Abstract
Sex ratio allocation has important fitness consequences, and theory predicts that parents should adjust offspring sex ratio in cases where the fitness returns of producing male and female offspring vary. The ability of fathers to bias offspring sex ratios has traditionally been dismissed given the expectation of an equal proportion of X- and Y-chromosome-bearing sperm (CBS) in ejaculates due to segregation of sex chromosomes at meiosis. This expectation has been recently refuted. Here we used Peromyscus leucopus to demonstrate that sex ratio is explained by an exclusive effect of the father, and suggest a likely mechanism by which male-driven sex-ratio bias is attained. We identified a male sperm morphological marker that is associated with the mechanism leading to sex ratio bias; differences among males in the sperm nucleus area (a proxy for the sex chromosome that the sperm contains) explain 22% variation in litter sex ratio. We further show the role played by the sperm nucleus area as a mediator in the relationship between individual genetic variation and sex-ratio bias. Fathers with high levels of genetic variation had ejaculates with a higher proportion of sperm with small nuclei area. This, in turn, led to siring a higher proportion of sons (25% increase in sons per 0.1 decrease in the inbreeding coefficient). Our results reveal a plausible mechanism underlying unexplored male-driven sex-ratio biases. We also discuss why this pattern of paternal bias can be adaptive. This research puts to rest the idea that father contribution to sex ratio variation should be disregarded in vertebrates, and will stimulate research on evolutionary constraints to sex ratios—for example, whether fathers and mothers have divergent, coinciding, or neutral sex allocation interests. Finally, these results offer a potential explanation for those intriguing cases in which there are sex ratio biases, such as in humans.
Keywords: Trivers and Willard hypothesis, male effects, inbreeding, sex allocation, sperm cell nucleus, sperm nucleus size
1. Introduction
Trivers & Willard's influential work on sex ratio allocation [1] predicts that parents should adjust offspring sex ratio in cases where the fitness returns of producing male and female offspring depend on parental condition. According to Trivers & Willard, mothers in better condition, who can afford the investment, are expected to invest more in the sex that has the potential to provide higher fitness returns, which typically is the sex that is more costly to produce. Mothers in worse condition should invest in the sex that is less costly to produce but has less variance in reproductive success. The Trivers & Willard hypothesis has successfully explained observed sex ratio patterns in some organisms [2,3], but its power to explain adaptive sex-ratio variation in vertebrates, and more specifically in mammals, has remained limited [4,5]. For instance, in ungulates, the most studied group, female condition accounts for 3–6% of variation in offspring sex ratio [6].
In taxa where males are the heterogametic sex, males can theoretically influence offspring sex ratio during sperm production. In mammals, however, the expectation of an equal proportion of X- and Y-chromosome-bearing sperm (CBS) in ejaculates has led to most research on sex allocation being focused on females [2,7] and to be interpreted in the light of maternal fitness returns [2,3]. Nevertheless, as with females, high-quality males should invest in the more costly sex (usually males). If high-quality males are more likely to produce high-quality offspring—either through contribution to the parental investment in offspring or via heritability of male quality—then it is adaptive for them to produce more of the sex that has the opportunity for higher reproductive success. Importantly, recent research shows that unbalanced proportions of X- versus Y-CBS pre-ejaculation are not rare [7–9].
Three studies conducted in sexually dimorphic ungulates with high variance in male reproductive success support the idea that fathers can bias sex ratio [10–12]. First, Gomendio et al. [10] used 14 red deer stags—a strongly sexually dimorphic species and a classic example for large variance across males in reproductive success—to show that fathers can bias sex ratio at birth [10]: more fertile fathers produce more sons and less fertile males produce more daughters, underscoring for the first time the effects of father quality on sex ratios. Second, Saragusty et al. [11] used seven pygmy hippos to show that variation in the ratio of X- and Y-chromosome bearing sperm in the ejaculate was associated with variation in the sex of the offspring produced. Third, Douhard et al. [12] recently used a large dataset on another sexually dimorphic species, the bighorn sheep, to show, first, that males with higher reproductive success have a higher proportion of male offspring, and second, that such sex ratio bias is adaptive. These three studies suggest that sex allocation is not an exclusive ability of mothers. High-quality fathers could accrue higher fitness benefits by biasing sex ratio towards sons who would inherit their quality and go on to produce more grand-offspring. Despite this seemingly straightforward argument, the adaptive meaning of sex ratio bias by males has not been well established. Moreover, we do not know whether the adaptive value of sex ratio adjustments varies depending on the species's life history [2]. Furthermore, regardless of the adaptive mechanism, whether this father effect is an exclusive feature of ungulates or whether it is common across the mammalian tree of life is not known.
Here we investigate the possibility of fathers driving sex-ratio bias in a species lying at the opposite extreme of the mammalian life-history spectrum. We used a non-domestic rodent model, the white-footed mouse (Peromyscus leucopus), to test three hypotheses related to sex allocation. First, we tested whether a male-specific sperm trait (the size of the sperm nucleus) explains variation in sex-ratio bias. Second, we tested if male genetic quality [13], as measured by inbreeding [14], predicted offspring sex ratio at birth. Third, we tested whether the size of the sperm nucleus is the trait that potentially links father inbreeding levels with offspring sex ratios at birth.
We also discuss the adaptive value of paternal sex ratio bias in rodents. We have previously shown that, in Peromyscus leucopus, fertility genetic load (lethal equivalents) [15] are higher in males than in females [16]. High-quality fathers (those with relatively low inbreeding) would presumably have a higher probability of fertilization in promiscuous contexts (e.g. see [17]). Inbreeding is heritable in some systems [18,19] (see electronic supplementary material S2)—with parental levels correlating with offspring inbreeding under non-random mating—allowing for paternal sex ratio bias driven by inbreeding to be adaptive. In addition, even in cases where inbreeding is not heritable, if males assess their quality and adjust offspring sex ratio accordingly, and if inbreeding depresses phenotypic quality [14], then more inbred fathers would be expected to shift the offspring sex ratio towards the less costly sex. On the other hand, as sons inherit their fathers' quality, high-quality fathers would be expected to bias sex ratio towards sons because high quality males have a higher probability of successfully fertilizing females and having more offspring than low-quality males. Thus, assessments of quality that might drive adaptive sex ratio bias would be influenced by inbreeding. Note that the mechanism proposed above, dependent on quality assessment, could rely on self-perceptions of quality, as well as on comparisons with nearby males that would be competing for mates.
Our study system needed to meet some critical requirements to properly test the predictions above. First, to make our results generalizable to other naturally evolved species, and relevant in the context of the evolution of sex ratios, we used a wild rodent model system where domestication has not depleted natural variation. Second, to have certainty of paternity when linking males to their offspring, and to account for maternal and paternal effects on sex-ratio bias, we used a captive facility and controlled pairings. Third, to ensure that the paternal effects on offspring sex ratio were not confounded with maternal effects, we identified a male-only trait (nucleus sperm area) as the candidate variable mediating the male sex ratio bias. Sperm head size is used as a discrimination criterion for sorting X- and Y-CBS in a variety of mammalian species, including humans and bovines [20–24], where it has been shown that X-CBS are larger than Y-CBS (box 1). To further minimize the effect of factors contributing to maternal sex-ratio bias, such as maternal diet or female body condition, food was provided at libitum. Also, constant food and environment conditions, together with consistent housing, minimize the effects of drivers such as seasonality, density or social interactions on female sex ratio bias [33]. This also reduces the scope for local resource competition, local mate competition and local resource enhancement [34,35] driving the observed results. Lastly, we recorded and included in the statistical models other confounding variables, such as male age and litter size, known to influence sex ratios [36,37].
Box 1. Evidence supporting that the presence of either sex chromosome drives variation in sperm nucleus size (area and length).
It has been recognized for a long time that internal and not external forces influence the shape of the sperm nucleus [25]. In sperm, DNA reaches highest degrees of compaction. The fact that there is no unused space inside sperm cells, together with the evolution of DNA-packaging mechanisms inside the sperm nucleus, are proof for the presence of very strong selective forces for volume reduction. In mice, if the DNA packaging in the sperm cells used nucleosomes (as in the rest of cell types) instead of protamines, it would require 213% of the total nuclear volume [26,27].
Variation in volume of CBS and chromatin can be reflected in the nucleus area. Likewise, differences in the length of the sperm head can reflect differences in X- versus Y-chromosomes and their compacting material because (1) protamine-DNA complex is arranged inside the nucleus lying lengthwise inside the minor groove [28] and (2) sex chromosomes are positioned in repeatable, non-intertwined positions along the antero-posterior axis of the sperm nucleus, which are conserved within individuals [29,30]. Furthermore, there is evidence that chromatin condensation influences the shape of the nucleus [25], and that in mice sperm nucleus size and elongation reflects the chromatin content [31], which in turn varies between X and Y chromosomes [32].
2. Material and methods
(a). Study population
We used a population of white-footed mice (Peromyscus leucopus) at the Chicago Zoological Society's research animal facility, derived from wild individuals trapped at Volo Bog State Natural Area (Illinois) (see electronic supplementary material S1.1. for details). Individuals from the 10th generation were used for this study. This population is expected to have retained, on average, 97.5% of the wild genetic diversity and, thus, nearly all additive genetic variance available in the natural population. In generation number one, experimental populations were established following three different breeding protocols (see electronic supplementary material S1.1 for details on the set-up of the founder population). Given that inbreeding accumulated in them at different rates (see electronic supplementary material S1.2. for details) [16,38], we checked that offspring sex ratio was not affected by breeding protocol (ANOVA; F2,35 = 2.40, p = 0.11), and further included this three-level categorical variable in the relevant models below.
(b). Sampling, sperm collection and sperm nuclei area
This study used 58 males. Each male was paired with a single female and allowed to reproduce for up to 70 days. To standardize the conditions of males before sperm collection, after separation from the female, and given that spermatogenesis takes 7–8 days, every male was left in an individual cage for at least 7 days to allow the sperm stores to be replenished. Immediately after euthanasia, the testicles were removed and sperm was collected and processed (see electronic supplementary material S1.3 and S1.4 for details on sperm sampling). We used the Feulgen stain and a microscope with a ×60 bright-field objective linked to a video camera to visualize the sperm nuclei and carry out the sperm head measurements using computer-assisted sperm head morphometric analysis (CASMA). The software automatically identifies sperm heads and calculates nucleus length and area (electronic supplementary material, figure S1). The nuclei of a minimum of 200 sperm were analysed per individual (see electronic supplementary material S1.5. for details on sperm nuclei measurements). We present full statistics for sperm nucleus area, and show the consistency of the results using another relevant measure of the nucleus (length), with which it is strongly correlated (electronic supplementary material, figure S2).
(c). Genetic quality, sex ratio at birth and offspring number
We used the Wright's f coefficient of inbreeding as an indicator of genetic quality [13]. The coefficient of inbreeding of the father and the mother (sire f and dam f) was calculated from the pedigree of the population. Offspring sex ratios at birth (secondary sex ratios; calculated as the proportion of sons, Nsons/[Nsons + Ndaughters]) were recorded for each father after his impregnated mate gave birth, as well as the number of pups produced. Male age was recorded to account for this factor in the models.
(d). Data analysis
Means and standard deviations are used to describe the variables included in the models (table 1). We used GLMs with either a Gaussian (offspring sex ratio as a proportion) or binomial (sons versus daughters, using a logit link function) to test for the predictors of secondary sex ratios. We also used Akaike's information criterion (AIC) to assess model support and to select the best model. Given that males from different breeding protocols [16,38] varied in the rate of inbreeding accumulation through the 10 generations (see electronic supplementary material S1.2 for details), we accounted for this variable in the relevant models. The models vary in sample sizes because the total number of males was 58, while the total number of fathers siring offspring was 40. Two of those males were outliers for sperm nucleus area (more than 2.85 × s.d. away from the mean), so they were excluded from the relevant models prior to analysis. Data were analysed using R software (v. 3.3.2, http://www.R-project.org/) and STATISTICA (v. 7.0, StatSoft Inc., Tulsa, OK, USA).
Table 1.
Descriptive statistics for the continuous variables included in the different models conducted. Sire f and dam f stand for the coefficient of inbreeding of the father and the mother, respectively. The models vary in sample sizes for the following reasons: total number of individuals = 58, total number males producing litters = 40, and two of the males that produced litters were outliers for the variable sperm nucleus area (described in the main text); these males were excluded from the analyses and consequently they are also excluded from the summary statistics.
| mean | s.d. | range min-max | n | |
|---|---|---|---|---|
| offspring sex ratio | 0.43 | 0.21 | 0–0.88 | 38 |
| sperm nucleus area (µm) | 12.54 | 0.51 | 11.3–13.5 | 38 |
| sperm nucleus length (µm) | 4.94 | 0.12 | 4.64–5.14 | 38 |
| sire f | 0.136 | 0.021 | 0.099–0.170 | 58 |
| dam f | 0.134 | 0.026 | 0.097–0.170 | 58 |
| total number of offspring | 9.7 | 2.7 | 5–17 | 40 |
| age sire (days) | 204 | 20.5 | 153–249 | 58 |
3. Results
We independently tested the effects of inbreeding (sire f, mean ± s.d. = 0.136 ± 0.021) and sperm nucleus size (table 1) on sex ratio (0.43 ± 0.21) using different GLM models.
(a). Male-specific trait (sperm nucleus size) and sex ratio bias
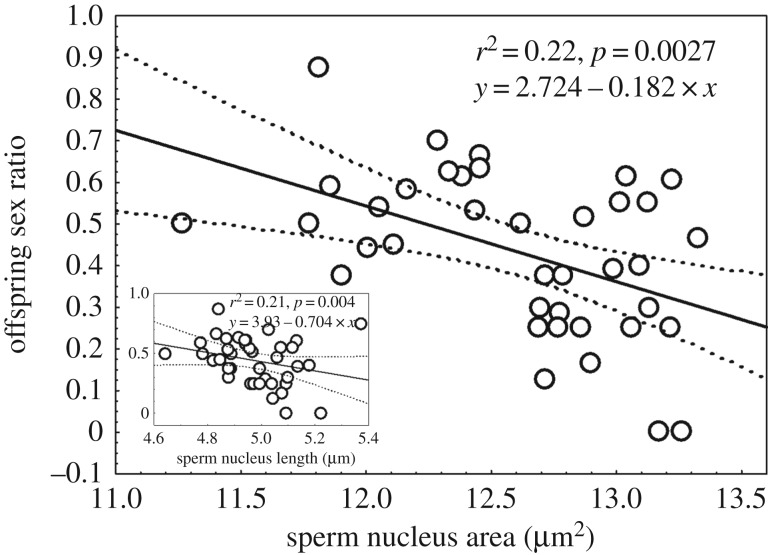
Results showed that fathers with smaller sperm head nuclei sired a higher proportion of sons than daughters (β ± s.e. = −0.18 ± 0.05, t = −3.22, d.f. = 38, p = 0.002; figure 1).
Figure 1.
Relationship between a father's mean sperm nucleus area (or nucleus length; inset graph) and its offspring sex ratio. Each data point reflects the mean calculated using a minimum of 200 sperm per male. Two outlier data points for sperm nucleus area were removed (2.85 s.d. and 3.12 s.d. away from the mean sperm nucleus area). n = 38.
(b). Father genetic quality (inbreeding) and sex ratio bias
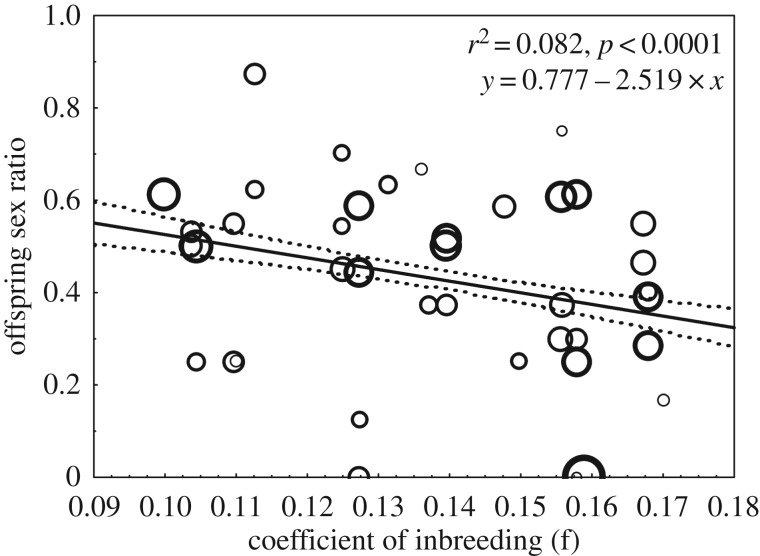
Likewise, a model conducted to test independently the association between sire f and offspring sex ratio showed that fathers with lower inbreeding sired a higher proportion of sons than daughters (β ± s.e. = −2.86 ± 1.33, t = −2.15, d.f. = 38, p = 0.038; figure 2).
Figure 2.
Relationship between the father's coefficient of inbreeding (f) and offspring sex ratio. Each data point is weighted by the total number of offspring that a male sired. n = 38.
(c). Sperm nucleus size mediates the effects of paternal inbreeding on sex ratio
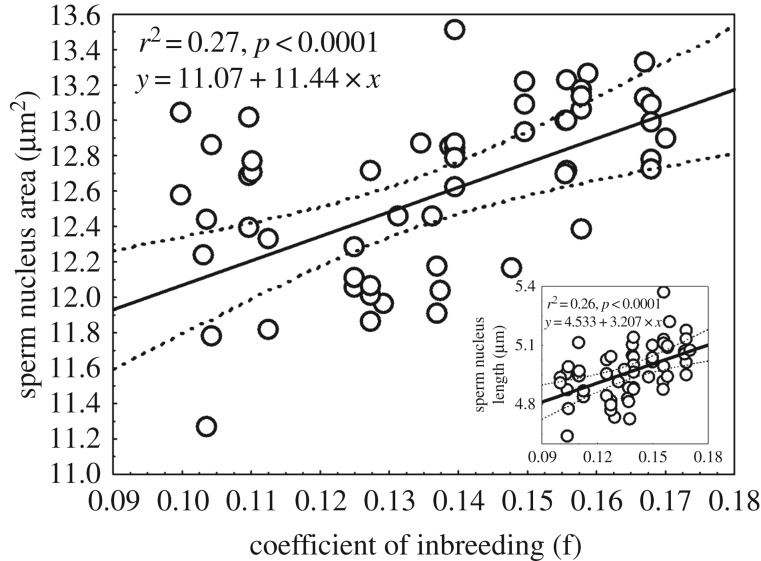
We tested whether father inbreeding effects on litter sex ratio were mediated through its effects on sperm nucleus area. We first ascertained whether sire f and sperm nucleus area had independent effects on sex ratio by running a GLM including these two predictors, plus other potentially explanatory variables (table 2). After stepwise deletion of non-significant terms, only sperm nucleus area remained as a significant predictor of mean sex ratio, explaining 22% of variation (β ± s.e. = −0.18 ± 0.06, t = −3.22, d.f. = 38, p = 0.003; figure 1). We then included inbreeding in the same statistical model as a predictor. In this model, inbreeding did not explain variation on sex ratio (β ± s.e. = 1.46 ± −1.30, t = −0.88, d.f. = 38, p = 0.38). This result was also confirmed in a different set of models run using an information theory approach (electronic supplementary material, table S1). Finally, we tested whether male inbreeding leads to an increase in sperm nucleus area, and showed that was indeed the case (β ± s.e. = 11.55 ± 2.75, t = 4.198, d.f. = 38, p < 0.0001; figure 3). This result was also confirmed in a different set of models run using an information theory approach (electronic supplementary material, table S2).
Table 2.
Full and minimum adequate (final) models testing the effects of different paternal and maternal drivers on litter sex ratio. Sire f and dam f represent the coefficient of inbreeding of the father and mother, respectively. Full model: null deviance: 1.390 on 37 d.f.; residual deviance: 0.959 on 30 d.f.; AIC: −13.97. Final model: residual deviance: 1.078 on 36 d.f.; AIC: −21.502. Statistically significant results are highlighted in italics.
| estimate | s.e. | t | p | |
|---|---|---|---|---|
| full model term | ||||
| intercept | 2.275 | 1.250 | 1.819 | 0.078 |
| sire f | −2.105 | 3.760 | −0.56 | 0.579 |
| sperm nucleus area | −0.158 | 0.073 | −2.17 | 0.038 |
| protocol | — | — | 0.19 | 0.819 |
| age | 0.003 | 0.001 | 1.50 | 0.143 |
| number offspring | 0.008 | 0.011 | 0.72 | 0.476 |
| dam f | −1.728 | 4.543 | −0.38 | 0.706 |
| final model term | ||||
| intercept | 2.723 | 0.711 | 3.826 | 0.0004 |
| sperm nucleus area | −0.181 | 0.056 | −3.222 | 0.0026 |
Figure 3.
Relationship between the father's coefficient of inbreeding (f) and the sperm nucleus area (or nucleus length; inlet graph). Each data point reflects the mean calculated using a minimum of 200 sperm per male. Two outlier data points for sperm nucleus area were removed (2.85 s.d. and 3.12 s.d. away from the mean sperm nucleus area). n = 58.
Overall, our results show that inbreeding does not have a direct effect on litter sex ratio, but rather an indirect one operating via its effects on sperm nucleus area. Males of higher genetic quality (i.e. lower inbreeding coefficients) have sperm with smaller nuclei (suggesting higher proportion of Y-CBS) that go on to produce more male-biased litters.
4. Discussion
Here we show, first, that fathers account for over 20% of variation in offspring sex ratio. Second, an increase in father's genetic quality (reduced inbreeding) translated into the production of a higher proportion of sons through changes in the proportion of X- and Y-bearing sperm (which we determined by a sperm morphological trait). Third, a father's individual genetic quality (level of inbreeding) explained 8% of the variance in offspring sex ratio. As explained below, the higher costs of increased inbreeding for males than for females (given their higher genetic load for fertility traits) suggest that highly inbred fathers can reduce the costs by having relatively more daughters, while outbred fathers can increase their fitness benefits by biasing sex ratio to sons.
A previous study in red deer, a species having singleton births, experimentally showed that males with higher fertility levels produce more males [10]. That bias could have been due to either a direct male-driven bias of the proportion of X- and Y-CBS in the ejaculate [39], or due to higher competitive ability of Y-bearing sperm in the more fertile males [40]. However, which of the two factors was responsible for the observed male-driven sex ratio bias could not be resolved in that study. Our present results on a woodland rodent suggest that offspring sex ratio biases are due to a direct effect of father genetic quality on the proportion of Y- versus X-CBS, and that such bias can be adaptive given its expected fitness benefits. Notwithstanding, other alternative explanations are possible, such as Y-CBS being more vulnerable, with their vulnerability further exacerbated under inbreeding. Similarly, the possibility of an X drive system that damages Y-CBS—suppressed in fit males, those with lower coefficient of inbreeding—could potentially cause more inbred males to produce more female-biased broods [41], so research on these possibilities is warranted. In any case, by identifying a male-only trait such as the sperm nucleus area, sperm size is a reliable marker of X- and Y-CBS [20–24] (box 1)—as the main driver of sex ratio, we can be confident that a male effect drives this result. Claiming that the observed effect is driven by mothers (e.g. due to female allocation based on mate quality assessment, or post-copulatory sexual selection including sperm choice) would not be parsimonious as the sperm trait is independent and lies first in the chain of events leading to sex ratio variation. Furthermore, the sperm was extracted from the epididymis post-mortem, which excludes the possibility of a maternal effect explaining the links that we have uncovered. Nevertheless, the possibility that after insemination or during gestation maternal effects also moderate sex ratio cannot be ruled out. However, these effects would impact sex ratios beyond the primary paternal effects that we have uncovered here.
The individual- and population-level mechanisms underlying the effects of inbreeding on sex ratio allocation in vertebrates remain far from being understood [42]. Partly, this may be because males' role on sex ratio bias has been consistently dismissed. Here, we show that father inbreeding—an indicator of genetic quality—influences sex ratio through its effects on the area of the sperm nucleus—an indicator of X- versus Y-CBS. However, inbreeding only accounts for roughly a quarter of variation in sperm head (27%), and for 8% overall on sex ratio, so a remaining approximately 14% of paternal (sperm-driven) variation in sex ratio is not related to inbreeding (22% − 8% = 14%). The identification of the area of the sperm nucleus narrows down the possible underlying mechanisms of sex-ratio bias, strongly pointing towards the existence of distortions in the proportions of X- versus Y-CBS before ejaculation. These could emerge after meiosis due to differences in longevity between X and Y sperm, which has been shown previously [43].
In species with high variation in reproductive success between males there is a relative higher scope for the evolution of father sex ratio bias [10,12]. In contrast, the lack of strong sexual dimorphism in rodents and the expected absence of large differences between sexes in intra-sexual variance in reproductive success could argue against the expectation of adaptive sex ratio bias in this group. However, Peromyscus leucopus are polygamous, and males will aggressively exclude other males from home ranges that overlap those of up to several females [44]. In an ecologically equivalent woodland rodent species [45], variance in reproductive success was over two times higher in males than in females (B. Godsall, T. Coulson, A. F. Malo 2015, unpublished results).
We argue that father influences on sex ratio should be taken into account in sex allocation studies. In monotocous species (producing a single offspring at a time), high-ranking females could produce more males not only because they themselves may be biasing sex ratio towards sons, but also because they mate with high-quality males, who may be biasing sex ratio in the same direction. In polytocous species (producing multiple offspring at a time), such as mice [36,46,47], the failure to acknowledge that fathers can contribute to sex ratio bias can explain the lack of support of the Trivers & Willard hypothesis [4,48,49].
Mice present a fast life history compared with the slow life history exhibited by species such as red deer or pygmy hippopotamus [50]. The finding that rodent fathers, which lay at the opposite extreme of the mammalian life-history continuum, can also bias sex ratio at birth suggests that this is a common feature of eutherian mammals. Our results stress the need for a formal extension of sex-ratio theory to include the drivers and consequences of paternal sex ratio allocation in vertebrates.
We hope our paper will help stimulate research on whether fathers and mothers have divergent (antagonistic), coinciding or neutral sex allocation interests. This question has remained largely unexplored in the study of evolutionary constraints to sex ratios [10,11]. By identifying the area of the sperm cell nucleus—indicator of the proportion of X- versus Y-CBS in their ejaculates—as the morphometric trait responsible for the observed sex-ratio bias we show that the first causal factor for biases in offspring sex ratio lies in males. This finding points towards paternal distortions in the proportions of X- versus Y-CBS at meiosis, or at sperm maturation, as an important driver underlying sex ratio variation in nature, although the underlying mechanism is largely unknown. This work challenges the traditional view in sex allocation theory, which largely disregards the role of fathers in sex ratio allocation and offers a new potential explanation for those cases in which there is sex ratio bias, such as in humans [51]. A shift from only maternal-oriented research to both paternal- and maternal-oriented research—both empirical and theoretical—will provide new insights into the evolution of sex ratio and sex-ratio allocation.
Supplementary Material
Acknowledgements
We dedicate this paper to Professor JoGayle Howard (deceased). We are grateful to Glen Alaks for assistance with data collection, and to Jean Dubach for her help with sample storage and shipping. We also thank Stuart West, Tom Pizzari and Alex Kacelnik for their comments on a previous draft.
Ethics
The animal care protocols and experiments described here comply with all current laws and were approved by the Animal Care and Use Committee of the Chicago Zoological Society.
Data accessibility
Raw data have been uploaded in the Dryad Digital Repository (http://dx.doi.org/10.5061/dryad.948kq) [52].
Authors' contributions
A.F.M. conceived the project, analysed the data and wrote the manuscript, with contributions from all authors. A.F.M. and F.M.-P. initiated the research, analysed sperm samples and wrote the supplementary materials section. R.C.L., F.M.-P., J.G. and J.D.B. provided equipment, laboratory space and facilities. R.C.L. provided the study system, contributed data and insights on the study system. All co-authors contributed valuable discussions and relevant edits to the manuscript.
Competing interests
We have no competing interests.
Funding
A.F.M. was supported by a MEC/Fulbright fellowship (FU2005-0893), a travel grant from the Ministry of Education and Science, a Marie Curie fellowship (PIEF-GA-2008-220322) and an ERC grant (249872). F.M.-P. was supported by the Juan de la Cierva program (Spanish Ministry of Education and Science), Ramon y Cajal program (RYC-2008-02560, MICINN) and two travel grants from the University of Castilla-La Mancha (Spain). F.G.-G. was supported by the Ramon y Cajal program, the Spanish Severo Ochoa Program (SEV-2012-0262) and by grant no. CGL2016-76173-P (co-funded by the European Regional Development Fund) from the Spanish Ministry of Economy.
References
- 1.Trivers RL, Willard DE. 1973. Natural-selection of parental ability to vary sex-ratio of offspring. Science 179, 90–92. ( 10.1126/science.179.4068.90) [DOI] [PubMed] [Google Scholar]
- 2.West SA. 2009. Sex allocation. Princeton, NJ: Princeton University Press. [Google Scholar]
- 3.Charnov EL. 1982. The theory of sex allocation. Princeton, NJ: Princeton University Press. [Google Scholar]
- 4.Festa-Bianchet M. 1996. Offspring sex ratio studies of mammals: does publication depend upon the quality of the research or the direction of the results? Écoscience 3, 42–44. ( 10.1080/11956860.1996.11682313) [DOI] [Google Scholar]
- 5.Hardy ICW. 1997. Possible factors influencing vertebrate sex ratios: an introductory overview. Appl. Anim. Behav. Sci. 51, 217–241. ( 10.1016/S0168-1591(96)01106-9) [DOI] [Google Scholar]
- 6.Ferriere R, Belthoff JR, Olivieri I, Krackow S. 2000. Evolving dispersal: where to go next? Trends Ecol. Evol. 15, 5–7. ( 10.1016/S0169-5347(99)01757-7) [DOI] [PubMed] [Google Scholar]
- 7.Edwards AM, Cameron EZ. 2014. Forgotten fathers: paternal influences on mammalian sex allocation. Trends Ecol. Evol. 29, 158–164. ( 10.1016/j.tree.2013.12.003) [DOI] [PubMed] [Google Scholar]
- 8.Berry DP, Kearney JF, Roche JR. 2011. Evidence of genetic and maternal effects on secondary sex ratio in cattle. Theriogenology 75, 1039–1044. ( 10.1016/j.theriogenology.2010.11.011) [DOI] [PubMed] [Google Scholar]
- 9.Chandler JE, Taylor TM, Canal AL, Cooper RK, Moser EB, McCormick ME, Willard ST, Rycroft HE, Gilbert GR. 2007. Calving sex ratio as related to the predicted Y-chromosome-bearing spermatozoa ratio in bull ejaculates. Theriogenology 67, 563–571. ( 10.1016/j.theriogenology.2006.09.006) [DOI] [PubMed] [Google Scholar]
- 10.Gomendio M, Malo AF, Soler AJ, Fernandez-Santos MR, Esteso MC, García AJ, Roldan ERS, Garde J. 2006. Male fertility and sex ratio at birth in red deer. Science 314, 1445–1447. ( 10.1126/science.1133064) [DOI] [PubMed] [Google Scholar]
- 11.Saragusty J, Hermes R, Hofer H, Bouts T, Goritz F, Hildebrandt TB. 2012. Male pygmy hippopotamus influence offspring sex ratio. Nat. Commun. 3, 697 ( 10.1038/ncomms1700) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Douhard M, Festa-Bianchet M, Coltman DW, Pelletier F. 2016. Paternal reproductive success drives sex allocation in a wild mammal. Evolution 70, 358–368. ( 10.1111/evo.12860) [DOI] [PubMed] [Google Scholar]
- 13.Neff BD, Pitcher TE. 2005. Genetic quality and sexual selection: an integrated framework for good genes and compatible genes. Mol. Ecol. 14, 19–38. ( 10.1111/j.1365-294X.2004.02395.x). [DOI] [PubMed] [Google Scholar]
- 14.Keller LF, Waller DM. 2002. Inbreeding effects in wild populations. Trends Ecol. Evol. 17, 230–241. ( 10.1016/S0169-5347(02)02489-8) [DOI] [Google Scholar]
- 15.Saccheri IJ, Lloyd HD, Helyar SJ, Brakefield PM. 2005. Inbreeding uncovers fundamental differences in the genetic load affecting male and female fertility in a butterfly. Proc. R. Soc. B 272, 39–46. ( 10.1098/rspb.2004.2903). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malo AF, Martinez-Pastor F, Alaks G, Dubach J, Lacy RC. 2010. Effects of genetic captive-breeding protocols on sperm quality and fertility in the white-footed mouse. Biol. Reprod. 83, 540–548. ( 10.1095/biolreprod.110.085316) [DOI] [PubMed] [Google Scholar]
- 17.Michalczyk L, Martin OY, Millard AL, Emerson BC, Gage MJG. 2010. Inbreeding depresses sperm competitiveness, but not fertilization or mating success in male Tribolium castaneum. Proc. R. Soc. B 277, 3483–3491. ( 10.1098/rspb.2010.0514). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reid JM, Arcese P, Keller LF. 2006. Intrinsic parent-offspring correlation in inbreeding level in a song sparrow (Melospiza melodia) population open to immigration. Am. Nat. 168, 1–13. ( 10.1086/504852) [DOI] [PubMed] [Google Scholar]
- 19.Nietlisbach P, Keller LF, Postma E. 2016. Genetic variance components and heritability of multiallelic heterozygosity under inbreeding. Heredity 116, 1–11. ( 10.1038/hdy.2015.59) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson LA, Flook JP, Hawk HW. 1989. Sex preselection in rabbits—live births from X-sperm and Y-sperm separated by DNA and cell sorting. Biol. Reprod. 41, 199–203. ( 10.1095/biolreprod41.2.199). [DOI] [PubMed] [Google Scholar]
- 21.Zhuang XJ, Lu YQ, Zhang M, Lu SS, Lu KH. 2011. Microisolation and microcloning of bovine X-chromosomes for identification of sorted buffalo (Bubalus bubalis) spermatozoa. Anim. Reprod. Sci. 126, 32–36. ( 10.1016/j.anireprosci.2011.04.021). [DOI] [PubMed] [Google Scholar]
- 22.Parati K, Bongioni G, Aleandri R, Galli A. 2006. Sex ratio determination in bovine semen: a new approach by quantitative real time PCR. Theriogenology 66, 2202–2209. ( 10.1016/j.theriogenology.2006.07.007). [DOI] [PubMed] [Google Scholar]
- 23.Cui K. 1997. Size differences between human X and Y spermatozoa and prefertilization diagnosis. Mol. Hum. Reprod. 3, 61–67. ( 10.1093/molehr/3.1.61) [DOI] [PubMed] [Google Scholar]
- 24.Penfold LM, Holt C, Holt WV, Welch GR, Cran DG, Johnson LA. 1998. Comparative motility of X and Y chromosome-bearing bovine sperm separated on the basis of DNA content by flow sorting. Mol. Reprod. Dev. 50, 323–327. ( 10.1002/(SICI)1098-2795(199807)50:3%3C323::AID-MRD8%3E3.0.CO;2-L) [DOI] [PubMed] [Google Scholar]
- 25.Fawcett DW, Anderson WA, Phillips DM. 1971. Morphogenetic factors influencing shape of sperm head. Dev. Biol. 26, 220–253. ( 10.1016/0012-1606(71)90124-2) [DOI] [PubMed] [Google Scholar]
- 26.Pogany GC, Corzett M, Weston S, Balhorn R. 1981. DNA and protein content of mouse sperm: Implications regarding sperm chromatin structure. Exp. Cell Res. 136, 127–136. ( 10.1016/0014-4827(81)90044-6) [DOI] [PubMed] [Google Scholar]
- 27.Ward WS, Coffey DS. 1991. DNA packaging and organization in mammalian spermatozoa: comparison with somatic cells. Biol. Reprod. 44, 569–574. ( 10.1095/biolreprod44.4.569) [DOI] [PubMed] [Google Scholar]
- 28.Balhorn R. 1982. A model for the structure of chromatin in mammalian sperm. J. Cell Biol. 93, 175–181. ( 10.1083/jcb.93.2.298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noblanc A, Kocer A, Drevet JR. 2014. Recent knowledge concerning mammalian sperm chromatin organization and its potential weakness when facing oxidative challenge. Basic Clin. Androl. 24, 1–12. ( 10.1186/2051-4190-24-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zalenskaya IA, Zalensky AO. 2004. Non-random positioning of chromosomes in human sperm nuclei. Chromosome Res. 12, 163–173. ( 10.1023/B:CHRO.0000013166.04629.97) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cacciola G, Chioccarelli T, Altucci L, Viggiano A, Fasano S, Pierantoni R, Cobellis G. 2013. Nuclear size as estrogen-responsive chromatin quality parameter of mouse spermatozoa. Gen. Comp. Endocrinol. 19, 201–209. ( 10.1016/j.ygcen.2013.07.018) [DOI] [PubMed] [Google Scholar]
- 32.Silkaitis K, Lemos B. 2014. Sex-biased chromatin and regulatory cross-talk between sex chromosomes, autosomes, and mitochondria. Biol. Sex Differ. 5, 1–14. ( 10.1186/2042-6410-5-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lambin X. 1994. Sex-ratio variation in relation to female philopatry in Townsend voles. J. Anim. Ecol. 63, 945–953. ( 10.2307/5271) [DOI] [Google Scholar]
- 34.Lambin X. 1994. Natal philopatry, competition for resources, and inbreeding avoidance in Townsend's voles (Microtus townsendii). Ecology 75, 224–235. ( 10.2307/1939396) [DOI] [Google Scholar]
- 35.Armitage KB. 1987. Do female yellow-bellied marmots adjust the sex-ratios of their offspring? Am. Nat. 129, 501–519. ( 10.1086/284654). [DOI] [Google Scholar]
- 36.Rosenfeld CS, Roberts RM. 2004. Maternal diet and other factors affecting offspring sex ratio: a review. Biol. Reprod. 71, 1063–1070. ( 10.1095/biolreprod.104.030890) [DOI] [PubMed] [Google Scholar]
- 37.Kruuk LEB, Clutton-Brock TH, Albon SD, Pemberton JM, Guinness FE. 1999. Population density affects sex ratio variation in red deer. Nature 399, 459–461. ( 10.1038/20917) [DOI] [PubMed] [Google Scholar]
- 38.Lacy RC, Alaks G, Walsh A. 2013. Evolution of Peromyscus leucopus mice in response to a captive environment. PLoS ONE 8, e72452 ( 10.1371/journal.pone.0072452) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chandler JE, Canal AM, Paul JB, Moser EB. 2002. Collection frequency affects percent Y-chromosome bearing sperm, sperm head area and quality of bovine ejaculates. Theriogenology 57, 1327–1346. ( 10.1016/S0093-691x(01)00721-X) [DOI] [PubMed] [Google Scholar]
- 40.LaMunyon CW, Ward S. 1997. Evolution increased competitiveness of nematode sperm bearing the male X chromosome. Proc. Natl Acad. Sci. USA 94, 185–189. ( 10.1073/pnas.94.1.185) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jaenike J. 2001. Sex chromosome meiotic drive. Annu. Rev. Ecol. Evol. Syst. 32, 25–49. ( 10.1146/annurev.ecolsys.32.081501.113958) [DOI] [Google Scholar]
- 42.Frankham R, Wilcken J. 2006. Does inbreeding distort sex-ratios? Conserv. Genet. 7, 879–893. ( 10.1007/s10592-006-9129-6) [DOI] [Google Scholar]
- 43.Martinez F, Kaabi M, Martinez-Pastor F, Alvarez M, Anel E, Boixo JC, de Paz P, Anel L. 2004. Effect of the interval between estrus onset and artificial insemination on sex ratio and fertility in cattle: a field study. Theriogenology 62, 1264–1270. ( 10.1016/j.theriogenology.2004.01.002) [DOI] [PubMed] [Google Scholar]
- 44.Wolff JO. 1989. Social behavior. In Advances in the study of Peromyscus (Rodentia) (eds Kirkland GLJ, Layne JN), pp. 271–291. Lubbock, TX: Texas Tech University Press. [Google Scholar]
- 45.Malo AF, Godsall B, Prebble C, Grange Z, McCandless S, Taylor A, Coulson T. 2013. Positive effects of an invasive shrub on aggregation and abundance of a native small rodent. Behav. Ecol. 24, 759–767. ( 10.1093/beheco/ars202) [DOI] [Google Scholar]
- 46.Krackow S. 1997. Maternal investment, sex-differential prospects, and the sex ratio in wild house mice. Behav. Ecol. Sociobiol. 41, 435–443. ( 10.1007/s002650050404) [DOI] [Google Scholar]
- 47.Williams GC. 1979. The question of adaptive sex ratio in outcrossed vertebrates. Proc. R. Soc. Lond. B 205, 567–580. ( 10.1098/rspb.1979.0085) [DOI] [PubMed] [Google Scholar]
- 48.Brown GR, Silk JB. 2002. Reconsidering the null hypothesis: is maternal rank associated with birth sex ratios in primate groups? Proc. Natl Acad. Sci. USA 99, 11 252–11 255. ( 10.1073/pnas.162360599) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sheldon BC, West SA. 2004. Maternal dominance, maternal condition, and offspring sex ratio in ungulate mammals. Am. Nat. 163, 40–54. ( 10.1086/381003) [DOI] [PubMed] [Google Scholar]
- 50.Oli MK. 2004. The fast-slow continuum and mammalian life-history patterns: an empirical evaluation. Basic Appl. Ecol. 5, 449–463. ( 10.1016/j.baae.2004.06.002) [DOI] [Google Scholar]
- 51.Austad SN. 2015. The human prenatal sex ratio: a major surprise. Proc. Natl Acad. Sci. USA 112, 4839–4840. ( 10.1073/pnas.1505165112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Malo AF, Martinez-Pastor F, Garcia-Gonzalez F, Garde J, Ballou JD, Lacy RC. 2017. Data from: A father effect explains sex-ratio bias Dryad Digital Repository. ( 10.5061/dryad.948kq) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Malo AF, Martinez-Pastor F, Garcia-Gonzalez F, Garde J, Ballou JD, Lacy RC. 2017. Data from: A father effect explains sex-ratio bias Dryad Digital Repository. ( 10.5061/dryad.948kq) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Raw data have been uploaded in the Dryad Digital Repository (http://dx.doi.org/10.5061/dryad.948kq) [52].