shaddox et al. [23] demonstrated that biofilm growth reached a steady state after 7 days. In our study, the incubation time was 3 days for biofilm formation. The thickness and antiseptic resistance of the biofilms may have been influenced by the incubation time and maturation. Therefore, biofilm maturation at different incubation times should be considered in future studies.
In this study, HA disks were used as a substitution for human tooth sections. HA disks are ready-made, easy to obtain, and more homogenous than human tooth or bovine enamel. In several previous studies [24,25,26], HA disks were successfully used for biofilm formation, and Pratten et al. [27] claimed that the bacterial adhesion in HA and enamel were similar. Furthermore, the HA disks in our study were coated by saliva to ensure pellicle formation, and any effects due to the differences between HA disks and tooth sections were negligible.
The main limitation of this study was the absence of initial colonizers, such as S. gordonii or Streptococcus sanguinis. These streptococci bind to salivary pellicle [28,29,30] and provide binding sites for F. nucleatum and P. gingivalis [31,32,33]. In this work, however, we excluded streptococci because their excessive growth inhibited F. nucleatum and P. gingivalis in our pilot study. In future studies, we should consider an experimental design that contains these initial colonizers.
Within the limitations of this study, the dynamic oral biofilm model was found to result in significantly fewer bacteria in all species biofilms than the static model. However, there were no significant differences between the methods in terms of biofilm thickness and the bacterial reduction of each biofilm by chlorhexidine. Therefore, both methods were useful to reproduce an oral biofilm model as a laboratory biofilm model system.
Footnotes
Funding: This study was supported by a faculty research grant of Gangneung-Wonju National University for 2015.
Author Contributions: Conceptualization: Jae-Kwan Lee, Si Young Lee, Heung-Sik Um, Beom-Seok Chang; Formal analysis: Won sub Song, Si Young Lee, Jae-Kwan Lee; Investigation: Won sub Song, Se Hwan Park, Si Young Lee, Heung-Sik Um, Beom-Seok Chang, Jae-Kwan Lee; Methodology: Won sub Song, Si Young Lee, Jae-Kwan Lee; Project administration: Si Young Lee, Heung-Sik Um, Beom-Seok Chang, Jae-Kwan Lee; Writing - original draft: Won sub Song, Se Hwan Park, Jae-Kwan Lee; Writing - review & editing: Se Hwan Park, Si Young Lee, Heung-Sik Um, Beom-Seok Chang, Jae-Kwan Lee.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 2.Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol. 2004;2:95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- 3.Filoche S, Wong L, Sissons CH. Oral biofilms: emerging concepts in microbial ecology. J Dent Res. 2010;89:8–18. doi: 10.1177/0022034509351812. [DOI] [PubMed] [Google Scholar]
- 4.Kolenbrander PE, Andersen RN, Blehert DS, Egland PG, Foster JS, Palmer RJ., Jr Communication among oral bacteria. Microbiol Mol Biol Rev. 2002;66:486–505. doi: 10.1128/MMBR.66.3.486-505.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ximénez-Fyvie LA, Haffajee AD, Socransky SS. Comparison of the microbiota of supra- and subgingival plaque in health and periodontitis. J Clin Periodontol. 2000;27:648–657. doi: 10.1034/j.1600-051x.2000.027009648.x. [DOI] [PubMed] [Google Scholar]
- 6.Paster BJ, Boches SK, Galvin JL, Ericson RE, Lau CN, Levanos VA, et al. Bacterial diversity in human subgingival plaque. J Bacteriol. 2001;183:3770–3783. doi: 10.1128/JB.183.12.3770-3783.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blanc V, Isabal S, Sánchez MC, Llama-Palacios A, Herrera D, Sanz M, et al. Characterization and application of a flow system for in vitro multispecies oral biofilm formation. J Periodontal Res. 2014;49:323–332. doi: 10.1111/jre.12110. [DOI] [PubMed] [Google Scholar]
- 8.Park JH, Lee JK, Um HS, Chang BS, Lee SY. A periodontitis-associated multispecies model of an oral biofilm. J Periodontal Implant Sci. 2014;44:79–84. doi: 10.5051/jpis.2014.44.2.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walker C, Sedlacek MJ. An in vitro biofilm model of subgingival plaque. Oral Microbiol Immunol. 2007;22:152–161. doi: 10.1111/j.1399-302X.2007.00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guggenheim B, Giertsen E, Schüpbach P, Shapiro S. Validation of an in vitro biofilm model of supragingival plaque. J Dent Res. 2001;80:363–370. doi: 10.1177/00220345010800011201. [DOI] [PubMed] [Google Scholar]
- 11.Thomas WE, Trintchina E, Forero M, Vogel V, Sokurenko EV. Bacterial adhesion to target cells enhanced by shear force. Cell. 2002;109:913–923. doi: 10.1016/s0092-8674(02)00796-1. [DOI] [PubMed] [Google Scholar]
- 12.Ding AM, Palmer RJ, Jr, Cisar JO, Kolenbrander PE. Shear-enhanced oral microbial adhesion. Appl Environ Microbiol. 2010;76:1294–1297. doi: 10.1128/AEM.02083-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goeres DM, Loetterle LR, Hamilton MA, Murga R, Kirby DW, Donlan RM. Statistical assessment of a laboratory method for growing biofilms. Microbiology. 2005;151:757–762. doi: 10.1099/mic.0.27709-0. [DOI] [PubMed] [Google Scholar]
- 14.Rudney JD, Chen R, Lenton P, Li J, Li Y, Jones RS, et al. A reproducible oral microcosm biofilm model for testing dental materials. J Appl Microbiol. 2012;113:1540–1553. doi: 10.1111/j.1365-2672.2012.05439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saunders KA, Greenman J. The formation of mixed culture biofilms of oral species along a gradient of shear stress. J Appl Microbiol. 2000;89:564–572. doi: 10.1046/j.1365-2672.2000.01148.x. [DOI] [PubMed] [Google Scholar]
- 16.Haffajee AD, Socransky SS. Microbial etiological agents of destructive periodontal diseases. Periodontol 2000. 1994;5:78–111. doi: 10.1111/j.1600-0757.1994.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 17.Okuda T, Kokubu E, Kawana T, Saito A, Okuda K, Ishihara K. Synergy in biofilm formation between Fusobacterium nucleatum and Prevotella species. Anaerobe. 2012;18:110–116. doi: 10.1016/j.anaerobe.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Fonseca AP, Sousa JC. Effect of shear stress on growth, adhesion and biofilm formation of Pseudomonas aeruginosa with antibiotic-induced morphological changes. Int J Antimicrob Agents. 2007;30:236–241. doi: 10.1016/j.ijantimicag.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 19.Cotter JJ, O'Gara JP, Stewart PS, Pitts B, Casey E. Characterization of a modified rotating disk reactor for the cultivation of Staphylococcus epidermidis biofilm. J Appl Microbiol. 2010;109:2105–2117. doi: 10.1111/j.1365-2672.2010.04842.x. [DOI] [PubMed] [Google Scholar]
- 20.Saito Y, Fujii R, Nakagawa KI, Kuramitsu HK, Okuda K, Ishihara K. Stimulation of Fusobacterium nucleatum biofilm formation by Porphyromonas gingivalis . Oral Microbiol Immunol. 2008;23:1–6. doi: 10.1111/j.1399-302X.2007.00380.x. [DOI] [PubMed] [Google Scholar]
- 21.Abiko Y, Sato T, Mayanagi G, Takahashi N. Profiling of subgingival plaque biofilm microflora from periodontally healthy subjects and from subjects with periodontitis using quantitative real-time PCR. J Periodontal Res. 2010;45:389–395. doi: 10.1111/j.1600-0765.2009.01250.x. [DOI] [PubMed] [Google Scholar]
- 22.Loozen G, Ozcelik O, Boon N, De Mol A, Schoen C, Quirynen M, et al. Inter-bacterial correlations in subgingival biofilms: a large-scale survey. J Clin Periodontol. 2014;41:1–10. doi: 10.1111/jcpe.12167. [DOI] [PubMed] [Google Scholar]
- 23.Shaddox LM, Alfant B, Tobler J, Walker C. Perpetuation of subgingival biofilms in an in vitro model. Mol Oral Microbiol. 2010;25:81–87. doi: 10.1111/j.2041-1014.2009.00549.x. [DOI] [PubMed] [Google Scholar]
- 24.Fraud S, Maillard JY, Kaminski MA, Hanlon GW. Activity of amine oxide against biofilms of Streptococcus mutans: a potential biocide for oral care formulations. J Antimicrob Chemother. 2005;56:672–677. doi: 10.1093/jac/dki325. [DOI] [PubMed] [Google Scholar]
- 25.Hope CK, Petrie A, Wilson M. In vitro assessment of the plaque-removing ability of hydrodynamic shear forces produced beyond the bristles by 2 electric toothbrushes. J Periodontol. 2003;74:1017–1022. doi: 10.1902/jop.2003.74.7.1017. [DOI] [PubMed] [Google Scholar]
- 26.Thurnheer T, Gmür R, Guggenheim B. Multiplex FISH analysis of a six-species bacterial biofilm. J Microbiol Methods. 2004;56:37–47. doi: 10.1016/j.mimet.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Pratten J, Wills K, Barnett P, Wilson M. In vitro studies of the effect of antiseptic-containing mouthwashes on the formation and viability of Streptococcus sanguis biofilms. J Appl Microbiol. 1998;84:1149–1155. doi: 10.1046/j.1365-2672.1998.00462.x. [DOI] [PubMed] [Google Scholar]
- 28.Dû LD, Kolenbrander PE. Identification of saliva-regulated genes of Streptococcus gordonii DL1 by differential display using random arbitrarily primed PCR. Infect Immun. 2000;68:4834–4837. doi: 10.1128/iai.68.8.4834-4837.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahn SJ, Kho HS, Lee SW, Nahm DS. Roles of salivary proteins in the adherence of oral streptococci to various orthodontic brackets. J Dent Res. 2002;81:411–415. doi: 10.1177/154405910208100611. [DOI] [PubMed] [Google Scholar]
- 30.Morris EJ, McBride BC. Adherence of Streptococcus sanguis to saliva-coated hydroxyapatite: evidence for two binding sites. Infect Immun. 1984;43:656–663. doi: 10.1128/iai.43.2.656-663.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lamont RJ, El-Sabaeny A, Park Y, Cook GS, Costerton JW, Demuth DR. Role of the Streptococcus gordonii SspB protein in the development of Porphyromonas gingivalis biofilms on streptococcal substrates. Microbiology. 2002;148:1627–1636. doi: 10.1099/00221287-148-6-1627. [DOI] [PubMed] [Google Scholar]
- 32.Maeda K, Nagata H, Yamamoto Y, Tanaka M, Tanaka J, Minamino N, et al. Glyceraldehyde-3-phosphate dehydrogenase of Streptococcus oralis functions as a coadhesin for Porphyromonas gingivalis major fimbriae. Infect Immun. 2004;72:1341–1348. doi: 10.1128/IAI.72.3.1341-1348.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaplan CW, Lux R, Haake SK, Shi W. The Fusobacterium nucleatum outer membrane protein RadD is an arginine-inhibitable adhesin required for inter-species adherence and the structured architecture of multispecies biofilm. Mol Microbiol. 2009;71:35–47. doi: 10.1111/j.1365-2958.2008.06503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]