Abstract
Key points
Meldonium inhibits endogenous carnitine synthesis and tissue uptake, and accelerates urinary carnitine excretion, although the impact of meldonium‐mediated muscle carnitine depletion on whole‐body fuel selection, and muscle fuel metabolism and its molecular regulation is under‐investigated.
Ten days of oral meldonium administration did not impact on food or fluid intake, physical activity levels or body weight gain in the rat, whereas it depleted muscle carnitine content (all moieties), increased whole‐body carbohydrate oxidation and muscle and liver glycogen utilization, and reduced whole‐body fat oxidation.
Meldonium reduced carnitine transporter protein expression across muscles of different contractile and metabolic phenotypes. A TaqMan PCR low‐density array card approach revealed the abundance of 189 mRNAs regulating fuel selection was altered in soleus muscle by meldonium, highlighting the modulation of discrete cellular functions and metabolic pathways.
These novel findings strongly support the premise that muscle carnitine availability is a primary regulator of fuel selection in vivo.
Abstract
The body carnitine pool is primarily confined to skeletal muscle, where it regulates carbohydrate (CHO) and fat usage. Meldonium (3‐(2,2,2‐trimethylhydrazinium)‐propionate) inhibits carnitine synthesis and tissue uptake, although the impact of carnitine depletion on whole‐body fuel selection, muscle fuel metabolism and its molecular regulation is under‐investigated. Male lean Zucker rats received water (control, n = 8) or meldonium‐supplemented water (meldonium, n = 8) for 10 days [1.6 g kg−1 body mass (BM) day−1 days 1–2, 0.8 g kg−1 BM day−1 thereafter]. From days 7–10, animals were housed in indirect calorimetry chambers after which soleus muscle and liver were harvested. Food and fluid intake, weight gain and physical activity levels were similar between groups from days 7 to 10. Compared to control, meldonium depleted muscle total carnitine (P < 0.001) and all carnitine esters. Furthermore, whole‐body fat oxidation was less (P < 0.001) and CHO oxidation was greater (P < 0.05) compared to the control, whereas soleus and liver glycogen contents were less (P < 0.01 and P < 0.01, respectively). In a second study, male Wistar rats received water (n = 8) or meldonium‐supplemented water (n = 8) as above, and kidney, heart and extensor digitorum longus muscle (EDL) and soleus muscles were collected. Compared to control, meldonium depleted total carnitine content (all P < 0.001), reduced carnitine transporter protein and glycogen content, and increased pyruvate dehydrogenase kinase 4 mRNA abundance in the heart, EDL and soleus. In total, 189 mRNAs regulating fuel selection were differentially expressed in soleus in meldonium vs. control, and a number of cellular functions and pathways strongly associated with carnitine depletion were identified. Collectively, these data firmly support the premise that muscle carnitine availability is a primary regulator of fuel selection in vivo.
Keywords: carnitine, fat and carbohydrate metabolism, muscle fuel selection
Key points
Meldonium inhibits endogenous carnitine synthesis and tissue uptake, and accelerates urinary carnitine excretion, although the impact of meldonium‐mediated muscle carnitine depletion on whole‐body fuel selection, and muscle fuel metabolism and its molecular regulation is under‐investigated.
Ten days of oral meldonium administration did not impact on food or fluid intake, physical activity levels or body weight gain in the rat, whereas it depleted muscle carnitine content (all moieties), increased whole‐body carbohydrate oxidation and muscle and liver glycogen utilization, and reduced whole‐body fat oxidation.
Meldonium reduced carnitine transporter protein expression across muscles of different contractile and metabolic phenotypes. A TaqMan PCR low‐density array card approach revealed the abundance of 189 mRNAs regulating fuel selection was altered in soleus muscle by meldonium, highlighting the modulation of discrete cellular functions and metabolic pathways.
These novel findings strongly support the premise that muscle carnitine availability is a primary regulator of fuel selection in vivo.
Abbreviations
- AMPK
AMP‐activated protein kinase
- BM
body mass
- CHO
carbohydrates
- CoA
coenzyme A
- CPT1
carnitine palmitoyl transferase 1
- EDL
extensor digitorum longus muscle
- IPA
ingenuity pathway analysis
- meldonium
3‐(2,2,2‐trimethylhydrazinium)‐propionate
- OCTN2
organic cation/carnitine transporter 2
- PDC
pyruvate dehydrogenase complex
- PDK4
pyruvate dehydrogenase kinase 4
- PPAR
peroxisome proliferator‐activated receptor
- PXR/RXR
pregnane X receptor
- YWHAZ
tyrosine 3‐monooxygenase/tryptophan 5‐monooxygenase activation protein
Introduction
Carnitine synthesis occurs mainly in the liver, although its storage is localized almost exclusively to the skeletal muscle (> 90%) where it is involved in two discreet metabolic functions (Stephens et al. 2007). Carnitine acts as an acetyl group buffer when the rate of oxidative carbohydrate (CHO) derived pyruvate decarboxylation to acetyl‐CoA, which is controlled by the mitochondrial pyruvate dehydrogenase complex, exceeds the rate of acetyl‐coenzyme A (CoA) utilization in the tricarboxylic acid cycle (Harris et al. 1987; Constantin‐Teodosiu et al. 1991a). Carnitine also plays a mandatory role in translocating cytosolic fatty acid derived long‐chain acyl‐CoAs across the otherwise impermeable inner mitochondrial membrane (Fritz & McEwen, 1959; Fritz & Yue, 1963). Furthermore, more recent evidence from studies in which muscle carnitine availability has been increased via insulin‐mediated stimulation of muscle carnitine accumulation (Stephens et al. 2006; Wall et al. 2011; Stephens et al. 2013), or pharmacologically depleted (Spaniol et al. 2001), suggests that carnitine availability per se is a key regulator of muscle fuel selection. In brief, a 20% increase in muscle carnitine content in young, healthy volunteers modulated changes in whole‐body energy expenditure and quadriceps muscle fuel metabolism and gene expression, entirely consistent with a carnitine‐mediated increase in muscle fat oxidation as a consequence of increased muscle long‐chain acyl‐group translocation via carnitine palmitoyl transferase 1 (CPT1) (Stephens et al. 2006; Wall et al. 2011; Stephens et al. 2013). Conversely, it has also been proposed that the decline in muscle free carnitine availability that occurs in parallel with increasing exercise intensity [as a result of its acetylation by increased pyruvate dehydrogenase complex (PDC) flux] will ultimately limit CPT1 flux and thereby muscle fat oxidation (van Loon et al. 2001). In keeping with this, the upsurge in muscle glycolytic and PDC flux that occurs during exercise as a consequence of increasing muscle glycogen availability prior to exercise, has been proposed to result in greater acetylation of muscle free carnitine, leading to a reduction in long‐chain fatty acid oxidation (Roepstorff et al. 2005).
Despite its significance, little information is available concerning the impact of reduced muscle total carnitine content on fuel selection in vivo. Meldonium (3‐(2,2,2‐trimethylhydrazinium)‐propionate), a γ‐butyrobetaine (carnitine precursor) analogue, has been shown to: (i) impair liver carnitine biogenesis (Simkhovich et al. 1988; Spaniol et al. 2001); (ii) accelerate urinary free carnitine excretion (Kuwajima et al. 1999; Spaniol et al. 2001); (iii) reduce muscle carnitine transport in vitro (Georges et al. 2000; Grigat et al. 2009); and (iv) reduce muscle carnitine availability and whole‐body palmitate oxidation in vivo (Spaniol et al. 2001). More than 90% of the body carnitine pool is restricted to skeletal muscle but, to the best of our knowledge, no research has collectively quantified the impact of meldonium administration on muscle carnitine content, daily energy and fluid intake, physical activity levels, whole‐body fuel selection, and muscle energy metabolism.
Skeletal muscle is composed of fibre types differing in their contractile and metabolic properties. We have previously shown that there were no differences in the content of carnitine moieties between fibre types in human vastus lateralis muscle at rest (Constantin‐Teodosiu et al. 1996). However, the magnitude of carnitine acylation in human skeletal muscle during prolonged submaximal exercise was markedly greater in type I compared to type II fibres, supporting the concept of fibre type‐specific functional roles for carnitine in skeletal muscle connected to acetyl group buffering and mitochondrial long‐chain acyl‐CoA translocation, which will change depending on exercise intensity (Constantin‐Teodosiu et al. 1996). Although meldonium‐mediated carnitine depletion has been reported in mixed‐fibred rodent gastrocnemius (Tsoko et al. 1995) and quadriceps femoris (Spaniol et al. 2001) muscles, little is known regarding its relative impact on muscles of widely different metabolic and physiological characteristics, particularly from the same animal. Roberts et al. (2015) reported that, compared to the control, meldonium‐mediated carnitine depletion was associated with reduced tension development and increased glycogen hydrolysis over 5 min of fatiguing contraction in muscle composed of predominantly Type II fibres [extensor digitorum longus (EDL)]. This contrasted with the response seen in the slow contracting soleus muscle where contractile function and energy metabolism during contraction were apparently unaffected compared to the control.
In the context of this background, the first aim of the present study was to test the hypothesis that, compared to the control, daily meldonium administration would have no impact on daily energy and fluid intake or physical activity levels in vivo in the rat. However, given the reciprocal relationship between lipid and CHO oxidation, it was also hypothesized that meldonium administration would attenuate whole‐body fat oxidation compared to the control animals as a result of depleting tissue carnitine stores, at the same time as increasing whole‐body CHO oxidation and reducing muscle and liver glycogen availability. A further aim was to highlight potential mechanisms by which meldonium‐mediated carnitine depletion may have such metabolic effects, by specifically targeting analysis genes and proteins assumed to control carnitine linked‐cellular fuel metabolism (e.g. carnitine transporter (OCTN2), pyruvate dehydrogenase kinase 4 (PDK4; carbohydrate oxidation) and CPT1 (fat oxidation), along with aspects of intermediary metabolism. Moreover, these analyses were performed in muscles of different contractile and metabolic phenotypes (heart, soleus and EDL muscles) to determine fibre specific effects of meldonium‐mediated carnitine depletion. The final aim of the present study was to identify changes in cellular functions and metabolic pathways resulting from meldonium‐induced carnitine depletion in soleus muscle [using low‐density microarray cards and ingenuity pathway analysis (IPA)], thereby providing novel insight of genes, cellular functions and metabolic pathways underpinning the comprehensive switch in fuel selection that we hypothesized would be seen at the muscle and whole body level in meldonium vs. control animals.
Methods
Two studies were performed to test the stated aims and hypotheses. The first was conducted at AstraZeneca Pharmaceuticals (Alderley Park, UK), where whole‐animal open circuit indirect calorimetry facilities were available, and the second was conducted in the animal research facility of the Biomedical Services Unit (University of Nottingham, Nottingham, UK). All tissue analyses were performed at the University of Nottingham. Both studies were approved by a local ethical review committee and had UK Home Office approval. All procedures were carried out in accordance with the Animals Scientific Act (1986). All animals were given free access to drinking water and standard chow throughout the study unless otherwise stated and body mass (BM) and fluid intake were monitored daily.
Study 1: The impact of meldonium administration on muscle carnitine content, whole‐body fuel oxidation, muscle and liver glycogen availability, daily energy and fluid intake and physical activity levels in vivo in the rat
Sixteen male, lean Zucker rats (Alderley Park colony; AstraZeneca Pharmaceuticals), with a body weight of 283 ± 6 g (mean ± S.E.M.), were singly housed in standard cages in temperature and humidity controlled animal holding rooms at AstraZeneca Pharmaceuticals (Alderley Park, UK). Following a 7 day period of acclimatization, animals were randomly assigned to either the control (n = 8) or meldonium (n = 8) treatment group. The control group received standard drinking water for 10 days, whereas the meldonium group received meldonium (Shandong Bangda Pharmaceutical Co, Ltd, ShanDong, China) supplemented drinking water over the same time period [priming dose of 1.6 g kg−1 BM day−1 on days 1–2, followed by 0.8 g kg−1 BM day−1 thereafter]. Meldonium solutions were prepared daily and the concentration prepared was based on an animal's body weight and fluid intake, which were monitored daily for 1 week before the study commenced. A similar dosing protocol was shown to result in a 13‐fold reduction in hepatic carnitine content in Wistar rats (Degrace et al. 2007), although we used a priming dose to ensure a steady‐state blood meldonium concentration was be reached relatively quickly.
From days 7 to 10, control and meldonium‐treated animals were housed in Oxymax (Columbus Instruments, Columbus, OH, USA) open circuit indirect calorimetry cages equipped with the Comprehensive Laboratory Animal Monitoring System (CLAMS), which allowed continuous measurement of O2 consumption () and CO2 production (). In addition, food hoppers and volumetric drinkers allowed precise measurement of feeding and drinking behaviour and total daily dietary intake. The CLAMS system was also configured to measure tri‐axis physical activity levels using a Columbus Instruments Opto‐M3 activity monitor. Each chamber was fitted with two rows of infrared beams. Physical activity was scored as either single beam breaks or consecutive beam breaks representing ambulation, thereby allowing differentiation between rearing, grooming and ambulatory related physical activity. Grooming and ambulatory related activity were summed to allow calculation of total activity. These measures allowed the differentiation between metabolic and physical activity‐related changes in and occurring as a result of any reduction in muscle carnitine content.
Fat and CHO oxidation rates were calculated from rates of and according to equations described previously (Frayn, 1983). Because urinary nitrogen excretion was not measured in the present study, fat and CHO oxidation data are not presented as non‐protein rates of substrate oxidation. Animals were allowed to acclimatize to the metabolic cages over the course of days 7–10 to ensure metabolic stability and, for the final 12 h period, were fasted to circumvent confounding effects of between‐group differences in food intake on metabolic rate and patterns of substrate oxidation over this 12 h period.
On the morning of day 10 of the study subsequent to this 12 h measurement period, animals were terminally anaesthetized with sodium thiobutabarbital (125 mg kg−1 i.p. Inactin; Sigma‐Aldrich, Poole, UK). The soleus muscle from each hind limb and a portion of the lateral hepatic lobe were then excised when still blood‐perfused in situ and immediately (< 5 s) submerged in liquid nitrogen.
Study 2: Mechanistic insight of the impact of meldonium‐mediated carnitine depletion on tissue fuel metabolism
Sixteen male Wistar rats (Charles River, Margate, UK), with a body weight 309 ± 5 g (mean ± S.E.M.), were housed in pairs in standard cages in temperature and humidity controlled animal holding rooms in the Biomedical Services Unit at the University of Nottingham. Following acclimatization to the experimental conditions, animals were randomly divided into two experimental groups that received the same interventions as described for Study 1 above: either standard drinking water (control, n = 8) or drinking water supplemented with meldonium (meldonium, n = 8) for 10 days (1.6 g kg−1 BM day−1 for 2 days and 0.8 g kg−1 BM day−1 thereafter). Meldonium solutions were prepared daily. After 10 days (i.e. morning of day 10), the EDL and soleus muscles of each hindlimb, along with the heart and a kidney, were harvested under terminal anaesthesia (sodium pentobarbital, 120 mg kg−1 i.p.; Sagatal; Rhone Merieux, Harlow, UK), and snap‐frozen in liquid nitrogen within 5 s of collection.
Metabolite measurements in soleus and liver (Study 1) and in the heart, EDL and soleus (Study 2)
Frozen tissue muscle samples were crushed under liquid nitrogen and a homogenate of wet tissue was freeze‐dried for 24 h. All visible blood and connective tissue was removed before each sample was powdered with forceps and a pestle and mortar. Metabolites were extracted from 6 to 10 mg of tissue powder in 0.5 mmol l−1 perchloric acid (containing 1 mmol l−1 EDTA) and, following centrifugation, the extracts were neutralized with 2.2 mmol l−1 KHCO3. To determine long‐chain acylcarnitine content, the acid insoluble tissue pellet was re‐suspended in 0.2 mmol l−1 KOH to alkali hydrolyse acylcarnitine bonds at 50°C for 2 h before neutralization with 5 mmol l−1 perchloric acid. Tissue free, long‐chain acylcarnitine and acetylcarnitine content were subsequently determined using radio‐enzymatic methods described previously (Cederblad & Lindstedt, 1972; Cederblad et al. 1990). Free carnitine and carnitine esters were summed in order to calculate tissue total carnitine content. In addition, ∼2 mg of powdered tissue was alkali‐extracted in 0.1 m NaOH to determine tissue glycogen content spectrophotometrically as described previously (Harris et al. 1974). In Study 2, the neutralized perchloric acid extracts were also used to determine muscle ATP and PCr content using a modified spectrophotometric version of the method of Harris et al. (1974) to accommodate the use of a plate reader.
Targeted mRNA expression measurements in EDL, soleus and heart in Study 2
Total RNA was extracted from ∼25 mg of EDL, soleus and heart muscle using Tri Reagent (Sigma‐Aldrich), and subsequently quantified spectrophotometrically at 260 nm with RNA purity being determined as the ratio of 260/280 nm readings. Thereafter, first‐strand cDNA synthesis was carried out in accordance with a methodology described previously (Constantin et al. 2007). OCTN2, PDK4 and CPT1 transcripts were quantified by TaqMan PCR using an ABI prism 7000 sequence detector (Applied Biosystems, Foster City, CA, USA), with 2 μl of cDNA, 18 μm of each primer and 5 μm probe and Universal TaqMan 2x PCR Mastermix (Eurogentec, Liege, Belgium). Each sample was run in duplicate. Primers and MGB TaqMan probes (Applied Biosystems) are designed such that probes span over exon–exon boundaries to avoid genomic amplification. Hydroxymethylbilane synthase was used as internal control, and all genes of interest were labelled with the fluorescent reporter 5‐FAM (5‐carboxyfluorescein). The thermal cycling conditions used were: 2 min at 50°C and then 10 min at 95°C, followed by 40 cycles at 95°C for 15 s and 60°C for 1 min.
Soleus muscle mRNA expression levels using TaqMan low‐density microarray cards in Study 2
Multiple mRNA expression measurements were made in accordance with the manufacturer's instructions on 100 ng of cDNA obtained from total mRNA isolated from the soleus muscle collected from seven animals in each treatment group using Applied Biosystems 384‐well microfluidics TaqMan array cards (format 192 duplicates). The genes investigated were considered to be representative of CHO, fat and cellular energy metabolism according to a search of the literature, as well as SA Biosciences (http://www.sabiosciences.com/) and IPA databases (https://analysis.ingenuity.com/). The Ct values generated by the Applied Biosystems 7900HT Fast‐Real Time PCR system were analysed and normalized to the geometric mean of the β‐microglobulin and tyrosine 3‐monooxygenase/tryptophan 5‐monooxygenase activation protein (YWHAZ) mRNA expression values using Real‐Time StatMiner (Integromics, Granada, Spain) software before being uploaded in Ingenuity Pathway Analysis (IPA) software (Redwood City, CA, USA) for pathway analysis of gene expression data. IPA is a web‐based commercial software application that enables analysis, integration and understanding of data obtained from gene expression, miRNA and SNP microarrays, as well as metabolomics, proteomics and RNA sequencing experiments. It can also be used for analysis of small‐scale experiments that generate gene and chemical lists. Data analysis and interpretation with IPA builds on the comprehensive, manually curated content of the Ingenuity Knowledge Base (https://analysis.ingenuity.com/). Powerful algorithms identify regulators, relationships, mechanisms, functions and pathways relevant to changes observed in an analysed dataset. Analytics go beyond pathway analysis to understand the experimental results within the context of biological systems (https://www.qiagen.com/ie/products/life-science-research/research-applications/gene-expression-analysis/analysis/ingenuity-pathway-analysis/).
Carnitine transporter (OCTN2) protein expression in Study 2
Samples of EDL, soleus, heart and kidney (positive control) were lysed in the presence of phosphatase and protease inhibitors and protein content was quantitated using a Bradford assay. Protein lysates were run on a 4–12% Bis‐Tris acrylamide gel (Invitrogen, Paisley, UK) for 2 h at constant voltage (200 V) and transferred to a polyvinylidene difluoride membrane overnight at a constant 100 mA in ice‐cold buffer (4°C) as described by Constantin et al. (2007). The protein transfer was checked using Ponceau S red staining before blocking the membrane in BSA‐Tris buffer saline‐tween (TBS‐T) for 1 h at room temperature. The membranes were then probed with the primary antibodies against OCTN2 (Santa Cruz Biotechnology; Santa Cruz, CA, USA) and the endogenous α‐actin (Sigma‐Aldrich, St Louis, MO, USA) overnight at 4°C. The next day, membranes were washed in TBS‐T, incubated with an IRDye 800 labelled anti‐goat secondary antibody and further quantitated by using an Odyssey® Infrared Imaging System (Li‐Cor, Lincoln, NE, USA).
Statistical and bioinformatics analysis
All values are presented as the mean ± SEM unless otherwise stated. Comparison of mean values between treatment groups was performed using a Student's unpaired t test, as well as one‐way or two‐way ANOVA with a Bonferroni post hoc test analysis where appropriate (Prism, version 4; GraphPad, San Diego, CA, USA). For the individual mRNA expression data depicted in Fig. 4, the fold‐change difference in gene expression in the meldonium group relative to control was calculated with the 2−ΔΔCt method, and an independent two‐group Mann–Whitney U test was used to identify between‐group differences. In all cases, P < 0.05 was considered statistically significant.
Figure 4. Muscle mRNA expression.
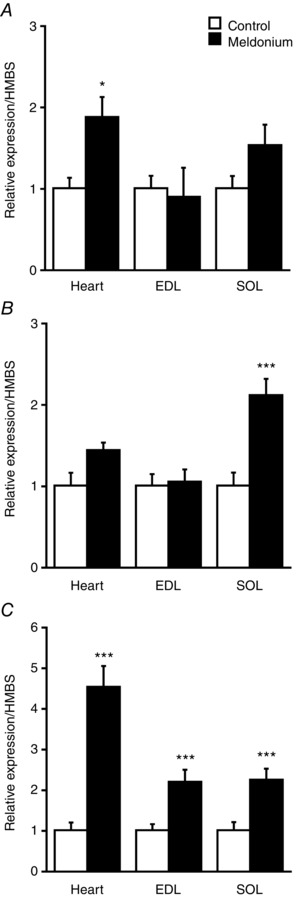
Muscle OCTN2 (A), CPT1 (B) and PDK4 (C) mRNA expression in the heart, EDL and soleus in control (n = 8) and meldonium (n = 8) treated Wistar rats. Values are the mean ± SEM. Significantly different from control: * P < 0.05 and *** P < 0.001, respectively.
StatMiner software (Thermo Fisher, Waltham, MA, USA) was used to generate a hierarchical clustering heat map (Fig. 6), depicting soleus muscle mRNA expression in the form of colours relative to housekeeping mRNAs (β‐microglobulin and YWHAZ) for individual animals in each experimental group. This was performed using Z‐score normalization, Ward's method for clustering and Euclidian distance as a similarity measurement. The selection criteria used were: calibrator group = control; selected target = meldonium; adjustment method for false discovery rate = Benjamin–Hochberg with the adjusted P value threshold set at 0.05; the relative quantification visualization scale = logarithmic. Regarding the Z‐score normalization, expression values in the hierarchical clustering were calculated as: x[i,j] Z‐score‐normalized = (x[i,j] ‐ mean[i]) / stdv[i]. Data in each row are centred on zero because the mean was subtracted from all values, and the results were divided by the SD, to prevent those rows with little variation losing contrast.
Figure 6. Data reflecting mRNA abundance.
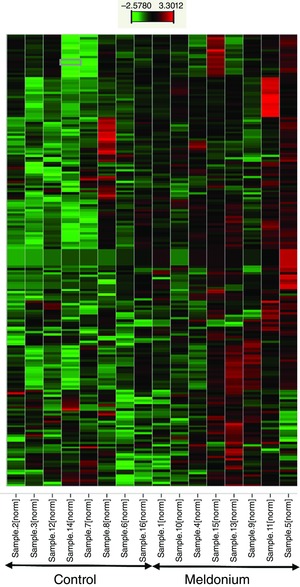
Representation of data reflecting mRNA abundance in the form of colours (heat map) relative to housekeeping genes in soleus muscle of individual control animals (left on the x‐axis, n = 8) and individual meldonium‐treated animals (right on the x‐axis, n = 8). Red indicates a greater relative abundance, whereas green indicates a less relative abundance.
Differences in cellular functions from control associated with meldonium administration are depicted in Fig. 8. This was produced by IPA, utilizing soleus muscle mRNA expression data generated in conjunction with microfluidic TaqMan array cards. The x‐axis displays cellular functions most affected by meldonium administration, whereas the y‐axis displays the –log of the P values. The P value associated with each cellular function is a measurement of the likelihood that the association between a set of focus transcripts and a given function is the result of random chance. The –log of P value was calculated using Fisher's exact test (right‐tailed).
Figure 8. Cellular functions identified by IPA as being altered from control (n = 8) in soleus muscle of meldonium‐treated animals (n = 8) based on mRNA expression data generated using the low‐density microarray cards.
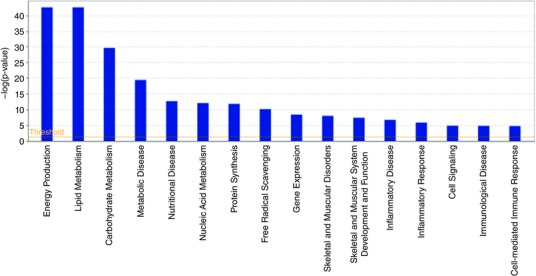
The x‐axis displays cellular functions most affected by meldonium administration, whereas the y‐axis displays the –log of the P value. The –log of the P value was calculated by Fisher's exact test right‐tailed (P < 0.05).
Metabolic pathways different from control in meldonium‐treated animals is shown in Fig. 9. Again, this was produced by IPA utilizing soleus muscle mRNA expression data generated in conjunction with microfluidic TaqMan array cards. The x‐axis displays those pathways most different from control following meldonium administration. The y‐axis on the left displays the –log of the P value for any given metabolic pathway. This was calculated by considering the number of measured transcripts that contributed to that pathway and the total number of transcripts known to be associated with that pathway in the Ingenuity Knowledge Base. The –log of the P value was calculated using Fisher's exact test (right‐tailed). The threshold was set at P < 0.05. The y‐axis on the right depicts the ratio of the number of genes in a given pathway that meet cut‐off criteria (P < 0.05), divided by the total number of genes that make up that pathway.
Figure 9. Metabolic pathways identified by IPA as being altered from control (n = 8) in soleus muscle of meldonium‐treated animals (n = 8) based on mRNA expression data generated using the low‐density microarray cards.
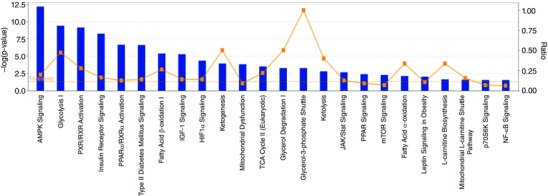
The x‐axis displays those pathways most affected by meldonium administration. The y‐axes display: (i) On the left, the –log of the P values. The P value for a given metabolic pathway was calculated by considering the number of focus transcripts that participated in the pathway and the total number of transcripts known to be associated with that pathway in the Ingenuity Knowledge Base. The –log of the P value was calculated using Fisher's exact test (right‐tailed). The threshold was set at P < 0.05. (ii) On the right, the ratio of the number of genes in a given pathway that meet cut‐off criteria (P < 0.05) divided by the total number of genes that make up that pathway.
Results
Study 1: The impact of meldonium administration on muscle carnitine content, whole‐body fuel oxidation, muscle and liver glycogen availability, daily energy and fluid intake and physical activity levels in vivo in the rat
Food and fluid intake, physical activity levels and BM
Daily food intake (20.5 ± 0.4 g day−1 vs. 19.3 ± 0.6 g day−1), fluid intake (32.3 ± 0.9 ml day−1 vs. 38.4 ± 1.5 ml day−1) and physical activity levels (10 305 ± 875 vs. 9219 ± 588 scores.day−1) were no different between control and meldonium‐treated groups, respectively, over the 3 days (days 7–10) that animals were housed in indirect calorimetry cages equipped with CLAMS. Furthermore, meldonium had no effect on BM gains during the 10 day study period compared to the control: control animals gained 6.2 ± 1.2 g in BM and meldonium‐treated animals gained 5.9 ± 2.0 g.
Metabolite measurements in soleus and liver
Soleus muscle carnitine esters in control and meldonium‐treated groups at the end of the 10 day study period are presented in Table 1. Meldonium‐treated animals had markedly less muscle free (90%, P < 0.001), acyl (65%, P < 0.05), acetyl (51%, P < 0.05) and total (80%, P < 0.001) carnitine content compared to the control. In addition, muscle and liver glycogen content in the meldonium group was less than in the control (22.4 ± 2.6 vs. 37.4 ± 4.0 mmol kg−1 dm, P < 0.01 and 6.0 ± 1.9 vs. 22.1 ± 3.8 mmol kg−1 dm, P < 0.01, respectively) (Fig. 1).
Table 1.
Carnitine ester content in soleus muscle of control (n = 8) and meldonium (n = 8) treated lean Zucker rats
| Control | Meldonium | |
|---|---|---|
| Free carnitine | 3.47 ± 0.24 | 0.35 ± 0.03*** |
| Long‐chain acylcarnitine | 0.27 ± 0.06 | 0.10 ± 0.03* |
| Acetylcarnitine | 1.11 ± 0.20 | 0.54 ± 0.06* |
| Total carnitine | 4.86 ± 0.13 | 0.99 ± 0.05*** |
Values are the mean ± SEM. Carnitine content expressed as mmol kg−1 dry muscle. Significant differences from the corresponding control value are indicated (* P < 0.05, *** P < 0.001 respectively).
Figure 1. Muscle and liver glycogen content.
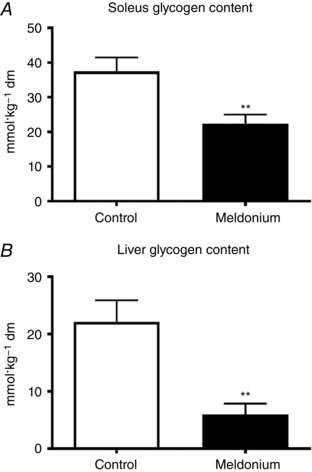
Soleus (A) and liver (B) glycogen content in control (n = 8) and meldonium (n = 8) treated lean Zucker rats. Values are expressed as the mean ± SEM. Significantly different from control: ** P < 0.01.
Whole‐body fuel oxidation
The rate of whole‐body fat oxidation on day 10 over the 12 h period during which animals were fasted is shown in Fig. 2. The rate of fat oxidation in the meldonium‐treated group was significantly less than in the control throughout (P < 0.001) (Fig. 2 A), such that total fat oxidation was 19% less in meldonium than in the control (7027 ± 123 vs. 5647 ± 127 μmol kg−1 BM, P < 0.001) (Fig. 2 B). Conversely, and in keeping with the muscle and liver glycogen responses reported (Fig. 1), the rate of CHO oxidation in the meldonium‐treated group over the initial 2 h period was greater than in the control (P < 0.05) (Fig. 3 A), resulting in the total CHO oxidation over the 12 h period being 16% greater in meldonium (3617 ± 155 vs. 4191 ± 202 μmol kg−1 BM, P < 0.05) (Fig. 3 B).
Figure 2. Rate of whole‐body fat oxidation.
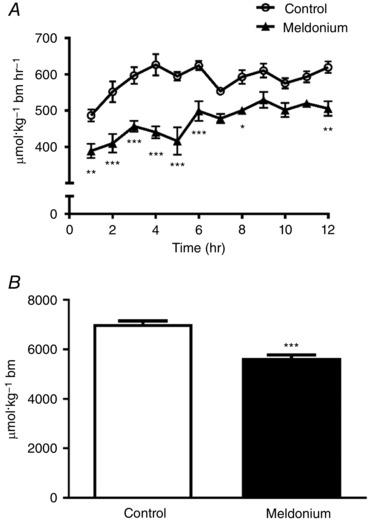
A, rate of whole‐body fat oxidation in control (n = 8) and meldonium (n = 8) treated lean Zucker rats over a 12 h period. Animals were allowed to acclimatize to their metabolic cages over the course of days 7–10 to ensure metabolic stability and, for the final 12 h period, were fasted to circumvent confounding effects of between‐group differences in food intake on metabolic rate and patterns of substrate oxidation over this 12 h period. Values are the mean ± SEM. Fat oxidation was significantly less throughout in meldonium compared to the control (Main effect treatment, P < 0.001; two‐way ANOVA). Significantly different from control: * P < 0.05, ** P < 001 and *** P < 0.001, respectively. B, total fat oxidation in control (n = 8) and meldonium (n = 8) treated lean Zucker rats. Values are the mean ± SEM. Significantly different from control: *** P < 0.001.
Figure 3. Rate of whole‐body carbohydrate oxidation.
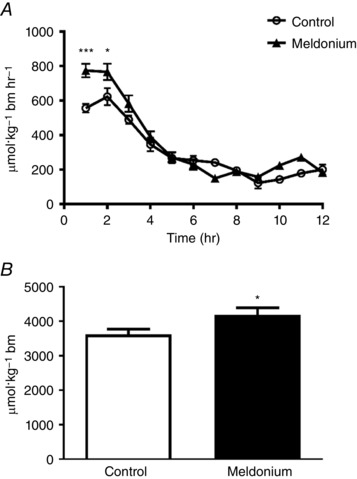
A, rate of whole‐body carbohydrate oxidation in control (n = 8) and meldonium (n = 8) treated lean Zucker rats over a 12 h period. Animals were allowed to acclimatize to their metabolic cages over the course of days 7–10 to ensure metabolic stability and, for the final 12 h period, were fasted to circumvent confounding effects of between‐group differences in food intake on metabolic rate and patterns of substrate oxidation over this 12 h period. Values are the mean ± SEM. Carbohydrate oxidation was significantly greater in meldonium compared to the control (main effect of treatment P < 0.05; two‐way ANOVA). Significantly different from control: * P < 0.05 and *** P < 0.001, respectively. B, total carbohydrate oxidation in control (n = 8) and meldonium (n = 8) treated lean Zucker rats. Values are the mean ± SEM. Significantly different from control: * P < 0.05.
Study 2: Mechanistic insight of the potential impact of meldonium‐mediated carnitine depletion on tissue fuel metabolism
Food and fluid intake, physical activity levels and BM
Meldonium supplementation had no impact on daily food or water consumption compared to the control. Meldonium administration did not affect BM over the course of the present study. The mean ± SEM mass gain was 70 ± 3 g in the control group compared to 68 ± 3 g in the meldonium group.
Metabolite measurements in the heart, EDL and soleus muscle
Tissue free carnitine content and carnitine esters are presented in Table 2. Free carnitine content was noticeably less in all tissues from meldonium‐treated animals compared to the control (by 90%, P < 0.001; 86%, P < 0.001; and 84%, P < 0.001 in the heart, EDL and soleusm, respectively). Long‐chain acylcarnitine content was also markedly less in the heart, EDL and soleus of meldonium‐treated animals compared to the control (by 72%, P < 0.001; 64%, P < 0.01; and 31%; P < 0.05, respectively), as was the acetylcarnitine content in the heart (39%, P < 0.05), EDL (55%, P < 0.05) and soleus (25%, P < 0.05). As expected from these observations, muscle total carnitine content was less in all tissues from meldonium‐treated animals compared to the control (by 81%, P < 0.001; 81%, P < 0.001; and 73%, P < 0.001, in the heart, EDL and soleus, respectively).
Table 2.
Muscle carnitine ester content in the heart, extensor digitorum longus (EDL) and soleus of control (n = 8) and meldonium (n = 8) treated Wistar rats
| Free carnitine | Long‐chain acylcarnitine | Acetylcarnitine | Total carnitine | |||||
|---|---|---|---|---|---|---|---|---|
| Control | Meldonium | Control | Meldonium | Control | Meldonium | Control | Meldonium | |
| Heart | 4.29 ± 0.27 | 0.44 ± 0.03*** | 0.39 ± 0.05 | 0.11 ± 0.04*** | 0.84 ± 0.11 | 0.49 ± 0.10* | 5.52 ± 0.25 | 1.04 ± 0.11*** |
| EDL | 3.59 ± 0.17 | 0.49 ± 0.04*** | 0.50 ± 0.08 | 0.18 ± 0.05** | 0.46 ± 0.09 | 0.19 ± 0.03* | 4.55 ± 0.19 | 0.86 ± 0.10*** |
| Soleus | 3.03 ± 0.10 | 0.47 ± 0.03*** | 0.29 ± 0.05 | 0.20 ± 0.04* | 0.51 ± 0.05 | 0.38 ± 0.03* | 3.84 ± 0.08 | 1.05 ± 0.08*** |
Values are the mean ± SEM. Carnitine content expressed as mmol kg−1 dry muscle. Significant differences from the corresponding control value are indicated (* P < 0.05, ** P < 0.01 and *** P < 0.001, respectively).
Tissue ATP, PCr and glycogen content is presented in Table 3. No differences in ATP or PCr content were observed when comparing carnitine‐depleted and control animals. Glycogen content was less in the heart, EDL and soleus in the carnitine‐depleted animals compared to the control (29%, P < 0.05, 26%, P < 0.05 and 26%, P < 0.05, respectively).
Table 3.
Muscle ATP, PCr and glycogen content in the heart, extensor digitorum longus (EDL), and soleus of control (n = 8) and meldonium (n = 8) treated Wistar rats
| ATP | PCr | Glycogen | ||||
|---|---|---|---|---|---|---|
| Control | Meldonium | Control | Meldonium | Control | Meldonium | |
| Heart | 16.6 ± 0.5 | 18.3 ± 1.1 | 6.8 ± 0.8 | 11.4 ± 1.1 | 93.3 ± 7.9 | 66.6 ± 4.8* |
| EDL | 25.4 ± 1.0 | 26.0 ± 1.0 | 72.8 ± 4.8 | 76.2 ± 7.2 | 113.5 ± 10.4 | 83.5 ± 8.4* |
| soleus | 14.4 ± 1.1 | 15.3 ± 1.3 | 40.2 ± 3.1 | 38.3 ± 3.7 | 80.1 ± 5.9 | 59.5 ± 8.1* |
Values are the mean ± SEM. Contents are expressed as mmol kg−1 dry muscle. Significant differences from the corresponding control value are indicated (* P < 0.05)
Targeted mRNA expression in the heart, EDL and soleus muscle
OCTN2, CPT‐1B and PDK4 mRNA expression in the heart, EDL and soleus muscle from control and meldonium‐treated animals is shown in Fig. 4. OCTN2 mRNA expression was greater in the heart from meldonium‐treated animals compared to the control (1.9‐fold, P < 0.05) but not in EDL and soleus. Expression of CPT‐1 mRNA in the heart and EDL was no different between treatment groups, although its expression was greater in the soleus of meldonium‐treated animals (2.1‐fold, P < 0.001). PDK4 mRNA expression was greater than in the control in the heart (4.5‐fold, P < 0.001), EDL (2.2‐fold, P < 0.001) and soleus (2.2‐fold, P < 0.001) from meldonium‐treated animals.
OCTN2 protein expression
Carnitine transporter protein expression and representative western blots of OCTN2 protein detected at 63 kDa are presented in Fig. 5. OCTN2 protein expression in the heart, soleus, EDL and kidney (with the latter used as a positive internal control because of its high OCTN2 expression (Tamai et al. 1998) was less in meldonium‐treated animals than in the control (78%, P < 0.001; 54%, P < 0.05; 56%, P < 0.05; 36%, P < 0.05; respectively).
Figure 5. Muscle protein expression.
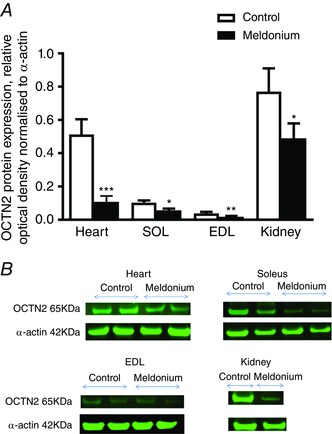
A, muscle OCTN2 protein expression in the heart, EDL, soleus and kidney in control (n = 8) and meldonium (n = 8) treated Wistar rats. Values are the mean ± SEM. Kidney (n = 2) is present as a positive internal control. Values are expressed as relative optical density normalized to α‐actin. Significantly different from control: * P < 0.05, ** P < 0.01 and *** P < 0.001, respectively. B, representative blots of OCTN2 located at 63 kDa in the heart, EDL, soleus and kidney.
Soleus muscle mRNA expression levels using TaqMan low‐density microarray cards
Figure 6 depicts a hierarchical clustering heat map illustrating relative soleus muscle mRNA expression in individual animals of the two treatment groups (data were normalized to the geometric mean of the housekeeping genes β‐microglobulin and YWHAZ (tyrosine 3‐monooxygenase/tryptophan 5‐monooxygenase activation protein) for all 189 transcripts investigated; see Methods). Although between animal variation was present, a pattern of between treatment group divergence was also apparent, such that soleus muscle mRNA expression in meldonium‐treated animals was generally greater (red) relative to housekeeping genes, compared to control animals, where generally less mRNA expression relative to the same housekeeping genes was observed (green). Overall, 158 transcripts were up‐regulated and 31 transcripts were down‐regulated in meldonium‐treated animals compared to the control (−2.6 ≤ range ≤ 3.3‐fold change). Figure 7 shows that of those genes up‐regulated in meldonium‐treated animals compared to the control, insulin receptor (INSR), C2‐12 straight‐chain acyl‐CoA dehydrogenase (ACADM), acetyl‐CoA carboxylase (MLYCD), hydroxysteroid (17‐β) dehydrogenase (HSD17B4) and, to a lesser extent, PDK4, carnitine palmitoyl‐transferase 2 (CTP2) Akt1 and IL‐6, were the most notably affected. Of those genes down‐regulated in meldonium relative to control, FOXO1, peroxisome proliferator‐activated receptor α, δ, γ, peroxisome proliferator‐activated receptor (PPAR) γ, coactivator 1α (PPARGC1A), transforming growth factor, β (TGFB1) and, to a lesser extent, carnitine transporter (SLC22A5) and sterol regulatory element binding transcription factor 1 (SREBF1), were the most affected (Fig. 7). Based on the collective differences in mRNA expression between meldonium and control animals, IPA predicted a comprehensive inhibition of soleus muscle fat oxidation, and particularly long‐chain fatty acid and palmitic acid oxidation, along with a prediction of an increase in glucose oxidation, albeit to a lesser extent (Fig. 7).
Figure 7. IPA prediction of energy metabolism in soleus muscle based upon changes in mRNA expression from control in meldonium‐treated animals.
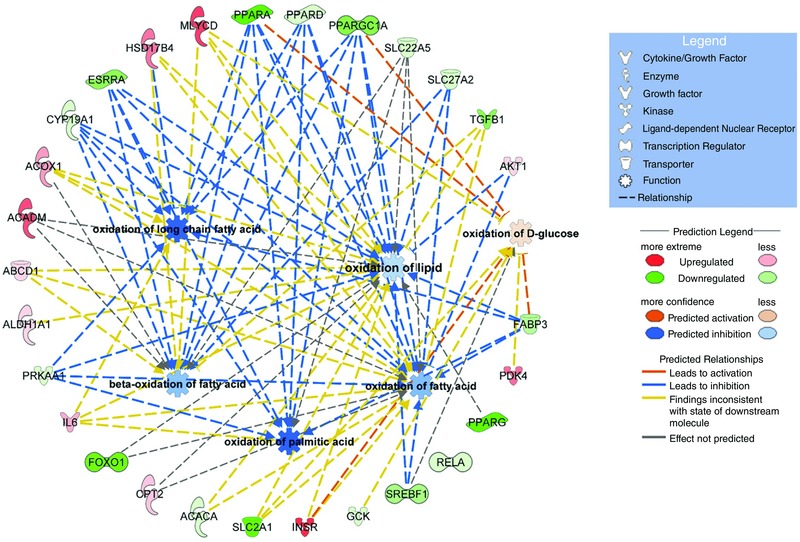
Schematic highlighting the most differentially regulated mRNAs (outer ring) and the predicted metabolic events resulting from these changes. PPARA, PPRD, PPARG, peroxisome proliferator‐activated receptor α, δ and γ; PPARGC1A, PPARG coactivator 1α; SLC22A5, solute carrier family 22 member 5 (carnitine transporter); SL27A2, very long‐chain‐fatty‐acid‐CoA ligase; TGFB1, transforming growth factor β1; AKT1, Akt serine‐threonine protein kinase 1; FABP3, fatty acid binding protein 3; SREBF1, sterol regulatory element binding transcription factor 1; GCK, glucokinase, INSR, insulin receptor; SLC2A1, solute carrier family 2 member 1; ACACA, acetyl‐CoA carboxylase α; FOXO1, forkhead box O1; IL6, interleukin 6; PRKAA1, protein kinase AMP‐activated catalytic subunit α1; ALDH1A1, aldehyde dehydrogenase 1 family member A1; ABCD1, ATP binding cassette subfamily D member 1; ACADM, acyl‐CoA dehydrogenase C4 to C12 straight chain; ACOX1, acyl‐CoA oxidase 1; CYP19A1, cytochrome P450 family 19 subfamily A member 1.
Another way of considering the data is highlighted in Fig. 8, which shows predicted changes in cellular functions as a result of meldonium administration generated by IPA (using the soleus mRNA expression from the TaqMan array cards). In keeping with Fig. 7, the most noticeably affected cellular functions associated with meldonium administration were energy production, lipid metabolism and CHO metabolism [–log (P value) ≥ 30]. Figure 9 highlights those metabolic pathways that are most different from control (up or down) in meldonium‐treated animals and that also probably underpin the shifts in cellular functions predicted in Fig. 8. AMP‐activated protein kinase (AMPK) signalling, glycolysis, nuclear receptor pregnane X receptor (PXR/RXR) activation, insulin receptor signalling, PPARα activation and fatty acid β‐oxidation were the most obviously affected [–log (P value) > 5].
Discussion
Carnitine plays well‐documented roles in muscle energy metabolism, and the ability to alter the muscle carnitine pool is central to furthering our understanding of the role of carnitine availability in the regulation of muscle fuel selection. Several studies have investigated the impact of increasing muscle carnitine availability on muscle energy metabolism in humans (Stephens et al. 2006; Wall et al. 2011; Stephens et al. 2013), although little is known about the metabolic consequences of muscle carnitine depletion. Meldonium is considered to inhibit hepatic carnitine biogenesis, tissue carnitine transport and renal carnitine retention (Spaniol et al. 2001). In the present study, we demonstrate that 10 days of oral meldonium administration was associated with markedly lower muscle carnitine content relative to control (an effect seen across all carnitine esters and in several muscle phenotypes in two independent experiments). Moreover, this was paralleled by lower rates of whole‐body fat oxidation relative to control animals and, in keeping with this, greater rates of whole‐body CHO oxidation, as well as muscle and liver glycogen depletion. Meldonium did not impact upon daily energy or fluid intake, habitual physical activity levels or BM gains. Collectively, these novel findings support the premise that muscle carnitine availability is a primary regulator of muscle fuel selection in vivo.
Although carnitine is the primary substrate for the carnitine transporter OCTN2, it is known that efficacy of transmembrane transport of meldonium by OCTN2 is greater than that of carnitine (Grigat et al. 2009). Moreover, administration of meldonium to rodents in vivo increased urinary free carnitine excretion by 30‐fold (Kuwajima et al. 1999; Spaniol et al. 2001). In the present study, 10 days of meldonium treatment resulted in substantial differences in muscle total carnitine content compared to the control in two rodent species, which is consistent with the observations reported elsewhere (Spaniol et al. 2001; Roberts et al. 2015). Furthermore, we demonstrated that this effect was distributed across all muscle carnitine esters, and in the heart and muscles of widely different metabolic phenotypes (Tables 1 and 2). Of importance, this meldonium‐mediated carnitine depletion was accompanied by less expression of carnitine transporter protein (OCTN2) in all tissues relative to control (Fig. 5). Heart, in particular, showed the most marked (70%) difference in OCTN2 protein expression from control, which was paralleled by greater OCTN2 mRNA expression (1.9‐fold) relative to control (not evident in skeletal muscle), pointing to the induction of a transcriptional response to restore carnitine transporter protein in this tissue. It appears therefore that OCTN2 mRNA and protein expression levels in the heart are more tightly regulated by carnitine availability than in muscle. Indeed, given heart carnitine content can be acutely increased by dietary or i.v. carnitine administration (Bartels et al. 1992), which is not the case for skeletal muscle (Stephens et al. 2006), this also supports the suggestion that heart tissue exhibits greater sensitivity to acute changes in plasma and tissue carnitine availability. The present observations are also consistent with the report that meldonium does not affect OCTN2 mRNA expression in mixed‐fibre skeletal muscles (quadriceps femoris; Schurch et al. 2010), suggesting that muscle OCTN2 transcription does not respond to acute plasma and tissue carnitine depletion, nor to increased OCTN2 substrates, such as meldonium and γ‐butyrobetaine, which are elevated in the plasma as a consequence of meldonium administration (Liepinsh et al. 2006; Liepinsh et al. 2011b). However, under conditions of chronically reduced plasma and muscle carnitine availability, as occurs in long‐standing human vegetarianism, a decline in muscle OCTN2 protein expression can be expected in humans (Stephens et al. 2011).
Although pharmacological carnitine depletion has been documented in gastrocnemius, quadriceps, soleus and EDL muscles, as well as the heart and liver (Tsoko et al. 1995; Spaniol et al. 2001; Liepinsh et al. 2006; Degrace et al. 2007; Liepinsh et al. 2008; Liepinsh et al. 2009a), the metabolic impact of muscle carnitine depletion on lipid and CHO oxidation remains under‐investigated. Collectively, our observations strongly advocate that the meldonium‐mediated reduction in muscle free carnitine content observed was limiting to muscle CPT1 flux, as indicated by the lower long‐chain muscle acylcarnitine (end product of CPT1 reaction) content compared to the control (Tables 1 and 2) and, ultimately, the lower rate of whole‐body fat oxidation in meldonium‐treated animals vs. control (Fig. 2). In line with our findings, Spaniol et al. (2001) reported that muscle carnitine content was reduced after 3 weeks of meldonium administration in the rat, whereas expired 14CO2 following acute 14C palmitate administration was also reduced. Spaniol et al. (2001) attributed this acute reduction in 14C palmitate oxidation to a decline in hepatic fat oxidation. However, this could perhaps be expected given the palmitate tracer was administered i.p. Indeed, given that > 90% of the body total carnitine pool is restricted to the skeletal muscle (Brass, 1995), the major part of the change in tissue fuel selection was probably muscle specific. In vitro experiments have reported the Michaelis–Menten constant (K m) of CPT1 for carnitine is 0.5 mmol l−1 (McGarry et al. 1983) and, in the present study, muscle free carnitine content in meldonium‐treated animals was 0.35 ± 0.03 mmol kg−1 dm (or < 0.1 mmol l−1 intracellular water), which is well below this reported K m value and would have markedly reduced muscle CPT1 enzyme kinetics. Further evidence that muscle free carnitine depletion plays a key role in reducing CPT1 flux and regulating fuel selection comes from studies of patients with systemic carnitine deficiency, in whom muscle carnitine is depleted and the capacity to oxidize long‐chain fatty acids is reduced (Engel & Angelini, 1973). The observation that both muscle long‐chain acylcarnitine content and long‐chain fatty acid oxidation are reduced under conditions of exercise‐mediated carnitine acetylation in healthy volunteers also lends support to this premise (Sidossis et al. 1996b; van Loon et al. 2001; Rasmussen et al. 2002). The current data also complement studies proposing that increasing, rather than decreasing, muscle carnitine availability increases lipid utilization at rest and spares muscle glycogen use during low‐intensity exercise (Stephens et al. 2006; Wall et al. 2011; Stephens et al. 2013) in healthy, young volunteers.
The reduction in whole‐body fat oxidation observed in meldonium‐mediated carnitine‐depleted animals compared to the control (Fig. 2) was accompanied by a concurrent increase in whole‐body CHO oxidation (16% greater in the meldonium group compared to the control) (Fig. 3), as well as lower muscle and liver glycogen content (Fig. 1). This is not surprising given the well‐documented reciprocal relationship between muscle fat and CHO oxidation (Randle et al. 1963; Sidossis & Wolfe, 1996a). Interestingly, PDK4 mRNA expression was greater in the heart, soleus and EDL muscles of meldonium‐treated animals relative to control (Fig. 4 C), which we suggest could reflect an adaptive response aimed at inhibiting PDC activation and sparing CHO as a consequence of a carnitine depletion mediated increase in tissue CHO use. Indeed, muscle PDK4 mRNA expression has been reported to be increased under conditions of reduced muscle glycogen availability (exercise‐mediated), which was proposed to be attributable to glycogen availability per se up‐regulating PDK4 transcription (Pilegaard et al. 2002). Furthermore, we have previously reported that increased PDK4 mRNA expression is accompanied by an increase in PDK4 protein expression in rodents (Constantin et al. 2007; Crossland et al. 2008; Crossland et al. 2010) and in humans (Constantin et al. 2011) under a variety of experimental conditions, and we we have no reason to assume that this did not occur in the present study. However, because whole‐body fat oxidation was lower in the meldonium‐treated animals compared to the control, we acknowledge that allosteric regulation of PDC could have also played a role in the increase in whole‐body CHO oxidation and muscle glycogen use. Of note, unlike the effect on fat oxidation, the impact of muscle carnitine depletion on CHO oxidation dissipated over the 12 h study period (Fig. 3 A), such that CHO oxidation declined as body CHO stores became exhausted (indicated by the very low muscle and liver glycogen content in the meldonium‐treated animals (Fig. 1 and Table 2). This is probably attributable to the animals being fasted over this time, which was necessary to allow direct comparison of the whole body and tissue fuel utilization between meldonium and control treated animals. By contrast to humans, where an overnight fast will have minimal impact on skeletal muscle glycogen stores (∼400 mmol kg−1 dry muscle), rodents are metabolically active, as reflected by a relatively high metabolic turnover requiring liver and muscle glycogen utilization during fasting (Freminet & Leclerc, 1980), and as corroborated by the fasted state low liver and muscle glycogen levels even in the control animals of the present study (Fig. 1). Notably, meldonium treatment did not affect food intake, water consumption or physical activity levels over the 3 day period when animals were housed in the calorimetric chambers, demonstrating that the meldonium‐induced changes in whole‐body fuel oxidation during the final 12 h of the study, when animal access to food was removed, occurred independent of diet and/or habitual physical activity level related changes in energy metabolism.
An important objective of the present study was to identify changes in cellular functions and metabolic pathways from the control as a result of meldonium‐induced carnitine depletion in soleus muscle using low‐density microarray cards and IPA. Based on fold changes in mRNA expression of 192 targeted transcripts (158 up‐regulated and 31 down‐regulated relative to control), IPA predicted a comprehensive inhibition of fat oxidation in soleus muscle, and particularly long‐chain fatty acid and palmitic acid oxidation, along with an increase in glucose oxidation (Fig. 7). In keeping with this, the cellular functions most notably affected by carnitine depletion were energy production, lipid metabolism and CHO metabolism (Fig. 8). Furthermore, IPA highlighted AMPK signalling, glycolysis, nuclear receptor PXR/RXR activation, insulin receptor signalling, PPARα activation and fatty acid β‐oxidation as being the most probable pathways mediating these changes in cellular functions [–log(P valued) ≥ 30] (Fig. 9). Although AMPK is accepted to be activated by a decrease in the cellular ATP/ADP ratio (Fimland et al. 2010), we found no evidence of a meldonium‐mediated reduction in ATP or PCr (a highly sensitive marker of cellular energy status) content in any tissue investigated (Table 3). However, AMPK is also activated by several drugs and xenobiotics, including anti‐diabetic drugs (such as metformin and rosiglitazone) (Fryer et al. 2002) or, more pertinent to the present study, by low muscle glycogen availability (Richter & Ruderman, 2009). Of note, carnitine depletion markedly suppressed the expression of several PPARs (Fig. 7), which is in keeping with the observation that carnitine supplementation increases the level of muscle PPARα mRNA in human skeletal muscle (Stephens et al. 2013). Our approach has therefore generated novel insight of mechanisms associated with the comprehensive differences in fuel use at a whole body and tissue level in meldonium‐treated animals vs. control. Whether these mRNA expression differences occurred as a direct result of meldonium administration markedly reducing carnitine availability and/or indirectly as a result of a change in tissue fuel utilization cannot be determined from the results of the present study.
Carnitine depletion resulted in a two‐fold increase in CPT‐1 mRNA expression in the soleus, although not in the heart or EDL. Although carnitine depletion has been reported to impair CPT‐1 activity in the heart (Liepinsh et al. 2009a), heart tissue is known to express both CPT‐1A (hepatic) and CPT‐1B (muscle) isoforms, whereas skeletal muscle expresses only CPT‐1B. Because the K m of CPT‐1B for carnitine is ∼60‐fold greater than that of CPT‐1A (McGarry et al. 1983), this may explain why CPT‐1 mRNA expression was increased in the soleus relative to control, particularly when considering that free carnitine was reduced to 0.47 mmol kg−1 dry muscle (∼0.17 mmol l−1 intracellular water) in this muscle, which is well below the reported K m of CPT‐1 for carnitine (0.5 mmol l−1) (Brass, 1995). In keeping with this, oxfenicine (a muscle‐specific inhibitor of CTP‐1B activity) has been associated with decreased fat oxidation and accumulation and increased CHO use in mouse gastrocnemius muscle (Keung et al. 2013).
In summary, we have demonstrated that acute meldonium administration caused widespread and substantial tissue carnitine depletion in vivo, which was associated with reduced OCTN2 protein expression in the heart, soleus and EDL muscles compared to the control. Relative to control, carnitine depletion was also linked to lower glycogen content across these tissues of differing phenotype, and was accompanied by an increase in PDK4 mRNA expression in all tissues. Of further novelty, muscle carnitine depletion was associated with a lower rate of whole‐body fat oxidation in vivo compared to the control, and a concomitant greater rate of whole‐body CHO oxidation, which was associated with lower muscle and liver glycogen contents in these animals. Further scrutiny identified a comprehensive difference in soleus muscle expression of mRNAs regulating a number of fuel selection regulatory pathways in meldonium vs. control animals, which we propose was mechanistically linked to the differences seen in fuel oxidation and glycogen content between the treatment groups. These findings complement reports documenting that an increase in human muscle carnitine content produces adaptive metabolic and genomic responses consistent with an increase in muscle lipid oxidation relative to control (Stephens et al. 2013) and, collectively, they highlight a central role of muscle carnitine availability in the regulation of muscle fuel selection.
Additional information
Competing interests
The authors declare that they have no competing interests.
Funding
The present study was supported by AstraZeneca Pharmaceuticals, the University of Nottingham and a BBSRC CASE award. AstraZeneca Pharmaceuticals had a role in the study design.
Author contributions
CP, DCT, SP and PLG were responsible for generation of experimental hypothesis and study design. CP, DCT, DC were responsible for research data. DC, BL, SP reviewed the manuscript. CP, DCT, DC and PLG wrote and the manuscript. CP, DCT, DC, BL, SP and PLG edited the manuscript. All authors approved the final version of the manuscript; and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved; and all persons designated as authors qualify for authorship, and all those who are eligible for authorship are listed.
Acknowledgements
We would like to thank Professor Sheila M. Gardiner from The University of Nottingham for her valuable help with the animal work in study 2.
Linked articles This article is highlighted by a Perspective by Krähenbühl. To read this Perspective, visit https://doi.org/10.1113/JP274755.
This is an Editor's Choice article from the 1 September 2017 issue.
References
- Bartels GL, Remme WJ, Pillay M, Schonfeld DH, Cox PH, Kruijssen HA & Knufman NM (1992). Acute improvement of cardiac function with intravenous L‐propionylcarnitine in humans. J Cardiovasc Pharm 20, 157–164. [PubMed] [Google Scholar]
- Brass EP (1995). Pharmacokinetic considerations for the therapeutic use of carnitine in hemodialysis patients. Clin Ther 17, 176–185. [DOI] [PubMed] [Google Scholar]
- Cederblad G, Carlin J, Constantin‐Teodosiu D, Harper P & Hultman E (1990). Radioisotopic assays of CoASH and carnitine and their acetylated forms in human skeletal muscle. Anal Biochem 185, 274–278. [DOI] [PubMed] [Google Scholar]
- Cederblad G & Lindstedt S (1972). A method for the determination of carnitine in the picomole range. Clin Chim Acta 37, 235–243. [DOI] [PubMed] [Google Scholar]
- Constantin‐Teodosiu D, Carlin J, Cederblad G, Harris R & Hultman E (1991a). Acetyl group accumulation and pyruvate dehydrogenase activity in human muscle during incremental exercise. Acta Physiol Scand 143, 367–372. [DOI] [PubMed] [Google Scholar]
- Constantin‐Teodosiu D, Howell S & Greenhaff P (1996). Carnitine metabolism in human muscle fiber types during submaximal dynamic exercise. J Appl Physiol 80, 1061–1064. [DOI] [PubMed] [Google Scholar]
- Constantin D, Constantin‐Teodosiu D, Layfield R, Tsintzas K, Bennett A & Greenhaff PL (2007). PPARdelta agonism induces a change in fuel metabolism and activation of an atrophy programme, but does not impair mitochondrial function in rat skeletal muscle. J Physiol 583, 381–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantin D, McCullough J, Mahajan RP & Greenhaff PL (2011). Novel events in the molecular regulation of muscle mass in critically ill patients. J Physiol 589, 3883–3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossland H, Constantin‐Teodosiu D, Gardiner SM, Constantin D & Greenhaff PL (2008). A potential role for Akt/FOXO signalling in both protein loss and the impairment of muscle carbohydrate oxidation during sepsis in rodent skeletal muscle. J Physiol 586, 5589–5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossland H, Constantin‐Teodosiu D, Greenhaff PL & Gardiner SM (2010). Low‐dose dexamethasone prevents endotoxaemia‐induced muscle protein loss and impairment of carbohydrate oxidation in rat skeletal muscle. J Physiol 588, 1333–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degrace P, Demizieux L, Du Z, Gresti J, Caverot L, Djaouti L, Jourdan T, Moindrot B, Guilland J, Hocquette J & Clouet P (2007). Regulation of lipid flux between liver and adipose tissue during transient hepatic steatosis in carnitine‐depleted rats. J Biol Chem 20, 20816–20826. [DOI] [PubMed] [Google Scholar]
- Engel A & Angelini C (1973). Carnitine deficiency of human skeletal muscle with associated lipid storage myopathy: a new syndrome. Science 179, 899–902. [DOI] [PubMed] [Google Scholar]
- Fimland MS, Helgerud J, Gruber M, Leivseth G & Hoff J (2010). Enhanced neural drive after maximal strength training in multiple sclerosis patients. Eur J Appl Physiol 110, 435–443. [DOI] [PubMed] [Google Scholar]
- Frayn K (1983). Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol 55, 628–634. [DOI] [PubMed] [Google Scholar]
- Freminet A & Leclerc L (1980). Effect of fasting on liver and muscle glycogen in rats and guinea pigs. J Physiol (Paris) 76, 877–880. [PubMed] [Google Scholar]
- Fritz I & Yue K (1963). Long chain carnitine acyl transferase and the role of acylcarnitines in the catalytic increase of fatty acid oxidation by carnitine. J Lipid Res 4, 279–288. [PubMed] [Google Scholar]
- Fritz IB & McEwen B (1959). Effects of carnitine on fatty‐acid oxidation by muscle. Science 6, 334–335. [DOI] [PubMed] [Google Scholar]
- Fryer LG, Parbu‐Patel A & Carling D (2002). The Anti‐diabetic drugs rosiglitazone and metformin stimulate AMP‐activated protein kinase through distinct signaling pathways. J Biol Chem 277, 25226–25232. [DOI] [PubMed] [Google Scholar]
- Georges B, Le Borgnea F, Gallanda, IM , Ecossea D, Grand‐Jeana F & Demarquoy J (2000). Carnitine transport into muscular cells. inhibition of transport and cell growth by mildronate. Biochem Pharm 59, 1357–1363. [DOI] [PubMed] [Google Scholar]
- Grigat S, Fork C, Bach M, Golz S, Geerts A, Schömig E & Gründemann D (2009). The carnitine transporter SLC22A5 is not a general drug transporter, but it efficiently translocates mildronate. Drug Metab Dispos 37, 330–337. [DOI] [PubMed] [Google Scholar]
- Harris R, Foster C & Hultman E (1987). Acetylcarnitine formation during intense muscular contraction in humans. J Appl Physiol 63, 440–442. [DOI] [PubMed] [Google Scholar]
- Harris R, Hultman E & Nordesjö L (1974). Glycogen, glycolytic intermediates and high‐energy phosphates determined in biopsy samples of musculus quadriceps femoris of man at rest. Methods and variance of values. Scand J Clin Lab Invest 33, 109–120. [PubMed] [Google Scholar]
- Keung W, Ussher JR, Jaswal JS, Raubenheimer M, Lam VH, Wagg CS & Lopaschuk GD (2013). Inhibition of carnitine palmitoyltransferase‐1 activity alleviates insulin resistance in diet‐induced obese mice. Diabetes 62, 711–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwajima M, Harashima H, Hayashi M, Saori I, Masako S, Kang‐Mo L, Kiwada H, Sugiyama Y & Shima K (1999). Pharmacokinetic analysis of the cardioprotective effect of 3‐(2,2,2‐ trimethylhydrazinium) propionate in mice: inhibition of carnitine transport in kidney. J Pharm Exp Therap 289, 93–102. [PubMed] [Google Scholar]
- Liepinsh E, Kuka J, Svalbe B, Vilskersts R, Skapare E, Cirule H, Pugovics O, Kalvinsh I & Dambrova M (2009a). Effects of long‐term mildronate treatment on cardiac and liver functions in rats. Basic Clin Pharmacol Toxicol 105, 387–394. [DOI] [PubMed] [Google Scholar]
- Liepinsh E, Skapare E, Svalbe B, Makrecka M, Cirule H & Dambrova M (2011b). Anti‐diabetic effects of mildronate alone or in combination with metformin in obese Zucker rats. Eur J Pharm 658, 277–283. [DOI] [PubMed] [Google Scholar]
- Liepinsh E, Vilskersts R, Loca D, Kirjanova O, Pugovichs O, Kalvinsh I & Dambrova M (2006). Mildronate, an inhibitor of carnitine biosynthesis, induces an increase in gamma‐butyrobetaine contents and cardioprotection in isolated rat heart infarction. J Cardivasc Pharm 48, 314–319. [DOI] [PubMed] [Google Scholar]
- Liepinsh E, Vilskersts R, Skapare E, Svalbe B, Kuka J, Cirule H, Pugovics O, Kalvinsh I & Dambrova M (2008). Mildronate decreases carnitine availability and up‐regulates glucose uptake and related gene expression in the mouse heart. Life sci 83, 613–619. [DOI] [PubMed] [Google Scholar]
- McGarry J, Mills S, Long C & Foster D (1983). Observations on the affinity for carnitine, and malonyl‐CoA sensitivity, of carnitine palmitoyltransferase I in animal and human tissues. Demonstration of the presence of malonyl‐CoA in non‐hepatic tissues of the rat. Biochem J 15, 21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilegaard H, Keller C, Steensberg A, Helge JW, Pedersen BK, Saltin B & Neufer PD (2002). Influence of pre‐exercise muscle glycogen content on exercise‐induced transcriptional regulation of metabolic genes. J Physiol 541, 261–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randle P, Garland P, Hale C & Newsholme E (1963). The glucose fatty‐acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1, 785–789. [DOI] [PubMed] [Google Scholar]
- Rasmussen B, Holmbäck U, Volpi E, Morio‐Liondore B, Paddon‐Jones D & Wolfe R. (2002). Malonyl coenzyme A and the regulation of functional carnitine palmitoyltransferase‐1 activity and fat oxidation in human skeletal muscle. J Clin Invest 110, 1687–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter EA & Ruderman NB (2009). AMPK and the biochemistry of exercise: implications for human health and disease. Biochem J 418, 261–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts PA, Bouitbir J, Bonifacio A, Singh F, Kaufmann P, Urwyler A & Krahenbuhl S (2015). Contractile function and energy metabolism of skeletal muscle in rats with secondary carnitine deficiency. Am J Physiol Endocrinol Metab 309, E265–E274. [DOI] [PubMed] [Google Scholar]
- Roepstorff C, Halberg N, Hillig T, Saha AK, Ruderman NB, Wojtaszewski JF, Richter EA & Kiens B (2005). Malonyl‐CoA and carnitine in regulation of fat oxidation in human skeletal muscle during exercise. Am J Physiol Endocrinol Metab 288, E133–E142. [DOI] [PubMed] [Google Scholar]
- Schurch R, Todesco L, Novakova K, Mevissen M, Stieger B & Krahenbuhl S (2010). The plasma carnitine concentration regulates renal OCTN2 expression and carnitine transport in rats. Eur J Pharm 635, 171–176. [DOI] [PubMed] [Google Scholar]
- Sidossis L, Stuart C, Shulman G, Lopaschuk G & Wolfe R (1996b). Glucose plus insulin regulate fat oxidation by controlling the rate of fatty acid entry into the mitochondria. J Clin Invest 98, 2244–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidossis LS & Wolfe RR (1996a). Glucose and insulin‐induced inhibition of fatty acid oxidation: the glucose‐fatty acid cycle reversed. Am J Physiol Endocrinol Metab 270, E733–E738. [DOI] [PubMed] [Google Scholar]
- Simkhovich B, Shutenko Z, Meirena D, Khagi K, Mezapuķe R, Molodchina T, Kalviņs I & Lukevics E (1988). 3‐(2,2,2‐Trimethylhydrazinium)propionate (THP)–a novel gamma‐butyrobetaine hydroxylase inhibitor with cardioprotective properties. Biochem Pharm 15, 195–202. [DOI] [PubMed] [Google Scholar]
- Spaniol M, Brooks H, Auer L, Zimmermann A, Solioz M, Stieger B & Krähenbühl S (2001). Development and characterization of an animal model of carnitine deficiency. Eur J Biochem 268, 1876–1887. [PubMed] [Google Scholar]
- Stephens FB, Constantin‐Teodosiu D, Laithwaite D, Simpson EJ & Greenhaff PL (2006). An acute increase in skeletal muscle carnitine content alters fuel metabolism in resting human skeletal muscle. J Clin Endo Metab 91, 5013–5018. [DOI] [PubMed] [Google Scholar]
- Stephens FB, Constantin‐Teodosiu D & Greenhaff PL (2007). New insights concerning the role of carnitine in the regulation of fuel metabolism in skeletal muscle. J Physiol 581, 431–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens FB, Marimuthu K, Cheng Y, Patel N, Constantin D, Simpson EJ & Greenhaff PL (2011). Vegetarians have a reduced skeletal muscle carnitine transport capacity. Am J Clin Nutr 94, 938–944. [DOI] [PubMed] [Google Scholar]
- Stephens FB, Wall BT, Marimuthu K, Shannon CE, Constantin‐Teodosiu D, Macdonald IA & Greenhaff PL (2013). Skeletal muscle carnitine loading increases energy expenditure, modulates fuel metabolism gene networks and prevents body fat accumulation in humans. J Physiol 591, 4655–4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamai I, Ohashi R, Nezu J‐i, Yabuuchi H, Oku A, Shimane M, Sai Y & Tsuji A (1998). Molecular and functional identification of sodium ion‐dependent, high affinity human carnitine transporter OCTN2. J Biol Chem 273, 20378–20382. [DOI] [PubMed] [Google Scholar]
- Tsoko M, Beauseigneur F, Gresti J, Niot I, Demarquoy J, Boichot J, Bezard J, Rochette L & Clouet P (1995). Enhancement of activities relative to fatty acid oxidation in the liver of rats depleted of L‐carnitine by D‐carnitine and a gamma‐butyrobetaine hydroxylase inhibitor. Biochem Pharm 49, 1403–1410. [DOI] [PubMed] [Google Scholar]
- van Loon LJ, Greenhaff PL, Constantin‐Teodosiu D, Saris WH & Wagenmakers AJ (2001). The effects of increasing exercise intensity on muscle fuel utilisation in humans. J Physiol 536, 295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall BT, Stephens FB, Constantin‐Teodosiu D, Marimuthu K, Macdonald IA & Greenhaff PL (2011). Chronic oral ingestion of L‐carnitine and carbohydrate increases muscle carnitine content and alters muscle fuel metabolism during exercise in humans. J Physiol 589, 963–973. [DOI] [PMC free article] [PubMed] [Google Scholar]


