Abstract
Key points
Peripheral vascular endothelial growth factor (VEGF) is necessary for exercise to stimulate hippocampal neurogenesis.
Here we report that skeletal myofiber VEGF directly or indirectly regulates exercise‐signalled proliferation of neuronal precursor cells.
Our results found skeletal myofiber VEGF to be necessary for maintaining blood flow through hippocampal regions independent of exercise training state.
This study demonstrates that skeletal myofiber VEGF is required for the hippocampal VEGF response to acute exercise.
These results help to establish the mechanisms by which exercise, through skeletal myofiber VEGF, affects the hippocampus.
Abstract
Exercise signals neurogenesis in the dentate gyrus of the hippocampus. This phenomenon requires vascular endothelial growth factor (VEGF) originating from outside the blood–brain barrier, but no cellular source has been identified. Thus, we hypothesized that VEGF produced by skeletal myofibers plays a role in regulating hippocampal neuronal precursor cell proliferation following exercise training. This was tested in adult conditional skeletal myofiber‐specific VEGF gene‐ablated mice (VEGFHSA−/−) by providing VEGFHSA−/− and non‐ablated (VEGFf/f) littermates with running wheels for 14 days. Following this training period, hippocampal cerebral blood flow (CBF) was measured by functional magnetic resonance imaging (fMRI), and neuronal precursor cells (BrdU+/Nestin+) were detected by immunofluorescence. The VEGFf/f trained group showed improvements in both speed and endurance capacity in acute treadmill running tests (P < 0.05). The VEGFHSA−/− group did not. The number of proliferating neuronal precursor cells was increased with training in VEGFf/f (P < 0.05) but not in VEGFHSA−/− mice. Endothelial cell (CD31+) number did not change in this region with exercise training or skeletal myofiber VEGF gene deletion. However, resting blood flow through the hippocampal region was lower in VEGFHSA−/− mice, both untrained and trained, than untrained VEGFf/f mice (P < 0.05). An acute hypoxic challenge decreased CBF (P < 0.05) in untrained VEGFf/f, untrained VEGFHSA−/− and trained VEGFHSA−/− mice, but not trained VEGFf/f mice. VEGFf/f, but not VEGFHSA−/−, mice were able to acutely run on a treadmill at an intensity sufficient to increase hippocampus VEGF levels. These data suggest that VEGF expressed by skeletal myofibers may directly or indirectly regulate both hippocampal blood flow and neurogenesis.
Keywords: exercise, neurogenesis, vascular endothelial growth factor
Key points
Peripheral vascular endothelial growth factor (VEGF) is necessary for exercise to stimulate hippocampal neurogenesis.
Here we report that skeletal myofiber VEGF directly or indirectly regulates exercise‐signalled proliferation of neuronal precursor cells.
Our results found skeletal myofiber VEGF to be necessary for maintaining blood flow through hippocampal regions independent of exercise training state.
This study demonstrates that skeletal myofiber VEGF is required for the hippocampal VEGF response to acute exercise.
These results help to establish the mechanisms by which exercise, through skeletal myofiber VEGF, affects the hippocampus.
Abbreviations
- AMPK
AMP‐activated protein kinase
- BrdU
5‐bromo‐2′deoxyuridine
- CASL
continuous arterial spin labelling
- CBF
cerebral blood flow
- fMRI
functional magnetic resonance imaging
- GFP
green fluorescent protein
- PFA
paraformaldehyde
- PPARδ
peroxisome proliferator‐activated receptor delta
- VEGF
vascular endothelial growth factor
- VEGFHSA−/−
skeletal myofiber VEGF gene deletion mouse
- VEGFf/f
mice whose VEGF gene is floxed with LoxP sites without the CRE gene
Introduction
Exercise is well known to have many benefits for overall metabolism and locomotion. Exercise is also thought to improve memory and spatial learning and assuage the symptoms of anxiety and depression (Snyder et al. 2011; Kiuchi et al. 2012; Marlatt et al. 2012). Improvements in brain function stem from an ability to increase or maintain the number of new neurons in hippocampal regions (van Praag et al. 1999). This seems to be particularly important during ageing, and exercise has been shown to slow the loss of neuronal stem cells and promote their commitment to a neuronal‐specific lineage (Yang et al. 2015). New neurons within the dentate gyrus of the hippocampus reside in a highly vascularized environment in contact with endothelial cells (Shen et al. 2004). Thus, neuronal precursor cells are well positioned to continuously sense and respond to changes in circulating levels of oxygen, nutrients and neurotrophic factors.
The signals produced by exercise training, which may directly or indirectly regulate the number of neuronal precursor cells in the hippocampus, have not been well defined. In skeletal muscle, exercise enhances the ability to provide and utilize oxygen and nutrients by augmenting the number of capillaries that perfuse muscle, oxidative metabolic enzyme activities and mitochondrial biogenesis (Yan et al. 2011; Summermatter et al. 2013). Agonists of metabolic regulators, AMP‐activated protein kinase (AMPK) and peroxisome proliferator‐activated receptor delta (PPARδ), have been shown to induce neurogenesis in the adult mouse dentate gyrus within a similar time frame as exercise training (Kobilo et al. 2011). However, exercise training may also increase the availability of growth factors produced in the periphery that circulate and enter the cerebral vascular niche. For instance, elegant studies using heterochronic parabiotic mice demonstrate that blood supplied by young mice can induce vascular remodelling that promotes neurogenesis in the dentate gyrus. In particular GDF11, a TGFß family member, in young blood was shown to mediate this formation of new neurons (Katsimpardi et al. 2014). Interestingly, GDF11 has also been reported to rejuvenate the stem cell population in skeletal muscle (Sinha et al. 2014).
An additional exercise‐responsive growth factor, thought to play a role in both cerebral and peripheral neural function, is vascular endothelial growth factor (VEGF) (Oosthuyse et al. 2001; Sun et al. 2003; Tang et al. 2010). In skeletal muscle VEGF expression is rapidly and transiently increased in locomotor muscle following an acute, exhaustive exercise bout (Breen et al. 1996; Tang et al. 2010). VEGF expressed by skeletal myofibers is essential for improving exercise capacity and augmenting skeletal muscle capillary number with exercise training (Delavar et al. 2014). This latter finding is accompanied by a coordinated increase in Nes+/Pax7+ stem cells associated with capillary endothelial cells (Christov et al. 2007; Shefer et al. 2013). VEGF produced in the periphery has also been shown to play a role in exercise‐induced cerebral neurogenesis. In a study by Fabel et al. (2003), blocking antibodies against VEGF were introduced into the peripheral circulation and shown to completely abolish hippocampal neurogenesis induced by running exercise. However, the peripheral source of the circulating VEGF necessary for exercise‐induced cerebral neurogenesis has not been identified.
Most (60–90%) of the VEGF content in adult skeletal muscle is produced by skeletal myofibers (Delavar et al. 2014; Knapp et al. 2016). Therefore, we hypothesized that skeletal myofiber VEGF may either be a source of VEGF for the brain or may indirectly modify other exercise signals that regulate the fate of neuronal stem cells in the dentate gyrus. Thus, in this study the number of neuronal precursor cells in the dentate gyrus of the hippocampus was measured in four groups of mice: mice with normal skeletal myofiber VEGF production that were (a) exercise‐trained or (b) remained untrained (cage‐confined) and mice with reduced levels of skeletal myofiber VEGF that were (c) trained or (d) remained untrained. One important factor, which decreases with age and can be accentuated by exercise training, is cerebral blood flow (CBF). Changes in hippocampal blood flow were also evaluated to assess regulation of vasculature function as a VEGF‐mediated signalling mechanism and a hypoxic challenge was performed to evaluate the autoregulatory capabilities of the cerebral vasculature. The ability to acutely increase hippocampal VEGF levels with an exhaustive exercise bout was also tested.
Methods
Animals
All animal protocols were reviewed and approved by the University of California, San Diego, Animal Care and Use Committee and conducted in accordance with guidelines outlined in the Guide for the Care and Use of Laboratory Animals (National Research Council). Seventy‐two male mice on a C57BIL/6J background (Jackson Laboratory, Bar Harbor, ME, USA) were used for this study. Our methods comply with the animal ethics checklist outlined in the Journal of Physiology (Grundy, 2015). Mice were housed under standard laboratory conditions under a 12 h dark/light cycle with water and food available ad libidum. Body mass was measured on day 0 (before induction with tamoxifen), day 21 (following tamoxifen‐induced gene deletion) and day 36 (after the two‐week exercise training or cage confinement period).
VEGF deletion strategy
We used a strategy of tamoxifen‐driven conditional skeletal myofiber‐specific VEGF gene deletion in adult mice. Thus, this was not a lifelong gene deletion, nor was the VEGF gene deleted in any of the other cells residing in the muscle. Seventy‐two male mice, 30 positive and 42 negative for the HSA‐CRE‐ERT2 gene, all on a homozygous Nes‐GFP/VEGFLoxP background (Gerber et al. 1999; Yamaguchi et al. 2000; Schuler et al. 2005), were maintained for this study. Male mice were used to minimize estrogen non‐specific binding to the tamoxifen receptor. Presence of the HSA‐CRE‐ERT2 gene was determined using PCR analysis of tail DNA. All mice at 4 months of age were administered tamoxifen (1 mg per mouse, i.p.) for five consecutive days (days 0–4 of the experiment) to induce ablation of the VEGF gene specifically in myofibers of mice that expressed the HSA‐CRE‐ERT2 transgene (VEGFHSA−/−) as previously described (Schuler et al. 2005; Delavar et al. 2014; Knapp et al. 2016). The VEGF gene was not ablated in mice negative for HSA‐CRE‐ERT2 (VEGFf/f). VEGF protein levels were measured in the plantaris muscle to ensure efficient ablation of the VEGF gene in VEGFHSA−/− mice.
Exercise training and testing
Three weeks after initiating VEGF gene deletion with tamoxifen, a time when VEGF protein levels were previously found to be reduced by over 90% in skeletal muscle (Knapp et al. 2016), mice were randomly separated into cages without (untrained, groups a and c) or with (trained, groups b and d) running wheels for 2 weeks (days 21–35) and housed under the same standard laboratory conditions described previously. Trained mice had 24 h of voluntary access to an individual running wheel (wheel circumference: 35.8 cm) attached to a bike pedometer, which was used as a magnetic revolution counter (Sigma BC 906, St. Charles, IL, USA). Measurements (distance, time, average speed) were recorded every 24 h.
Exercise testing was performed to elucidate physiological differences in the training response. Prior to group separation, two treadmill (Model Cl‐4, Omnitech, Columbus, OH, USA) protocols were used to measure submaximal endurance and maximal speed. These tests were repeated after the two week period in which groups (a) and (c) remained confined to standard mouse cages while groups (b) and (d) were trained by being given access to a running wheel. The maximal speed test was performed the morning before the endurance capacity test. Maximal speed was measured on a treadmill at a 10 deg incline with speeds increasing 3–4 cm s−1 every minute until exhaustion. Endurance capacity was measured as time to exhaustion on a treadmill at a 10 deg incline at 33 cm s−1 (Billat et al. 2005). Mice were encouraged to run using an electric shock grid (≤4 mA) and manual air jets. Mice were deemed exhausted when they spent 10 continuous seconds on the shock grid.
Cerebral blood flow measurement
CBF was measured to assess functional changes in hippocampal vasculature. CBF in the hippocampus was non‐invasively measured using an arterial spin labelling magnetic resonance imaging (MRI) technique (Lei et al. 2011). Mice were naïve to isoflurane prior to the CBF measurements (Wegener & Wong, 2008). Mice were anaesthetized with 2% isoflurane and then maintained lightly anaesthetized with 0.8% isoflurane. Physiological parameters of body temperature (37°C), O2 saturation, respiration rate and heart rate were monitored throughout the functional MRI (fMRI) protocol. Mice were imaged in a 7T small animal magnet (Bruker Biosciences Corp., Billerica, MA, USA). The imaging coil system was a 7.2 cm transmit coil with a 1 cm receive‐only surface coil. In the magnet, mice were initially maintained on 21% O2. An anatomical scan was performed to locate the coronal section through the hippocampus. The anatomical scan was followed by a 6 min T1 relaxation scan and two 5 min CASL (continuous arterial spin labelling) scans. Next, the mice underwent a hypoxic challenge by lowering the inspired gas to 10% O2. Once physiological parameters were stabilized, one 5 min CASL scan was performed at 10% O2. CASL scans were analysed by a radiologist (M.S.) who was blinded to the mouse genotype, training condition and inspired O2 level during the scan. Blood flow was quantified from the CASL scan using MATLAB (MathWorks, Natick, MA, USA) to evaluate regions of interest that encompassed the left and right sections of the hippocampus. CBF is reported as the average CBF in ml min–1.
Detection of proliferating neuronal precursor and endothelial cells
To assess the potential initiation of hippocampal neurogenesis and angiogenesis, 5‐bromo‐2′deoxyuridine (BrdU, B5002 Sigma) was administered (i.p.) on eight of the days of wheel running or cage confinement (days 22–25 and 29–32 after the initial tamoxifen injection) at a dose of 50 mg kg–1 body mass. On day 37 the mice were anaesthetized with an i.p. injection of ketamine (50 mg kg−1) and xylazine (10 mg kg−1). Unresponsiveness to toe pinch was used to ensure the mice were adequately anaesthetized. Hind limb muscles were removed for analysis and measurement of VEGF protein levels in the plantaris. The animals were then killed and perfused with 120 ml of PBS followed by 40 ml of 4% paraformaldehyde (PFA). The brain was removed and stored in 2% PFA at 4°C for 24–48 h before being transferred to a solution consisting of 30% sucrose and 0.1% PFA at 4°C. The brain was divided into the left and right hippocampus and frontal cortex before being frozen on a cork in isopentane and protected in freezing medium. Consecutive 16 μm coronal sections were prepared with a cryostat, placed on slides and stored at −20°C. Neuronal precursor cells were detected by immunohistochemistry using antibodies specific for proliferating cells and the in vivo Nes‐GFP signal was amplified with a rabbit antibody against green fluorescent protein (GFP) (Encinas & Enikolopov, 2008). Endothelial cell proliferation was detected using immunohistochemistry for CD31, an endothelial cell marker, to investigate changes in hippocampal angiogenesis as a potential mechanism utilized by skeletal myofiber VEGF to up‐regulate neurogenesis in this region. All immunohistochemistry steps were performed at room temperature unless noted otherwise. Cerebral sections on slides were washed in PBS (3 × 5 min), permeabilized with 0.3% Triton X‐100 for 15 min, incubated in 2 m HCl for 30 min and neutralized in 0.1 m borate buffer (pH 8.5) for 10 min. The sections were then washed in PBS with 0.5% Tween 20 (3 × 5 min) before a 30 min incubation in blocking solution (3% BSA, 0.1% Triton X‐100 in PBS). Primary antibodies were applied overnight at 4°C. Primary antibodies used were anti‐BrdU (3:1000, B35128, Invitrogen, Molecular Probes, Carlsbad, CA, USA), anti‐GFP (1:1000, G10362, Invitrogen, Molecular Probes) and anti‐CD31 (1:200, Cat. No. 550274 BD Pharmingen, Franklin Lakes, NJ, USA). Signals were detected with AlexaFluor secondary antibodies (Invitrogen, Molecular Probes) at a 1:1000 dilution. Fluorescence was preserved with ProLong Gold reagent with DAPI. The entire dentate gyrus of one section was imaged using confocal microscopy. Co‐localization was assessed and quantified with ImageJ. BrdU+/Nes+ co‐localization was quantified and standardized to the total number of nestin positive cells (GFP+) and to the total number of cells (DAPI+). CD31+ cells were co‐localized with BrdU and standardized to the total number of CD31+ cells and to the total number of cells (DAPI+).
Hippocampal VEGF levels following an acute exercise bout
An acute exercise bout was utilized to determine the effect of skeletal muscle VEGF ablation on hippocampal VEGF levels during training. On day 21 of the experiment 41 additional untrained mice (27 VEGFf/f and 14 VEGFHSA−/−) were subjected to a 1 h treadmill bout or rest (control). The treadmill was set to a 10 deg incline and mice were randomized to a speed of 33 or 40 cm s−1. Immediately following the acute exercise bout the mice were anaesthetized with an i.p. injection of ketamine (50 mg kg−1) and xylazine (10 mg kg−1), the animals were killed and the brain was removed, and the hippocampus was isolated for measurement of VEGF protein levels.
VEGF protein levels
VEGF protein levels in the plantaris from all groups of mice and hippocampus from the acutely exercised mice were measured with an enzyme‐linked immunosorbent assay (ELISA; VEGF Mouse ELISA, R&D Systems, La Jolla, CA, USA) and standardized to total protein levels (Bio‐Rad DC protein assay, Bio‐Rad, Hercules, CA, USA).
Statistics
A two‐way ANOVA was used to detect differences between the genotypes and exercise conditions. A Tukey post hoc test was used to identify specific differences between the groups. CBF measurements in mice breathing 21% oxygen and then switched to a 10% oxygen gas mixture were analysed with a two‐way, repeated‐measures ANOVA and Fisher's least significant difference (LSD) post hoc test. P ≤ 0.05 was considered significant for all tests.
Results
Body mass
In the untrained VEGFf/f group there was a trend for body mass to increase over the time frame of the experiment (Table 1). No difference in body mass was detected between groups prior to training. Main effects of time (P < 0.05) and mouse group (P < 0.01) were detected with no interaction. Following the period of exercise training or cage confinement without running wheels the untrained VEGFHSA−/− mice weighed less than the untrained VEGFf/f mice (P < 0.05).
Table 1.
Body weights of the different mice groups
| Group | Day 0 (g) | Day 21 (g) | Day 36 (g) |
|---|---|---|---|
| Untrained VEGFf/f | 22.8 ± 0.6 | 23.5 ± 0.5 | 25.3 ± 1.6 |
| Trained VEGFf/f | 24.0 ± 0.7 | 25.3 ± 0.7 | 24.6 ± 0.5 |
| Untrained VEGFHSA−/− | 20.7 ± 0.7 | 22.0 ± 0.8 | 22.0 ± 0.7* |
| Trained VEGFHSA−/− | 22.8 ± 0.7 | 24.5 ± 0.6 | 24.5 ± 0.6 |
Data are mean ± SEM. *Difference from untrained VEGFf/f condition on day 36 (P < 0.05).
Conditional ablation of skeletal myofiber VEGF gene
To confirm efficient VEGF gene deletion, VEGF levels (Fig. 1) were measured in a representative skeletal muscle. VEGF levels (Fig. 1) were reduced 61.5% in the plantaris of VEGFHSA−/− mice relative to VEGFf/f (VEGF/total protein: VEGFf/f, 52.3 ± 4.9 pg μg−1; VEGFHSA−/−, 20.2 ± 6.7 pg μg−1; genotype effect, P = 0.001). Plantaris VEGF levels measured at the end of the two week voluntary training period were not increased in either genotype (VEGF/total protein: untrained VEGFf/f, 43.9 ± 6.9 pg μg−1; trained VEGFf/f, 59.1 ± 5.7 pg μg−1; untrained VEGFHSA−/−, 20.6 ± 3.1 pg μg−1; trained VEGFHSA−/−, 19.7 ± 5.9 pg μg−1).
Figure 1. Skeletal muscle VEGF levels in skeletal myofiber VEGF gene‐deleted mice are not recovered by exercise training.
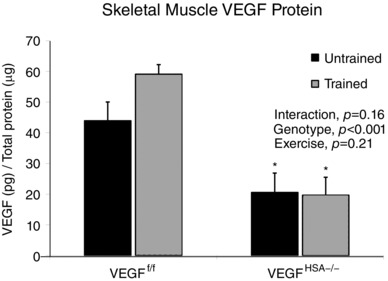
VEGF levels in the plantaris were measured by ELISA. Data are expressed as the mean ± SEM, n = 4–5.
Exercise training
No difference in daily voluntary wheel running distance, time or speed (Fig. 2) was observed between the VEGFf/f and VEGFHSA−/− mice over the two week exercise training protocol. The average time, distance and speed ran per day was not different between the groups over the two week period. The average time ran per day was 120 ± 46 min for trained VEGFf/f mice and 185 ± 28 min for trained VEGFHSA−/− mice. The average distance ran per day was 3.88 ± 1.71 km for trained VEGFf/f mice and 5.46 ± 1.02 km for trained VEGFHSA−/− mice. The average speed ran per day was 40.4 ± 9.7 cm s−1 for trained VEGFf/f mice and 44.7 ± 6.5 cm s−1 for trained VEGFHSA−/− mice. Total distances and times ran over the two weeks were also not different between the VEGFf/f and VEGFHSA−/− groups. The total time ran was 1680 ± 637 min for trained VEGFf/f mice and 2595 ± 393 min for trained VEGFHSA−/− mice. The total distance ran was 54.3 ± 24.0 km for trained VEGFf/f mice and 76.4 ± 14.3 km for trained VEGFHSA−/− mice.
Figure 2. Control and skeletal myofiber VEGF‐deficient mice voluntarily run for similar daily distance and time over a two week period.

The distance (km), time (min) and speed (cm s–1) that each mouse in the VEGFf/f or VEGFHSA−/− group voluntary ran on running wheels was recorded each day. No differences were observed between the groups. Data are expressed as the daily mean ± SEM, n = 5–7 mice per group.
Treadmill exercise testing
There was no difference in maximum treadmill speed or endurance (time to exhaustion at 33 cm s−1) between genotype or training groups prior to running wheel training (Fig. 3). The trained VEGFf/f mice showed a 9% increase in maximal speed (P < 0.05) and a 65% increase in time to exhaustion (P < 0.05). The trained VEGFHSA−/− mice did not significantly improve speed or endurance after running wheel training. Untrained VEGFf/f and VEGFHSA−/− mice showed no changes in speed or endurance relative to baseline after two weeks of rest.
Figure 3. Skeletal myofiber VEGF is essential for improving endurance capacity in response to voluntary exercise training.
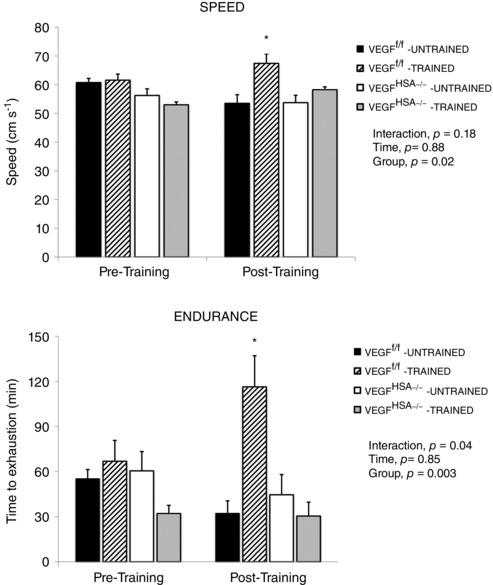
Mice were given access to running wheels (Trained) or cage‐confined without wheels (Untrained) for two weeks. Endurance and maximum speed were measured on a treadmill before (Pre‐Training) and after the training period (Post‐Training). The endurance data are presented as the time (min) before the mice reached exhaustion when run at 33 cm s−1. Maximum speed data are presented as the highest speed (cm s–1) the mice achieved. Data are expressed as the mean ± SEM, n = 4–8. The effects of genotype group, time (two week interval) and interaction were measured with a two‐way ANOVA. *Significant effect of training in the VEGFf/f mice compared to the other trained (Post‐Training) groups, P < 0.01.
Functional changes in hippocampal vasculature
Hippocampal blood flow was measured under normoxic and hypoxic conditions to assess functional changes in hippocampal vasculature. Normoxic, resting blood flow through the hippocampal region was 36% lower in untrained VEGFHSA−/− mice (P = 0.05) and 40% lower in trained VEGFHSA−/− mice (P = 0.04) than untrained VEGFf/f mice (Fig. 4). O2 saturation was maintained in the magnet at rest (O2 saturation: untrained VEGFf/f, 95.9 ± 0.6%; trained VEGFf/f, 93.6 ± 0.9%; untrained VEGFHSA−/−, 93.5 ± 1.2%; trained VEGFHSA−/−, 94.2 ± 0.7%). An acute hypoxic challenge with 10% inspired O2 decreased blood flow. Compared to each group's normoxic levels, flow fell by 58% in untrained VEGFf/f (P = 0.003), 73% in untrained VEGFHSA−/− (P = 0.02) and 80% in trained VEGFHSA−/− (P = 0.02) mice. There was a trend for an acute hypoxic challenge to lower CBF in trained VEGFf/f mice (P = 0.08). The mice were unable to maintain O2 saturation during the hypoxic challenge (O2 saturation: untrained VEGFf/f, 77.2 ± 3.4%; trained VEGFf/f, 79.2 ± 4.2%; untrained VEGFHSA−/−, 79.1 ± 2.4%; trained VEGFHSA−/−, 80.6 ± 3.0%).
Figure 4. Cerebral blood flow (CBF) to the hippocampus is decreased by inhibition of skeletal myofiber‐expressed VEGF independent of the exercise training state.
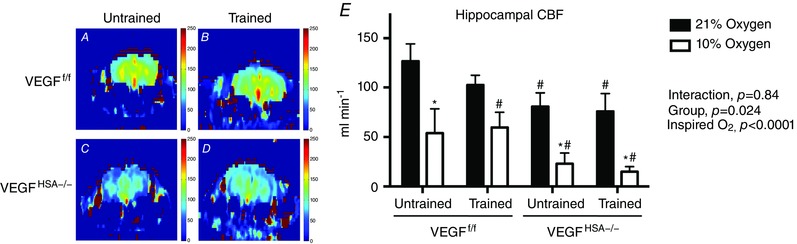
A–D, representative images of CBF generated with MRI detected by continuous arterial spin labelling in a coronal section in (A) untrained VEGFf/f, (B) trained VEGFf/f, (C) untrained VEGFHSA−/− or (D) trained VEGFHSA−/− anaesthetized mice breathing 21% oxygen. Scale is 0–250 ml min−1. E, blood flow in the hippocampal region computed as ml min–1 averaged over 6 min in anaesthetized mice spontaneously breathing 21% oxygen followed by 10% oxygen. Data are expressed as the mean ± SEM, n = 4–8. *Difference with 21% O2 within the same group, P < 0.05; #difference from untrained VEGFf/f under 21% oxygen, P < 0.05.
Confocal analysis of proliferating neuronal precursor cells
To measure the potential for exercise and skeletal muscle VEGF to stimulate hippocampal neurogenesis, proliferating neuronal precursor cells were quantified. The VEGFf/f mice showed a significant increase (Fig. 5) in proliferating neuronal precursor cells with training in both means of quantification: Nes+/BrdU+ co‐localized cells per total Nes+ cells and Nes+/BrdU+ co‐localized cells per total DAPI+ cells (P < 0.05). The proliferating neuronal precursor cell training response was absent in VEGFHSA−/− mice and both genotypes of untrained mice.
Figure 5. Increased number of nestin positive (Nes+) cells in VEGFf/f, but not VEGFHSA−/−, mice following two weeks of voluntary exercise training.
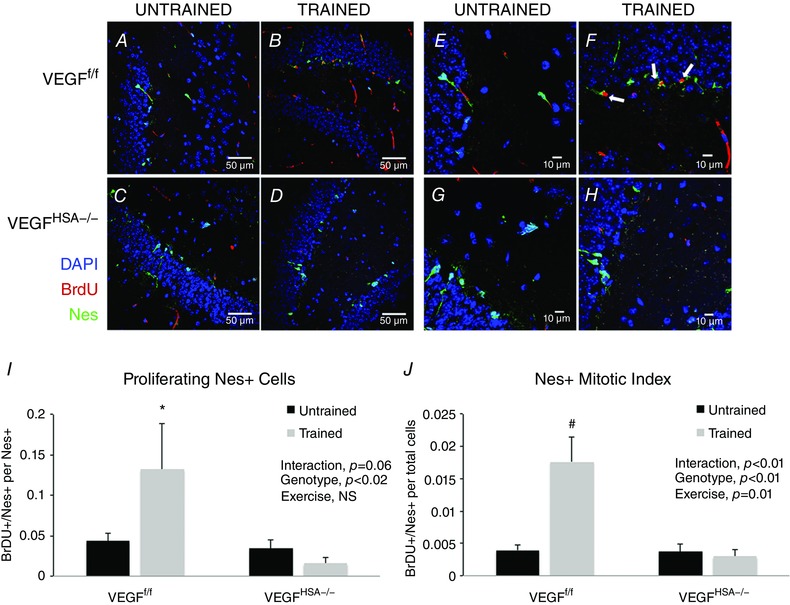
A–H, confocal images of the dentate gyrus sections revealing DAPI‐, BrdU‐ and Nestin‐positive cells. I, BrDU+/Nes+ cells per number of Nes+ cells. J, BrDU+/Nes+ cells per total DAPI‐labelled cells. Data are expressed as the mean ± SEM, n = 6–8. *Significant difference between VEGFf/f‐trained and VEGFHSA−/−‐trained, P < 0.05; #significant difference between VEGFf/f‐trained and the other three groups, P < 0.01. In A–D, scale bar = 50 μm; E–H, scale bar = 10 μm.
Endothelial changes in hippocampal vasculature
In addition to analysing functional blood flow changes, vasculature changes were assessed by measuring the number of proliferating endothelial cells. Training did not reveal a change in endothelial cell proliferation in the VEGFf/f groups. Likewise, there was no training response in the VEGFHSA−/− mice and endothelial cell proliferation was not different between genotypes (Fig. 6).
Figure 6. Skeletal myofiber VEGF gene deletion or exercise training does not alter the number of CD31‐positive capillaries.
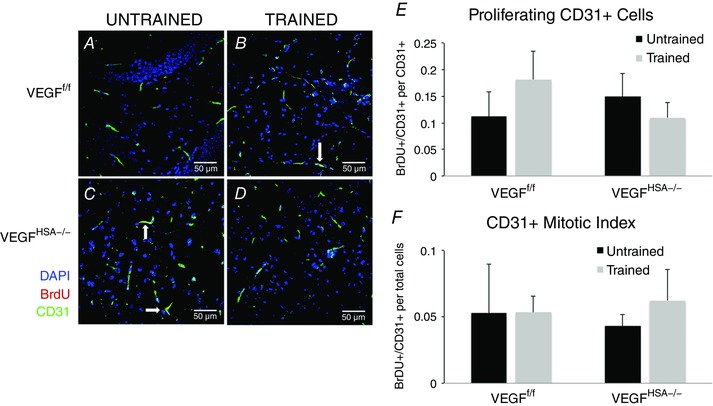
A–D, confocal images of the dentate gyrus sections revealing DAPI‐, BrdU‐ and CD31‐positive cells. E, BrDU+/CD31+ cells per number of CD31+ cells. F, BrDU+/CD31+ cells per total DAPI‐labelled cells. Data are expressed as the mean ± SEM, n = 5–8. No statistical differences were observed between the experimental groups. Scale bar = 50 μm.
Hippocampal VEGF levels in response to an acute exercise bout in untrained mice
Although neurogenesis is a phenomenon that occurs with chronic exercise, we sought to document the effect of skeletal myofiber VEGF ablation on hippocampal VEGF levels at rest and in response to an exercise bout. VEGFf/f mice were run at 33 or 40 cm s−1 for 1 h. VEGFHSA−/− mice could not maintain a speed of 40 cm s−1 for the 1 h period and as a result hippocampal VEGF levels at only 33 cm s−1 were collected in the VEGFHSA−/− mice (Fig. 7). The VEGFf/f mice that ran at 40 cm s−1 showed an increase (P < 0.05) in hippocampus VEGF levels (19.70 ± 1.55 pg μg−1) above the VEGFf/f rest group (11.45 ± 1.56 pg μg−1). Neither the VEGFHSA−/− mice (14.62 ± 2.50 pg μg−1) nor the VEGFf/f mice (14.05 ± 1.23 pg μg−1) that ran acutely at 33 cm s−1 showed an increase in hippocampal VEGF from their genotype respective resting group (rest VEGFHSA−/−, 11.11 ± 1.77 pg μg−1).
Figure 7. VEGF levels in the hippocampus in response to an acute exercise bout on the treadmill.
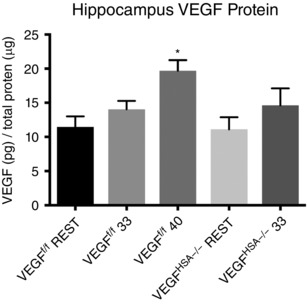
VEGFf/f and VEGFHSA−/− mice were subjected to a 1 h exercise session on the treadmill. The VEGFf/f mice could easily maintain a speed of 33 cm s−1 for 1 h. The VEGFHSA−/− mice had trouble completing the test. An additional group of VEGFf/f mice were run at a higher speed of 40 cm s−1 (approximately 60% of the average maximal speed for this group). VEGF levels were measured in the hippocampus dissected 1 h after the exercise session. Data are expressed as the mean ± SEM, n = 6–14. *Difference between VEGFf/f mice run at 40 cm s−1 and the VEGFf/f rest group, P < 0.05.
Discussion
In this study we found, as reported by several other laboratories (van Praag et al. 1999; Fabel et al. 2003; Yang et al. 2015), that voluntary wheel running for two weeks increases the number of neuronal precursor cells in the dentate gyrus of the hippocampus. The new findings in the present study are as follows. (1) VEGF expressed by peripheral skeletal myofibers is necessary for this initial step in the mechanism to enhance the production of new neuronal cells in response to exercise. (2) Inhibition of VEGF expression in skeletal myofibers is accompanied by reduced blood flow to the hippocampus and this effect is similar in both untrained and trained mice. (3) Blood flow changes cannot be attributed to a change in the number of vessels in this region (based on our CD31+ results). (4) Resting VEGF levels in the hippocampus are not affected by induced VEGF knockout in skeletal myofibers. (5) A bout of acute exercise increases hippocampal VEGF levels in VEGFf/f mice, but not VEGFHSA−/− mice. (6) A hypoxic challenge with 10% inspired O2 lowers CBF in trained and untrained VEGFHSA−/− mice and trained VEGFf/f mice from normoxia.
Thus, this study supports the work of Fabel et al. (2003) suggesting the peripheral VEGF originating from outside the blood–brain barrier is essential for the cerebral neurogenesis in response to exercise training, and expands upon it by showing that skeletal myofiber VEGF appears to be the key source.
Skeletal myofiber VEGF is required to signal neuronal precursor cell proliferation
The main finding of this study is that VEGF expressed by skeletal myofibers is essential for increasing the number of neuronal precursor cells, as defined by the proliferation marker, BrdU, and the neuronal precursor cell marker, nestin. VEGF is (1) a short lived protein (∼1 h) (Levy et al. 1996), (2) transiently increased with an exhaustive exercise bout (Breen et al. 1996; Tang et al. 2010) and (3) not differentially expressed in the hippocampus following chronic exercise training (Inoue et al. 2015). These findings support the possibility that bioactive VEGF may be produced by skeletal myofibers, enter the circulation and act to stimulate proliferation of neuronal precursor cells in the hippocampus. Alternatively, skeletal myofiber‐expressed VEGF may be important for the release or activation of additional exercise‐responsive factors, metabolites or signalling of skeletal muscle afferent nerve activity that indirectly play a role in signalling cerebral neurogenesis. These direct and indirect mechanisms would both be in agreement with the study of Fabel et al. (2003) in which VEGF blocking antibodies administered peripherally, and thought to be too large to cross the blood–brain barrier, prevented exercise‐induced neurogenesis. However, it is yet to be determined whether skeletal myofiber VEGF is transported across the blood–brain barrier and directly interacts with the neuronal precursor cell or has an indirect effect on blood flow or metabolism to signal cell proliferation. Alternatively, integrated functions signalled by VEGF in exercising skeletal myofibers may play a role in allowing exercise tolerance levels to achieve a threshold that stimulates cerebral VEGF expression or other factors that promote neuronal precursor cell proliferation in the hippocampus. VEGFHSA−/− mice could not acutely exercise at a sufficient intensity to increase VEGF expression in the hippocampus. Given the transient half‐life of the VEGF protein, it will take further labelling studies to distinguish these possibilities. Furthermore, whether the mechanism of increased neuronal precursor cells in response to exercise begins at a neural stem cell or progenitor cell is not known (Overall et al. 2016) and future studies will be necessary to determine how many neuronal precursor cells commit to a neuronal cell linage and form functional neurons (Laplagne et al. 2006; Vivar et al. 2012; Deshpande et al. 2013). Peripheral VEGF originating from outside the blood–brain barrier is essential for the cerebral neurogenesis in response to exercise training (Fabel et al. 2003), and the present study expands upon this observation by showing that skeletal myofiber VEGF may be the key source.
Voluntary wheel running parameters similar among groups
In this study, two weeks of voluntary wheel running was used to exercise train the mice (van Praag et al. 1999; Fabel et al. 2003). Angiogenesis and neurogenesis have been reported to occur in the skeletal muscle and hippocampus, respectively, after just seven days (Fabel et al. 2003; Waters et al. 2004). Furthermore, voluntary exercise does not necessarily stimulate stress or the release of stress hormones that could influence cerebral plasticity as has been reported for forced treadmill exercise (Arida et al. 2004). One initial concern with the voluntary mode of exercise is that the experimental mouse groups may not run the same amount and thus receive different exercise stimuli than the control group. Mice were monitored for the daily and cumulative time, duration and speed that they spent on the running wheels. The daily and cumulative average for all parameters in the myofiber VEGF knockout mice were not different from the VEGFf/f mice, so the VEGFHSA−/− mice received at least a comparable training dose as the VEGFf/f mice.
Integrated cerebral and skeletal muscle adaptations to exercise training
Although the voluntary training parameters of running distance, time and speed between mice that do or do not express normal skeletal myofiber VEGF levels were not different and even tended to be greater in skeletal myofiber VEGF‐deficient mice, the training adaptations were clearly different. In tests for maximal running speed and endurance on a treadmill, the control group (VEGFf/f) showed improvements. In contrast, skeletal myofiber VEGF‐deficient mice were not able to improve their exercise performance. These findings are similar to previous studies in our laboratory in which mice were exercise trained using forced treadmill exercise (Delavar et al. 2014). Thus, while the mice were not limited in their daily activity and frequently went on and off the running wheels, when pushed to their limits a difference in exercise tolerance was revealed. Whether this is due to an insufficient central or peripheral blood flow in response to exercise or some other possibly neurogenesis‐limiting factor is unknown. The neuronal importance of an exercise training response is highlighted in findings by Nokia et al. (2016) that demonstrate that hippocampal neurogenesis after exercise training is increased in rats selectively bred for a high exercise performance response to aerobic training. Kobilo et al. (2011) reported that when factors known to be enhanced with endurance exercise, PPARδ and AMPK kinase, are activated with the peripheral agonists GW501516 and AICAR, respectively, in rodents spatial memory can be improved and neurogenesis detected in the dentate gyrus. The latter finding is especially true for the metabolic regulator, AICAR. In skeletal muscle PPARδ and AMPK regulate VEGF expression at the transcriptional and post‐transcriptional level, respectively, and as a result VEGF from skeletal muscle could be an intermediate in the cerebral changes induced by these metabolic agonists (Ouchi et al. 2005; Wang et al. 2006). Whether changes in cellular metabolism are taking place in both the peripheral skeletal myofibers and cells located in the dentate gyrus or these are indirect effects of changing muscle metabolism will require further investigations.
CBF requirement for exercise‐induced cerebral neurogenesis
An additional adaptation to exercise training, with respect to the oxygen transport system, is to augment vascular structure and function. Skeletal muscle angiogenesis allows more O2 and nutrients to be available for an increased number of mitochondria in trained muscle and this is accompanied by greater speed and endurance exercise capacities, and appears to be VEGF‐dependent (Olfert et al. 2009; Delavar et al. 2014; Tang et al. 2016). Regional blood flow is also known to increase in the dentate gyrus, CA3 region and hippocampal fimbria with treadmill training (Holschneider et al. 2007). VEGF‐dependent changes in vascular function that allow enhanced flow through arteries and perfusion of the muscle capillary bed during exercise may occur through nitric oxide or other metabolic signalling pathways (Tschakovsky et al. 1996; Thomas et al. 2003; Lee‐Young et al. 2009). In the present study VEGF‐dependent control of perfusion through capillary beds appears to extend beyond locomotor skeletal muscles (Knapp et al. 2016) and influence or regulate perfusion of the hippocampus. Blood flow through the hippocampal region at rest under normoxic conditions was 36% and 40% lower in untrained and trained myofiber VEGF null mice, respectively, relative to untrained VEGFf/f mice. This finding of reduced hippocampal perfusion in myofiber VEGF null mice suggests that poor perfusion at rest or during exercise may be a contributing factor in the unresponsiveness of mice with conditional, skeletal myofiber VEGF gene deletion to signal neurogenesis in response to exercise training. In the current study this change in blood flow is not accompanied by a change in the number of new endothelial cells (CD31+/BrdU+), a marker of angiogenesis (Zhang et al. 2014), in the dentate gyrus with skeletal myofiber VEGF gene deletion or exercise training. However, previous studies have found hippocampal vascular density to increase with exercise training for a longer duration (Clark et al. 2009). Furthermore, it has been reported that mice with HIF‐1α gene inactivation exhibit both regression of cerebral vasculature and loss of the nestin‐expressing neural stem cells (Li et al. 2014). The present study suggests that adequate skeletal myofiber VEGF is necessary to maintain resting dentate gyrus blood flow. Further studies will be required to assess the precise changes in hippocampal regional perfusion during an acute exercise bout.
A hypoxic challenge was used to stimulate an additional metabolic stress that could potentially occur during exercise. Untrained VEGFf/f mice and both untrained and trained VEGFHSA−/− mouse groups had significant, substantial decreases in hippocampal blood flow that ranged from 57 to 80% with a hypoxic challenge. This reduction in CBF under the period of low oxygen in our protocol is a predicted consequence of the fall in or hypocapnia (Duong, 2007). Interestingly, the trained VEGFf/f group did not show a significant decrease in CBF with the hypoxic challenge. Animal and human studies have found VEGF levels to be increased in the brain in response to chronic hypoxia and exercise (Dombrowski et al. 2008; Tang et al. 2010; Yang et al. 2016). Thus, additional training effects from skeletal myofiber VEGF may attenuate this decrement in CBF under hypoxic conditions.
Summary
This study has established that VEGF expressed by skeletal muscle myofibers is important for increasing the number of neuronal precursor cells in the hippocampus in response to exercise training. This has many implications for chronic diseases that exhibit impaired cognitive function. While there are many metabolites and growth factors that could play a role in the exercise response, this study suggests that adequate blood flow to the hippocampus is associated with the neurogenesis response. It also provides support for developing therapeutic strategies in which neurogenesis factors delivered through a peripheral or myofiber route work in conjunction with exercise to enhance neurogenesis and spatial memory. Identifying the precise factors that are diminished with age or altered with specific neurodegenerative disease would facilitate such an approach.
Additional information
Competing interests
The authors have no competing interests.
Author contributions
BR exercise‐trained the mice, collected the fMRI, histology and gene expression measurements and prepared the manuscript. MS performed and analysed the fMRI measurements of CBF. MY provided the Nestin‐GFP mice. PDW assisted with the research design and manuscript preparation. ECB designed the experiments, and assisted with data collection and manuscript preparation.
Funding
This study was funded by NIH/NHLBI 1 P01 HL091830‐01A1. BR was funded by a grant from the American Heart Association, AHA 13UFEL16580031.
Acknowledgements
We thank Janelle Fine for assistance with the experimental set up in the laboratory, and Robert Bussell for assistance with arterial spin labelling set up for MRI.
Linked articles This article is highlighted by a Perspective by Ballard. To read this Perspective, visit https://doi.org/10.1113/JP274658.
This is an Editor's Choice article from the 1 September 2017 issue.
References
- Arida RM, Scorza CA, da Silva AV, Scorza FA & Cavalheiro EA (2004). Differential effects of spontaneous versus forced exercise in rats on the staining of parvalbumin‐positive neurons in the hippocampal formation. Neurosci Lett 364, 135–138. [DOI] [PubMed] [Google Scholar]
- Billat VL, Mouisel E, Roblot N & Melki J (2005). Inter‐ and intrastrain variation in mouse critical running speed. J Appl Physiol (1985) 98, 1258–1263. [DOI] [PubMed] [Google Scholar]
- Breen EC, Johnson EC, Wagner H, Tseng HM, Sung LA & Wagner PD (1996). Angiogenic growth factor mRNA responses in muscle to a single bout of exercise. J Appl Physiol 81, 355–361. [DOI] [PubMed] [Google Scholar]
- Christov C, Chretien F, Abou‐Khalil R, Bassez G, Vallet G, Authier FJ, Bassaglia Y, Shinin V, Tajbakhsh S, Chazaud B & Gherardi RK (2007). Muscle satellite cells and endothelial cells: close neighbors and privileged partners. Mol Biol Cell 18, 1397–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark PJ, Brzezinska WJ, Puchalski EK, Krone DA & Rhodes JS (2009). Functional analysis of neurovascular adaptations to exercise in the dentate gyrus of young adult mice associated with cognitive gain. Hippocampus 19, 937–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delavar H, Nogueira L, Wagner PD, Hogan MC, Metzger D & Breen EC (2014). Skeletal myofiber VEGF is essential for the exercise training response in adult mice. Am J Physiol Regul Integr Comp Physiol 306, R586–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande A, Bergami M, Ghanem A, Conzelmann KK, Lepier A, Gotz M & Berninger B (2013). Retrograde monosynaptic tracing reveals the temporal evolution of inputs onto new neurons in the adult dentate gyrus and olfactory bulb. Proc Natl Acad Sci USA 110, E1152–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrowski SM, Deshpande A, Dingwall C, Leichliter A, Leibson Z & Luciano MG (2008). Chronic hydrocephalus‐induced hypoxia: increased expression of VEGFR‐2+ and blood vessel density in hippocampus. Neuroscience 152, 346–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong TQ (2007). Cerebral blood flow and BOLD fMRI responses to hypoxia in awake and anesthetized rats. Brain Res 1135, 186–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Encinas JM & Enikolopov G (2008). Identifying and quantitating neural stem and progenitor cells in the adult brain. Methods Cell Biol 85, 243–272. [DOI] [PubMed] [Google Scholar]
- Fabel K, Tam B, Kaufer D, Baiker A, Simmons N, Kuo CJ & Palmer TD (2003). VEGF is necessary for exercise‐induced adult hippocampal neurogenesis. Eur J Neurosci 18, 2803–2812. [DOI] [PubMed] [Google Scholar]
- Gerber HP, Hillan KJ, Ryan AM, Kowalski J, Keller GA, Rangell L, Wright BD, Radtke F, Aguet M & Ferrara N (1999). VEGF is required for growth and survival in neonatal mice. Development 126, 1149–1159. [DOI] [PubMed] [Google Scholar]
- Grundy D (2015). Principles and standards for reporting animal experiments in the Journal of Physiology and Experimental Physiology . J Physiol 593, 2547–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holschneider DP, Yang J, Guo Y & Maarek JM (2007). Reorganization of functional brain maps after exercise training: Importance of cerebellar‐thalamic‐cortical pathway. Brain Res 1184, 96–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K, Okamoto M, Shibato J, Lee MC, Matsui T, Rakwal R & Soya H (2015). Long‐term mild, rather than intense, exercise enhances adult hippocampal neurogenesis and greatly changes the transcriptomic profile of the hippocampus. PLoS One 10, e0128720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsimpardi L, Litterman NK, Schein PA, Miller CM, Loffredo FS, Wojtkiewicz GR, Chen JW, Lee RT, Wagers AJ & Rubin LL (2014). Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science 344, 630–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiuchi T, Lee H & Mikami T (2012). Regular exercise cures depression‐like behavior via VEGF‐Flk‐1 signaling in chronically stressed mice. Neuroscience 207, 208–217. [DOI] [PubMed] [Google Scholar]
- Knapp AE, Goldberg D, Delavar H, Trisko BM, Tang K, Hogan MC, Wagner PD & Breen EC (2016). Skeletal myofiber VEGF regulates contraction‐induced perfusion and exercise capacity but not muscle capillarity in adult mice. Am J Physiol Regul Integr Comp Physiol 311, R192–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobilo T, Yuan C & van Praag H (2011). Endurance factors improve hippocampal neurogenesis and spatial memory in mice. Learn Mem 18, 103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplagne DA, Esposito MS, Piatti VC, Morgenstern NA, Zhao C, van Praag H, Gage FH & Schinder AF (2006). Functional convergence of neurons generated in the developing and adult hippocampus. PLoS Biol 4, e409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee‐Young RS, Griffee SR, Lynes SE, Bracy DP, Ayala JE, McGuinness OP & Wasserman DH (2009). Skeletal muscle AMP‐activated protein kinase is essential for the metabolic response to exercise in vivo . J Biol Chem 284, 23925–23934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei H, Pilloud Y, Magill AW & Gruetter R (2011). Continuous arterial spin labeling of mouse cerebral blood flow using an actively‐detuned two‐coil system at 9.4T. Conf Proc IEEE Eng Med Biol Soc 2011, 6993–6996. [DOI] [PubMed] [Google Scholar]
- Levy AP, Levy NS & Goldberg MA (1996). Post‐transcriptional regulation of vascular endothelial growth factor by hypoxia. J Biol Chem 271, 2746–2753. [DOI] [PubMed] [Google Scholar]
- Li L, Candelario KM, Thomas K, Wang R, Wright K, Messier A & Cunningham LA (2014). Hypoxia inducible factor‐1α (HIF‐1α) is required for neural stem cell maintenance and vascular stability in the adult mouse SVZ. J Neurosci 34, 16713–16719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlatt MW, Potter MC, Lucassen PJ & van Praag H (2012). Running throughout middle‐age improves memory function, hippocampal neurogenesis, and BDNF levels in female C57BL/6J mice. Dev Neurobiol 72, 943–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nokia MS, Lensu S, Ahtiainen JP, Johansson PP, Koch LG, Britton SL & Kainulainen H (2016). Physical exercise increases adult hippocampal neurogenesis in male rats provided it is aerobic and sustained. J Physiol 594, 1855–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olfert IM, Howlett RA, Tang K, Dalton ND, Gu Y, Peterson KL, Wagner PD & Breen EC (2009). Muscle‐specific VEGF deficiency greatly reduces exercise endurance in mice. J Physiol 587, 1755–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosthuyse B, Moons L, Storkebaum E, Beck H, Nuyens D, Brusselmans K, Van Dorpe J, Hellings P, Gorselink M, Heymans S, Theilmeier G, Dewerchin M, Laudenbach V, Vermylen P, Raat H, Acker T, Vleminckx V, Van Den Bosch L, Cashman N, Fujisawa H, Drost MR, Sciot R, Bruyninckx F, Hicklin DJ, Ince C, Gressens P, Lupu F, Plate KH, Robberecht W, Herbert JM, Collen D & Carmeliet P (2001). Deletion of the hypoxia‐response element in the vascular endothelial growth factor promoter causes motor neuron degeneration. Nat Genet 28, 131–138. [DOI] [PubMed] [Google Scholar]
- Ouchi N, Shibata R & Walsh K (2005). AMP‐activated protein kinase signaling stimulates VEGF expression and angiogenesis in skeletal muscle. Circ Res 96, 838–846. [DOI] [PubMed] [Google Scholar]
- Overall RW, Walker TL, Fischer TJ, Brandt MD & Kempermann G (2016). Different mechanisms must be considered to explain the increase in hippocampal neural precursor cell proliferation by physical activity. Front Neurosci 10, 362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler M, Ali F, Metzger E, Chambon P & Metzger D (2005). Temporally controlled targeted somatic mutagenesis in skeletal muscles of the mouse. Genesis 41, 165–170. [DOI] [PubMed] [Google Scholar]
- Shefer G, Rauner G, Stuelsatz P, Benayahu D & Yablonka‐Reuveni Z (2013). Moderate‐intensity treadmill running promotes expansion of the satellite cell pool in young and old mice. FEBS J 280, 4063–4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K & Temple S (2004). Endothelial cells stimulate self‐renewal and expand neurogenesis of neural stem cells. Science 304, 1338–1340. [DOI] [PubMed] [Google Scholar]
- Sinha M, Jang YC, Oh J, Khong D, Wu EY, Manohar R, Miller C, Regalado SG, Loffredo FS, Pancoast JR, Hirshman MF, Lebowitz J, Shadrach JL, Cerletti M, Kim MJ, Serwold T, Goodyear LJ, Rosner B, Lee RT & Wagers AJ (2014). Restoring systemic GDF11 levels reverses age‐related dysfunction in mouse skeletal muscle. Science 344, 649–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Soumier A, Brewer M, Pickel J & Cameron HA (2011). Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature 476, 458–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summermatter S, Santos G, Perez‐Schindler J & Handschin C (2013). Skeletal muscle PGC‐1α controls whole‐body lactate homeostasis through estrogen‐related receptor α‐dependent activation of LDH B and repression of LDH A. Proc Natl Acad Sci USA 110, 8738–8743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Jin K, Xie L, Childs J, Mao XO, Logvinova A & Greenberg DA (2003). VEGF‐induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Invest 111, 1843–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang K, Gu Y, Dalton ND, Wagner H, Peterson KL, Wagner PD & Breen EC (2016). Selective life‐long skeletal myofiber‐targeted VEGF gene ablation impairs exercise capacity in adult mice. J Cell Physiol 231, 505–511. [DOI] [PubMed] [Google Scholar]
- Tang K, Xia FC, Wagner PD & Breen EC (2010). Exercise‐induced VEGF transcriptional activation in brain, lung and skeletal muscle. Respir Physiol Neurobiol 170, 16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas GD, Shaul PW, Yuhanna IS, Froehner SC & Adams ME (2003). Vasomodulation by skeletal muscle‐derived nitric oxide requires α‐syntrophin‐mediated sarcolemmal localization of neuronal nitric oxide synthase. Circ Res 92, 554–560. [DOI] [PubMed] [Google Scholar]
- Tschakovsky ME, Shoemaker JK & Hughson RL (1996). Vasodilation and muscle pump contribution to immediate exercise hyperemia. Am J Physiol Heart Circ Physiol 271, H1697–1701. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G & Gage FH (1999). Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci 2, 266–270. [DOI] [PubMed] [Google Scholar]
- Vivar C, Potter MC, Choi J, Lee JY, Stringer TP, Callaway EM, Gage FH, Suh H & van Praag H (2012). Monosynaptic inputs to new neurons in the dentate gyrus. Nat Commun 3, 1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Wang H, Guo Y, Ning W, Katkuri S, Wahli W, Desvergne B, Dey SK & DuBois RN (2006). Crosstalk between peroxisome proliferator‐activated receptor δ and VEGF stimulates cancer progression. Proc Natl Acad Sci USA 103, 19069–19074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters RE, Rotevatn S, Li P, Annex BH & Yan Z (2004). Voluntary running induces fiber type‐specific angiogenesis in mouse skeletal muscle. Am J Physiol Cell Physiol 287, C1342–1348. [DOI] [PubMed] [Google Scholar]
- Wegener S & Wong EC (2008). Longitudinal MRI studies in the isoflurane‐anesthetized rat: long‐term effects of a short hypoxic episode on regulation of cerebral blood flow as assessed by pulsed arterial spin labelling. NMR Biomed 21, 696–703. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Saito H, Suzuki M & Mori K (2000). Visualization of neurogenesis in the central nervous system using nestin promoter‐GFP transgenic mice. Neuroreport 11, 1991–1996. [DOI] [PubMed] [Google Scholar]
- Yan Z, Okutsu M, Akhtar YN & Lira VA (2011). Regulation of exercise‐induced fiber type transformation, mitochondrial biogenesis, and angiogenesis in skeletal muscle. J Appl Physiol 110, 264–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Shanahan KJ, Shriver LP & Luciano MG (2016). Exercise‐induced changes of cerebrospinal fluid vascular endothelial growth factor in adult chronic hydrocephalus patients. J Clin Neurosci 24, 52–56. [DOI] [PubMed] [Google Scholar]
- Yang TT, Lo CP, Tsai PS, Wu SY, Wang TF, Chen YW, Jiang‐Shieh YF & Kuo YM (2015). Aging and exercise affect hippocampal neurogenesis via different mechanisms. PloS One 10, e0132152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang RL, Chopp M, Roberts C, Liu X, Wei M, Nejad‐Davarani SP, Wang X & Zhang ZG (2014). Stroke increases neural stem cells and angiogenesis in the neurogenic niche of the adult mouse. PLoS One 9, e113972. [DOI] [PMC free article] [PubMed] [Google Scholar]


