Abstract
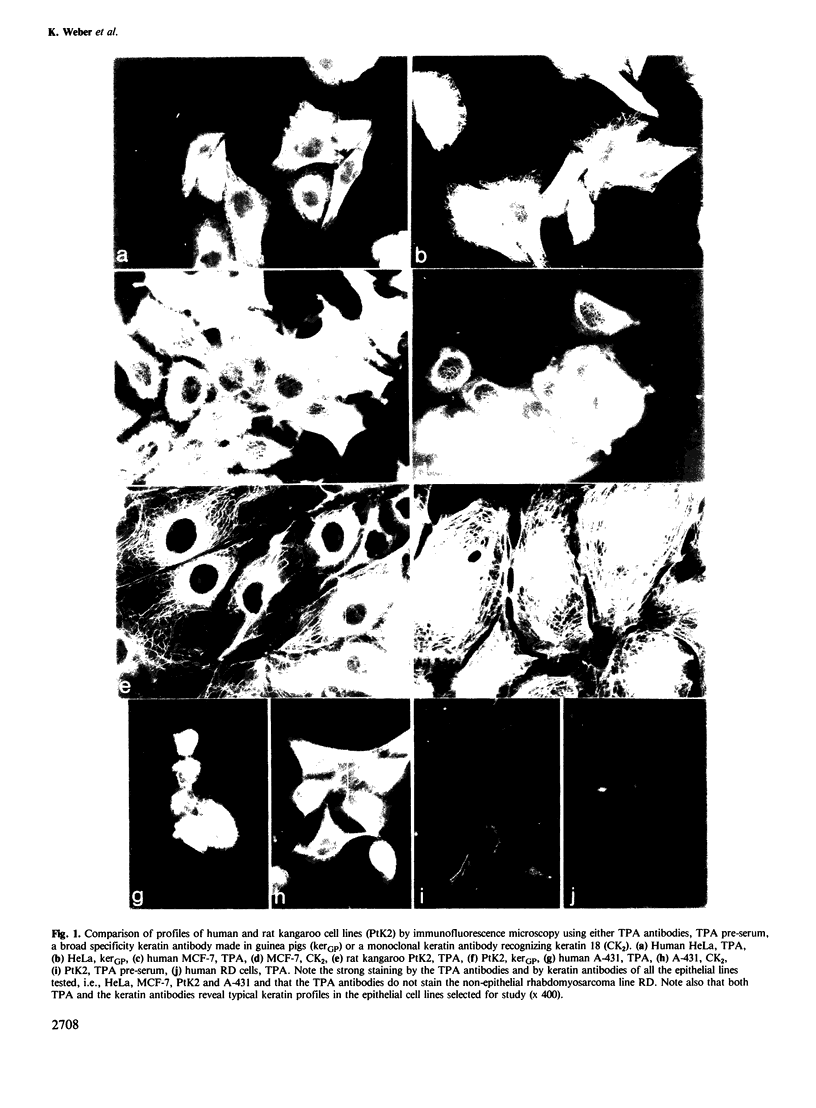
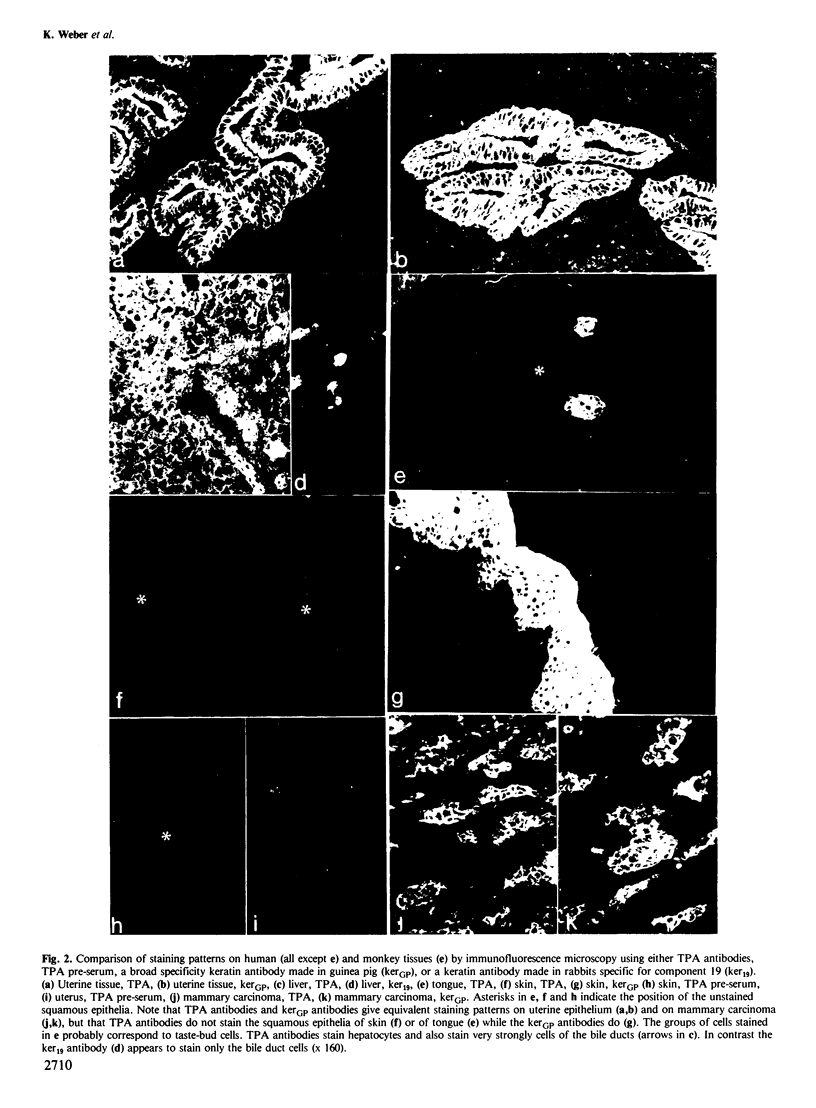
Because of the broad clinical interest which tissue polypeptide antigen (TPA) has attracted as a tumor marker, human cell lines and human tissues have been analyzed for TPA expression using immunofluorescence microscopy. Epithelial cell lines including HeLa, MCF-7, and A-431 are recognized by TPA antibodies whereas human lines of non-epithelial origin are not. The positive staining patterns coincide with keratin-type intermediate filaments of the cytoskeleton. On tissue sections a subset of epithelial cells including uterine epithelium, bile duct cells in liver and tumor cells in breast carcinoma are strongly positive; cells of the squamous epithelia of skin and tongue as well as cells of non-epithelial origin are negative. In immunoblots of human epidermis, human tongue mucosa, human hair follicles, Detroit 562 cells, HeLa cells, MCF-7 and RT-4 cells, only keratins 8, 18 and 19 show TPA antigenicity. Conversely a TPA preparation is recognized by various antibodies known to react with keratins, including alpha-IFA, KG 8.13.2 and two antibodies which recognize keratins 18 (CK2) and 19, respectively. Our results thus relate TPA to human keratins 8, 18 and 19 which are known cytoskeletal components in both normal and malignant epithelial cells of simple and non-squamous origin. We speculate that the elevated levels of circulating TPA antigenicity present in the sera of patients with carcinoma, which are often used to monitor tumor progression, correspond to soluble proteolytic fragments originating from this particular keratin subgroup.
Full text
PDF

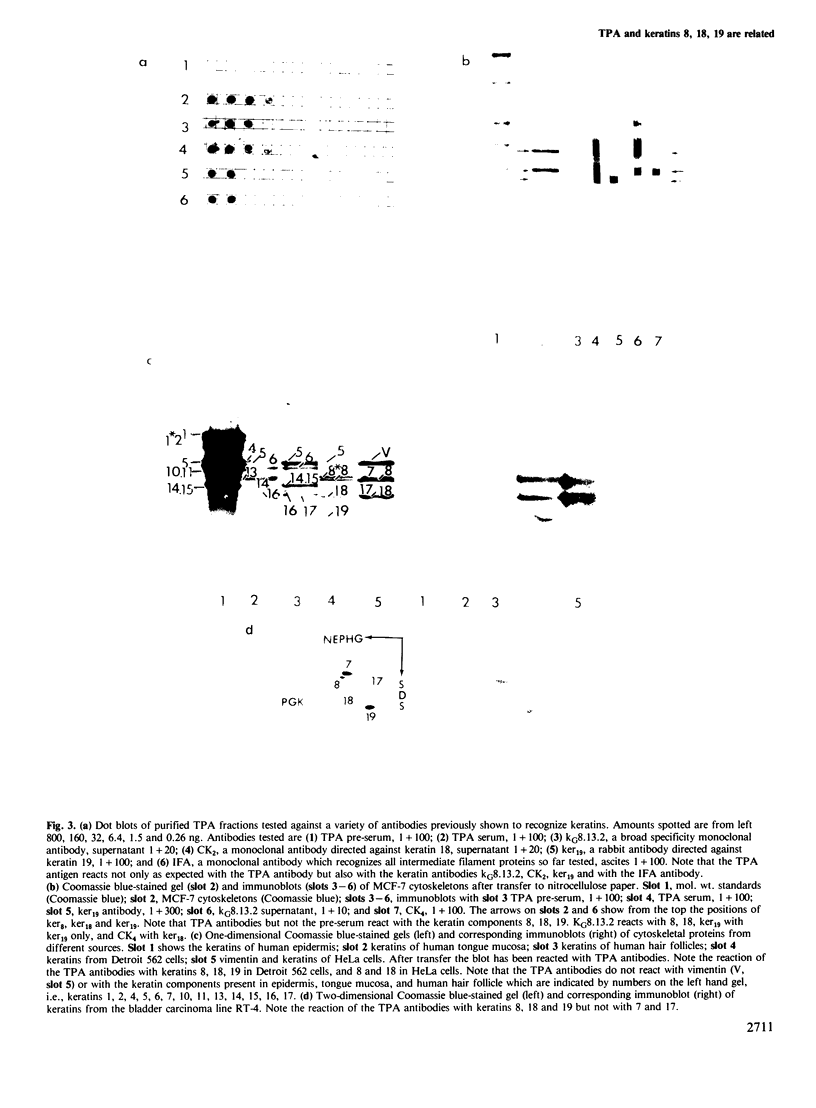



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altmannsberger M., Osborn M., Hölscher A., Schauer A., Weber K. The distribution of keratin type intermediate filaments in human breast cancer. An immunohistological study. Virchows Arch B Cell Pathol Incl Mol Pathol. 1981;37(3):277–284. doi: 10.1007/BF02892576. [DOI] [PubMed] [Google Scholar]
- BJORKLUND B., BJORKLUND V. Antigenicity of pooled human malignant and normal tissues by cyto-immunological technique; presence of an insoluble, heat-labile tumor antigen. Int Arch Allergy Appl Immunol. 1957;10(3):153–184. [PubMed] [Google Scholar]
- Björklund B. Tissue polypeptide antigen (TPA): Biology, biochemistry, improved assay methodology, clinical significance in cancer and other conditions, and future outlook. Antibiot Chemother (1971) 1978;22:16–31. [PubMed] [Google Scholar]
- Björklund V., Björklund B., Wittekind C., von Kleist S. Immuno-histochemical localization of tissue polypeptide antigen (TPA) and carcino-embryonic antigen (CEA) in breast cancer. A comparative study. Acta Pathol Microbiol Immunol Scand A. 1982 Nov;90(6):471–476. doi: 10.1111/j.1699-0463.1982.tb00124_90a.x. [DOI] [PubMed] [Google Scholar]
- Debus E., Moll R., Franke W. W., Weber K., Osborn M. Immunohistochemical distinction of human carcinomas by cytokeratin typing with monoclonal antibodies. Am J Pathol. 1984 Jan;114(1):121–130. [PMC free article] [PubMed] [Google Scholar]
- Debus E., Weber K., Osborn M. Monoclonal cytokeratin antibodies that distinguish simple from stratified squamous epithelia: characterization on human tissues. EMBO J. 1982;1(12):1641–1647. doi: 10.1002/j.1460-2075.1982.tb01367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Weber K., Osborn M. HeLa cells contain intermediate-sized filaments of the prekeratin type. Exp Cell Res. 1979 Jan;118(1):95–109. doi: 10.1016/0014-4827(79)90587-1. [DOI] [PubMed] [Google Scholar]
- Geisler N., Kaufmann E., Fischer S., Plessmann U., Weber K. Neurofilament architecture combines structural principles of intermediate filaments with carboxy-terminal extensions increasing in size between triplet proteins. EMBO J. 1983;2(8):1295–1302. doi: 10.1002/j.1460-2075.1983.tb01584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler N., Kaufmann E., Weber K. Proteinchemical characterization of three structurally distinct domains along the protofilament unit of desmin 10 nm filaments. Cell. 1982 Aug;30(1):277–286. doi: 10.1016/0092-8674(82)90033-2. [DOI] [PubMed] [Google Scholar]
- Geisler N., Weber K. The amino acid sequence of chicken muscle desmin provides a common structural model for intermediate filament proteins. EMBO J. 1982;1(12):1649–1656. doi: 10.1002/j.1460-2075.1982.tb01368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigi O., Geiger B., Eshhar Z., Moll R., Schmid E., Winter S., Schiller D. L., Franke W. W. Detection of a cytokeratin determinant common to diverse epithelial cells by a broadly cross-reacting monoclonal antibody. EMBO J. 1982;1(11):1429–1437. doi: 10.1002/j.1460-2075.1982.tb01334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanukoglu I., Fuchs E. The cDNA sequence of a human epidermal keratin: divergence of sequence but conservation of structure among intermediate filament proteins. Cell. 1982 Nov;31(1):243–252. doi: 10.1016/0092-8674(82)90424-x. [DOI] [PubMed] [Google Scholar]
- Holtzer H., Bennett G. S., Tapscott S. J., Croop J. M., Toyama Y. Intermediate-size filaments: changes in synthesis and distribution in cells of the myogenic and neurogenic lineages. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 1):317–329. doi: 10.1101/sqb.1982.046.01.033. [DOI] [PubMed] [Google Scholar]
- Kumar S., Costello C. B., Glashan R. W., Björklund B. The clinical significance of tissue polypeptide antigen (TPA) in the urine of bladder cancer patients. Br J Urol. 1981 Dec;53(6):578–581. doi: 10.1111/j.1464-410x.1981.tb03264.x. [DOI] [PubMed] [Google Scholar]
- Kurki P., Helve T., Virtanen I. Antibodies to cytoplasmic intermediate filaments in rheumatic diseases. J Rheumatol. 1983 Aug;10(4):558–562. [PubMed] [Google Scholar]
- Lane E. B. Monoclonal antibodies provide specific intramolecular markers for the study of epithelial tonofilament organization. J Cell Biol. 1982 Mar;92(3):665–673. doi: 10.1083/jcb.92.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löning T., Kühler C., Caselitz J., Stegner H. E. Keratin and tissue polypeptide antigen profiles of the cervical mucosa. Int J Gynecol Pathol. 1983;2(2):105–112. doi: 10.1097/00004347-198302000-00001. [DOI] [PubMed] [Google Scholar]
- Lüning B., Nilsson U. Sequence homology between tissue polypeptide antigen (TPA) and intermediate filament (IF) proteins. Acta Chem Scand B. 1983;37(8):731–733. doi: 10.3891/acta.chem.scand.37b-0731. [DOI] [PubMed] [Google Scholar]
- Lüning B., Wiklund B., Redelius P., Björklund B. Biochemical properties of tissue polypeptide antigen. Biochim Biophys Acta. 1980 Jul 24;624(1):90–101. doi: 10.1016/0005-2795(80)90228-7. [DOI] [PubMed] [Google Scholar]
- Lüthgens M., Schlegel G. Combined use of carcinoembryonic antigen and tissue polypeptide antigen in oncologic therapy and surveillance. Cancer Detect Prev. 1983;6(1-2):51–59. [PubMed] [Google Scholar]
- Menendez-Botet C. J., Oettgen H. F., Pinsky C. M., Schwartz M. K. A preliminary evaluation of tissue polypeptide antigen in serum or urine (or both) of patients with cancer or benign neoplasms. Clin Chem. 1978 Jun;24(6):868–872. [PubMed] [Google Scholar]
- Moll R., Franke W. W., Schiller D. L., Geiger B., Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982 Nov;31(1):11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- Moll R., Franke W. W., Volc-Platzer B., Krepler R. Different keratin polypeptides in epidermis and other epithelia of human skin: a specific cytokeratin of molecular weight 46,000 in epithelia of the pilosebaceous tract and basal cell epitheliomas. J Cell Biol. 1982 Oct;95(1):285–295. doi: 10.1083/jcb.95.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll R., Krepler R., Franke W. W. Complex cytokeratin polypeptide patterns observed in certain human carcinomas. Differentiation. 1983;23(3):256–269. doi: 10.1111/j.1432-0436.1982.tb01291.x. [DOI] [PubMed] [Google Scholar]
- Osborn M., Franke W. W., Weber K. Visualization of a system of filaments 7-10 nm thick in cultured cells of an epithelioid line (Pt K2) by immunofluorescence microscopy. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2490–2494. doi: 10.1073/pnas.74.6.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M., Geisler N., Shaw G., Sharp G., Weber K. Intermediate filaments. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 1):413–429. doi: 10.1101/sqb.1982.046.01.040. [DOI] [PubMed] [Google Scholar]
- Osborn M., Geisler N., Shaw G., Sharp G., Weber K. Intermediate filaments. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 1):413–429. doi: 10.1101/sqb.1982.046.01.040. [DOI] [PubMed] [Google Scholar]
- Osborn M., Weber K. The display of microtubules in transformed cells. Cell. 1977 Nov;12(3):561–571. doi: 10.1016/0092-8674(77)90257-4. [DOI] [PubMed] [Google Scholar]
- Osborn M., Weber K. Tumor diagnosis by intermediate filament typing: a novel tool for surgical pathology. Lab Invest. 1983 Apr;48(4):372–394. [PubMed] [Google Scholar]
- Pruss R. M., Mirsky R., Raff M. C., Thorpe R., Dowding A. J., Anderton B. H. All classes of intermediate filaments share a common antigenic determinant defined by a monoclonal antibody. Cell. 1981 Dec;27(3 Pt 2):419–428. doi: 10.1016/0092-8674(81)90383-4. [DOI] [PubMed] [Google Scholar]
- Rasmuson T., Björk G. R., Damber L., Holm S. E., Jacobsson L., Jeppsson A., Littbrand B., Stigbrand T., Westman G. Evaluation of carcinoembryonic antigen, tissue polypeptide antigen, placental alkaline phosphatase, and modified nucleosides as biological markers in malignant lymphomas. Recent Results Cancer Res. 1983;84:331–343. doi: 10.1007/978-3-642-81947-6_25. [DOI] [PubMed] [Google Scholar]
- Redelius P., Lüning B., Björklund B. Chemical studies of tissue polypeptide antigen (TPA). II. Partial amino acid sequences of cyanogen bromide fragments of TPA subunit B1. Acta Chem Scand B. 1980;34(4):265–273. doi: 10.3891/acta.chem.scand.34b-0265. [DOI] [PubMed] [Google Scholar]
- Schiller D. L., Franke W. W. Limited proteolysis of cytokeratin a by an endogeneous protease: removal of positively charged terminal sequences. Cell Biol Int Rep. 1983 Jan;7(1):3–3. doi: 10.1016/0309-1651(83)90098-x. [DOI] [PubMed] [Google Scholar]
- Steinert P. M., Rice R. H., Roop D. R., Trus B. L., Steven A. C. Complete amino acid sequence of a mouse epidermal keratin subunit and implications for the structure of intermediate filaments. Nature. 1983 Apr 28;302(5911):794–800. doi: 10.1038/302794a0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng S. C., Jarvinen M. J., Nelson W. G., Huang J. W., Woodcock-Mitchell J., Sun T. T. Correlation of specific keratins with different types of epithelial differentiation: monoclonal antibody studies. Cell. 1982 Sep;30(2):361–372. doi: 10.1016/0092-8674(82)90234-3. [DOI] [PubMed] [Google Scholar]
- Wiklund B., Lüning B., Björklund B. Chemical studies of tissue polypeptide antigen (TPA). III. on the nature of the antigenic determinant(s) of TPA subfraction B1. Acta Chem Scand B. 1981;35(5):325–336. doi: 10.3891/acta.chem.scand.35b-0325. [DOI] [PubMed] [Google Scholar]
- Wu Y. J., Parker L. M., Binder N. E., Beckett M. A., Sinard J. H., Griffiths C. T., Rheinwald J. G. The mesothelial keratins: a new family of cytoskeletal proteins identified in cultured mesothelial cells and nonkeratinizing epithelia. Cell. 1982 Dec;31(3 Pt 2):693–703. doi: 10.1016/0092-8674(82)90324-5. [DOI] [PubMed] [Google Scholar]