Abstract
Key points
Cerebral haemodynamic response to neural stimulation has been extensively studied in adults, but little is known about cerebral haemodynamic response in the fetal and neonatal brain.
The present study describes the cerebral haemodynamic response measured by near infrared spectroscopy to somatosensory stimulation in newborn lambs, in comparison to recent findings in fetal sheep.
The cerebral haemodynamic responses in the newborn lamb brain can involve an increase in oxyhaemoglobin (oxyHb), or a decrease of oxyHb suggestive of reduced perfusion and oxygenation.
Positive correlations between changes in oxyHb and mean arterial blood pressure were found in newborn but not fetal sheep, which suggests the result is unlikely to be due to immature autoregulation alone.
In contrast to adult studies, hypercapnia increased the changes in cerebral blood flow and oxyHb in most of the lambs in response to somatosensory stimulation.
Abstract
The neurovascular coupling response has been defined for the adult brain, but in the neonate non‐invasive measurement of local cerebral perfusion using near infrared spectroscopy or blood oxygen level‐dependent functional magnetic resonance imaging have yielded variable and inconsistent results, including negative responses suggesting decreased perfusion and localized tissue tissue hypoxia. Also, the impact of permissive hypercapnia ( > 50 mmHg) in the management of neonates on cerebrovascular responses to somatosensory input is unknown. Using near infrared spectroscopy to measure changes in cerebral oxy‐ and deoxyhaemoglobin (ΔoxyHb, ΔdeoxyHb) in eight anaesthetized newborn lambs, we studied the cerebral haemodynamic functional response to left median nerve stimulation using stimulus trains of 1.8, 4.8 and 7.8 s. Stimulation always produced a somatosensory evoked response, and superficial cortical perfusion measured by laser Doppler flowmetry predominantly increased following median nerve stimulation. However, with 1.8 s stimulation, oxyHb responses in the contralateral hemisphere were either positive (i.e. increased oxyHb), negative, or absent; and with 4.8 and 7.8 s stimulations, both positive and negative responses were observed. Hypercapnia increased baseline oxyHb and total Hb consistent with cerebral vasodilatation, and six of seven lambs tested showed increased Δtotal Hb responses after the 7.8 s stimulation, among which four lambs also showed increased ΔoxyHb responses. In two of three lambs, the negative ΔoxyHb response became a positive pattern during hypercapnia. These results show that instead of functional hyperaemia, somatosensory stimulation can evoke negative (decreased oxyHb, total Hb) functional responses in the neonatal brain suggestive of decreased local perfusion and vasoconstriction, and that hypercapnia produces both baseline hyperperfusion and increased functional hyperaemia.
Keywords: cerebral blood flow, functional NIRS, neonatal brain, neurovascular coupling, somatosensory stimulation
Key points
Cerebral haemodynamic response to neural stimulation has been extensively studied in adults, but little is known about cerebral haemodynamic response in the fetal and neonatal brain.
The present study describes the cerebral haemodynamic response measured by near infrared spectroscopy to somatosensory stimulation in newborn lambs, in comparison to recent findings in fetal sheep.
The cerebral haemodynamic responses in the newborn lamb brain can involve an increase in oxyhaemoglobin (oxyHb), or a decrease of oxyHb suggestive of reduced perfusion and oxygenation.
Positive correlations between changes in oxyHb and mean arterial blood pressure were found in newborn but not fetal sheep, which suggests the result is unlikely to be due to immature autoregulation alone.
In contrast to adult studies, hypercapnia increased the changes in cerebral blood flow and oxyHb in most of the lambs in response to somatosensory stimulation.
Abbreviations
- BOLD
blood oxygen level‐dependent
- CBF
cerebral blood flow
- deoxyHb
deoxyhaemoglobin
- EEG
electroencephalography
- HR
heart rate
- fMRI
functional magnetic resonance imaging
- LDF
laser Doppler flow
- MABP
mean arterial blood pressure
- NIRS
near infrared spectroscopy
- oxyHb
oxyhaemoglobin
arterial tension of carbon dioxide
- SEP
somatosensory evoked potential
- total Hb
total haemoglobin
Introduction
In the adult brain, increases in local neuronal activity result in a localized increase of cerebral blood flow (CBF), a process known as neurovascular coupling (Fox & Raichle, 1986). The influx of oxygenated arterial blood temporarily exceeds the consumption of oxygen by the tissue, leading to localized increases in oxygenation which can be detected as an increase in oxyhaemoglobin (oxyHb) and decrease in deoxyhaemoglobin (deoxyHb) by near‐infrared spectroscopy (NIRS), or as a positive blood oxygen level‐dependent (BOLD) signal by functional magnetic resonance imaging (fMRI).
The neurovascular coupling response has been relatively well defined for the adult brain. Fetal sheep studies have also shown increased CBF with the low voltage electroencephalography (EEG) and rapid eye movements indicative of active sleep (Richardson et al. 1985), and positive BOLD signals have been detected during auditory or vibratory stimulation in the human fetus (Hykin et al. 1999; Fulford et al. 2004). In contrast, functional imaging studies in early postnatal life in human neonates and rat pups have reported dramatically different responses that fall into two broad categories: (i) a positive response (i.e. a localized increase in oxyHb and/or decrease in deoxyHb) resembling adult functional hyperaemia; or (ii) a negative response (localized decrease in oxyHb and/or increase in deoxyHb) in early postnatal life, which then switches to a positive response at a later age (Kusaka et al. 2004; Kozberg et al. 2013; Zehendner et al. 2013). The first category of exclusively positive functional responses in all age groups from neonates to adults was shown in studies using fMRI in anaesthetized rats (Colonnese et al. 2008) and sedated human infants (Arichi et al. 2012), which further showed there was a decrease in the time to peak and increase in magnitude of the positive haemodynamic response with age. The age‐related changes may be due to development and refinement of synaptic connections, increased myelination and vasculature with age (Harris et al. 2011), i.e. increased maturation of some or all of the components of the neurovascular unit that is the anatomical basis of neurovascular coupling.
However, NIRS signals obtained from rat pups indicate the presence of the negative responses following somatosensory stimulation at postnatal days 7–15, contrasting with the positive functional response recorded in the adult rats and mice (Kozberg et al. 2013; Zehendner et al. 2013). Several studies in human infants report variable positive, negative and even biphasic responses in neonates and young infants (Meek et al. 1998; Muramoto et al. 2002; Kusaka et al. 2004; Erberich et al. 2006; Heep et al. 2009; Roche‐Labarbe et al. 2014), before switch to the typical positive response observed in adults (Harris et al. 2011). These data suggest that there is a developmental phase when oxygen demand can exceed local cerebral perfusion when cortical neural networks are activated, which then leads to localized tissue hypoxia (Muramoto et al. 2002). Clearly, this could be of consequence for the developing brain, particularly if it occurred frequently.
The differences between the studies discussed above may be due to the different species used, study protocols, methods used for recording the cerebral haemodynamic responses, or for neuronal stimulation. For example, newborn infants showed a negative haemodynamic response with reduced oxyHb in the olfactory cortex when exposed to the unpleasant odour of disinfectants used in the neonatal units, but a positive response when exposed to the smell of colostrum from the mother or to vanilla as a likely pleasant odour (Bartocci et al. 2001). Interestingly, the magnitude of the oxyHb increase during colostrum exposure was inversely related to postnatal age, but there were no similar age‐related differences for vanilla, suggesting the maternal influence on cortical responses (Bartocci et al. 2000). In addition, clinical studies in human neonates are often confounded by the limited number and heterogeneity of patients and the clinical conditions they present with. To our knowledge no studies have explored the difference in haemodynamic functional response to neuronal stimulation between fetal and postnatal life in an animal model using a consistent methodology. While a few experimental studies have used neonatal rats or mice from early (days 7–15) postnatal life (Colonnese et al. 2008; Kozberg et al. 2013; Zehendner et al. 2013), and may be said thereby to have investigated responses of the very immature brain, establishment of a controlled animal model for testing the haemodynamic functional response from fetal to early postnatal life is essential if we are to fully understand the impact of birth and gestational age on cerebral vasoreactivity.
Sheep provide an excellent basis for study of the basic physiology of the fetal and postnatal cerebral circulation and CBF reactivity in relation to cerebral metabolism (Richardson et al. 1989; Gleason et al. 1990, 2002; Raju, 1992). In fetal sheep we recently showed that somatosensory nerve stimulation provoked predominantly positive cerebral haemodynamic responses, but the response became negative (i.e. decrease in oxyHb) or biphasic when the nerve stimulation was prolonged (Nakamura et al. 2017). Moreover, hypercapnia increased the incidence and magnitude of positive haemodynamic responses in these fetuses, contrasting with adult studies where hypercapnia usually decreases activity‐related changes of CBF (Zappe et al. 2008; Maggio et al. 2014).
In the study presented here, we investigated the effect of forelimb median nerve stimulation of different durations on the cortical haemodynamic response in newborn lambs using NIRS. We hypothesized that in newborn lambs at approximately 1 week of age, peripheral nerve stimulation would provoke positive functional responses, signifying the presence of mature neurovascular coupling. In addition, we proposed that increased duration of somatosensory stimulation would provoke progressively higher changes in oxyHb and deoxyHb in the contralateral cortex, as observed in the adult rodent brain (Franceschini et al. 2010), and thus different from the responses observed previously by us in fetal sheep, which demonstrated negative and biphasic responses (Nakamura et al. 2017).
We also examined the effects of hypercapnia on the cerebral haemodynamic response to somatosensory stimulation in the newborn lamb brain. Permissive hypercapnia, involving the acceptance of high arterial tension of carbon dioxide ( > 50 mmHg) by using lower ventilator settings, has been found to reduce the incidence of chronic lung disease in preterm infants (Miller & Carlo, 2007), and it is accepted as standard clinical practice in many neonatal units. However, hypercapnia can trigger intraventricular haemorrhage in preterm infants through vasodilatation and engorgement of cerebral microvasculature (Kaiser et al. 2006), and some of the mediators (including H+, K+ and NO) implicated in the CBF response to neuronal activation are also involved when hypercapnia induces an increase of CBF (Maggio et al. 2013). Accordingly, it is reasonable to postulate that manipulation of could interfere with neural regulation of the cerebral microvasculature in neonates, thus affecting the haemodynamic functional response. Therefore, we hypothesized that hypercapnia would diminish the cerebral haemodynamic functional response in the newborn sheep, as shown in adults (Zappe et al. 2008; Maggio et al. 2014). We compared the findings in the newborn lamb with those we recently reported in the fetal sheep (Nakamura et al. 2017) using the same experimental protocol and NIRS techniques.
Methods
Ethics statement
Merino/Border–Leicester cross newborn lambs were used in this study. The use of these animals and all procedures were in accordance with the guidelines of the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes established by the National Health and Medical Research Council of Australia and was approved by the Monash University‐Monash Medical Centre Committee on Ethics in Animal Experimentation.
Animals and surgery
Eight newborn lambs were used, 5–8 days old and of mean (SEM) weight 6.1 (0.4) kg. General anaesthesia was induced by allowing the lamb to breath 4–5% isoflurane in O2 from a mask, and after intubation, anaesthesia was maintained by inhalation of 1–1.5% isoflurane with mechanical ventilation. A non‐occlusive intravenous catheter (Intracath 24 GA; Becton Dickinson, Sandy, UT, USA) was inserted into a peripheral vein on the hindlimb for administration of 5% glucose in 0.18% saline for fluid maintenance. Another non‐occlusive catheter (Insyte‐N 22–24 G; Becton Dickinson) was inserted into the femoral artery for blood pressure and blood gas monitoring; blood samples (0.3 ml) were taken at least hourly to monitor blood pH, oxygen saturation, partial pressures of oxygen and carbon dioxide, and acid–base balance (ABL5000, Radiometer, Brønshøj, Denmark) throughout the study. An oxygen saturation probe (, Radical 4, Masimo, Frenchs Forest, NSW, Australia) was placed on the right forelimb of the lamb. Ventilatory settings and fractional inspired oxygen were adjusted to maintain normoxia ( at 95–100%) and normocapnia (end‐tidal carbon dioxide at 35–45 mmHg, confirmed with arterial blood gas), except when deliberately changed to create arterial hypercapnia (see below). The experiment using the procedures described below usually took 4–5 h, at the end of which the lamb was killed by an i.v. overdose of pentobarbital (pentobarbitone sodium 325 mg ml−1, Virbac, Milperra, NSW, Australia).
Electroencephalography
Two channels of an EEG were recorded with silver cup electrodes placed on the scalp over the somatosensory cortex (C3–5 and C4‐–6) according to published coordinates for key land marks on the sheep's head (Cook et al. 1987). The EEG was recorded using differential AC amplifiers with bandpass filters set at 1.0–35 Hz (Grass Instrument, Quincy, MA, USA). The EEG was used to monitor depth of anaesthesia, in particular to avoid the burst‐suppression EEG pattern which indicates deep anaesthesia.
Near infrared spectroscopy
The light emitters and detectors (optodes) of a two channel NIRS (NIRO‐200, Hamamatsu Photonics) were positioned symmetrically on either side of the scalp over the somatosensory cortex. For each channel, the emitter and detector were placed 4 cm apart. The changes in total, oxy‐ and deoxyhaemoglobin (∆total Hb, ∆oxyHb and ∆deoxyHb, μm cm) were continuously recorded bilaterally, as described previously (Nakamura et al. 2017). The basic principles of this technique (Jobsis, 1977), its use in functional studies, and its reliability in studying cerebral cortical responses in newborn infants have been described in detail elsewhere (Wolf & Greisen, 2009).
Laser Doppler flowmetry
In five lambs a 1 cm diameter cranial window was created over the right parietal region (i.e. contralateral to the stimulated median nerve) just rostral to a line joining the anterior border of the ears and bregma. The dural surface was exposed onto which a laser Doppler flowmetry (LDF) probe (OxyFloTM2000, Oxford Optronix, Abingdon, UK) was placed to measure superficial local cortical blood flow to a depth of about 1 mm.
Somatosensory evoked potentials
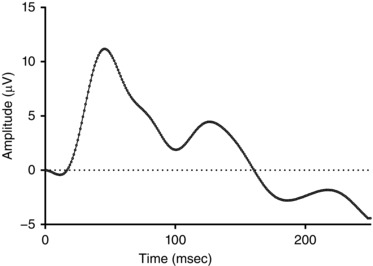
The median nerve was exposed at the cubital fossa of the left forelimb. A small silicon cuff mounted with two multi‐stranded copper wire electrodes approx. 1cm apart was placed around the median nerve. The electrodes were connected to an isolated constant‐current electrical pulse generator (ISOLATOR‐11 stimulator isolation unit; Axon Instruments Inc., Foster City, CA, USA) that delivered pre‐programmed trains of 2 ms pulses generated by software (LabChart 7, ADInstruments Pty Ltd, Bella Vista, NSW, Australia). For every experiment, the amplitude of the stimulus was set to the minimum necessary (usually 2–5 mA) to evoke a visible twitch of the left forelimb. Registration of median nerve stimulation as somatosensory stimulation was confirmed by recording the somatosensory evoked potential (SEP), using stimulus‐triggered signal averaging of the EEG over a 250 ms window following each pulse. Electrical pulses were delivered to the median nerve at 1 Hz over a number of repetitions (usually 60–80) necessary to identify a clear SEP response; an example is shown in Fig. 1. The mean (SEM) latency and amplitude of the first component in the SEP was 43.4 (13.1) ms and 7.9 (2.9) μV, respectively, consistent with the first negative or positive response component on the contralateral SEP (N/P1) at approximately 50 ms after the stimulation (Nicol et al. 1998; Abdel‐Rahman et al. 2000).
Figure 1. Example of the somatosensory evoked potential (SEPs) in a newborn lamb recorded from the contralateral cortex, in response to median nerve stimulation at 1 Hz for 80–100 s.
Experimental protocol and data recording
All the physiological signals (arterial pressure, heart rate, EEG, LDF, NIRS outputs (∆total Hb, ∆oxyHb, ∆deoxyHb)) were recorded digitally at a sampling rate of 1000 Hz using a data acquisition system (Powerlab, ADInstruments). To elicit and record the neurovascular coupling responses in each animal, the median nerve was stimulated with stimulus trains of 1.8, 4.8, and 7.8 s duration, using 2 ms pulses at 3.3 Hz repetition rate. The beginning of each stimulus train triggered the recording of all physiological signals over a 60 s window, preceded by a 6 s period of pre‐stimulus recording which was used as the baseline to determine the magnitude of change of the NIRS signals, mean arterial blood pressure (MABP), heart rate (HR), etc. following median nerve stimulation. Twenty repeats of each stimulus train were delivered to produce a signal‐averaged output of the NIRS, EEG and cardiovascular data, using Scope software (ADInstruments, Australia). The three train durations (1.8, 4.8, 7.8 s) were delivered in random order.
Hypercapnia
Hypercapnia was induced in seven of the eight lambs by reducing the ventilator rate and tidal volume to achieve a target end‐tidal CO2 of 60–70 mmHg, while maintaining of 95–100% by adjusting the fractional inspired oxygen. The 7.8 s stimulus train was repeated in the lamb during hypercapnia while following the protocols described above, and recording of all physiological signals was as for the normocapnia trials.
Data analysis
The MABP, LDF and NIRS data obtained during the 60 s post‐stimulus window of recording were averaged as consecutive 1 s duration bins, and expressed as changes from the pre‐stimulus baseline. Analysis and classification of the NIRS response patterns in the contralateral cortex was based on the ΔoxyHb signal, which gave a more robust signal‐to‐noise ratio than ΔdeoxyHb, as reported previously by us (Nakamura et al. 2017) and elsewhere (Bortfeld et al. 2007; Minagawa‐Kawai et al. 2007). The ‘positive’ response pattern was defined as an increase in oxyHb in the contralateral cortex after the stimulation, whereas a negative response was defined as a decrease in contralateral oxyHb. The magnitude in contralateral ΔoxyHb in either direction was required to be more than 2 standard deviations of its baseline data (6 s of pre‐stimulus recording) for the response to be defined as positive or negative.
Statistical analyses were performed with GraphPad Prism 5J (GraphPad Software, La Jolla, CA, USA). The non‐parametric Mann–Whitney U test was used to compare the time to peak and amplitude of MABP and ΔoxyHb signal, between the positive and negative response patterns, for the 1.8, 4.8 or 7.8 s stimulation, respectively. Non‐parametric repeated measures ANOVA on ranks was used to compare the contralateral ΔHb data in response to the 7.8 s stimulus train before and during hypercapnia. Data from fetal (as reported in Nakamura et al. 2017) and newborn sheep were also compared using the Mann–Whitney U test. P values of less than 0.05 were considered significant. All results are expressed as means ± SEM.
Results
Physiological status of the lambs
The entire experiment under anaesthesia and mechanical ventilation was of 4–5 h duration. With the exception of the arterial partial pressure of oxygen (), which fell slightly, all the other blood parameters, MABP and HR were stable over this time (Table 1).
Table 1.
Newborn lambs’ arterial blood gases, mean arterial blood pressure (MABP) and heart rate (HR) at the beginning and end of the experiment (values are means ± SEM)
| Parameter | Beginning of experiment | End of experiment |
|---|---|---|
| pH | 7.35 ± 0.03 | 7.36 ± 0.02 |
| (mmHg) | 41.9 ± 1.8 | 41.6 ± 1.6 |
| (mmHg) | 118.0 ± 9.4 | 96.0 ± 4.1 (P = 0.06) |
| HCO3 − (mmol l−1) | 22.5 ± 1.5 | 22.7 ± 1.3 |
| Base excess (mmol l−1) | −2.5 ± 1.7 | −2.1 ±1.6 |
| Glucose (mmol l−1) | 11.2 ± 2.3 | 9.8 ± 1.1 |
| Lactate (mmol l−1) | 1.9 ± 0.5 | 1.2 ± 0.1 |
| MABP (mmHg) | 67.3 ± 5.6 | 66.5 ± 4.4 |
| HR (beats min−1) | 213.5 ± 1.0 | 213.5 ± 12.3 |
Patterns of cerebral haemodynamic response to somatosensory stimulation
ΔoxyHb
Table 2 summarizes the incidence of the cerebral haemodynamic response patterns in the newborn lambs, which are based on changes in oxyHb signal above or below the baseline in the contralateral cortex following median nerve stimulation. These responses were classified as either ‘positive’ or ‘negative’, and occasionally, no distinct response at all (see Methods). A short (1.8 s) duration of stimulation produced a positive response in 3/8, a negative response in 2/8, and no response at all in 3/8 lambs (Table 2 and Fig. 2 A). In the lambs that showed no change in the ΔoxyHb signal, the SEP was not different from the lambs in which a positive or negative change in ΔoxyHb did occur. With 4.8 s of stimulation, 5/8 lambs showed the negative response with reduced contralateral oxyHb, and the other three lambs showed a positive response (Table 2 and Fig. 2 B). With 7.8 s of stimulation, an increase in oxyHb (i.e. positive response) occurred in 5/8 lambs, and the negative response occurred in another three lambs (Fig. 2 C).
Table 2.
Incidence of positive, negative or no response pattern in newborn lambs based on changes in oxyhaemoglobin (∆oxyHb) recorded by NIRS from the contralateral hemisphere following median nerve stimulation at 3.3 Hz for 1.8, 4.8 or 7.8 s
| Contralateral ΔoxyHb response pattern | ||||
|---|---|---|---|---|
| Duration of stimulation | No. of lambs studied | Positive | Negative | No response |
| 1.8 s | 8 | 3 | 2 | 3 |
| 4.8 s | 8 | 3 | 5 | 0 |
| 7.8 s | 8 | 5 | 3 | 0 |
Figure 2. Changes in oxy‐, deoxy‐ and total haemoglobin (ΔoxyHb, ΔdeoxyHb and Δtotal Hb), recorded from the contralateral hemispheres, and mean arterial blood pressure (ΔMABP), following a 3.3 Hz stimulus train of 1.8 (A), 4.8 (B), or 7.8 s (C) duration.
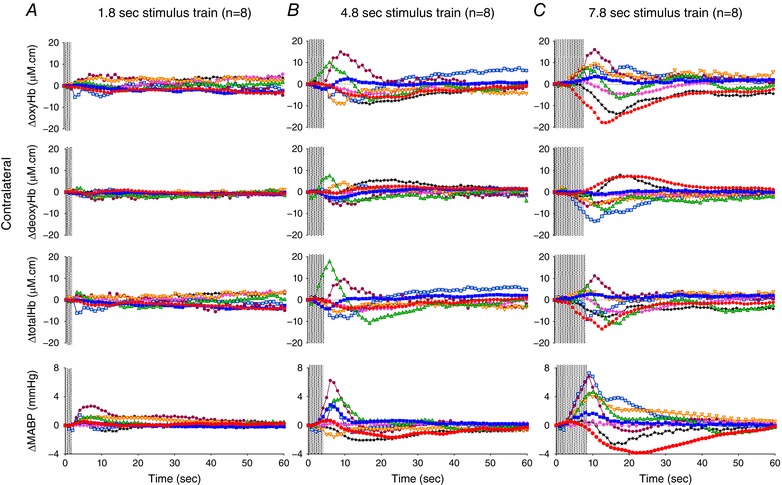
Individual lambs are shown by different colours. Shaded area in each graph shows the period of stimulation. [Color figure can be viewed at wileyonlinelibrary.com]
Δtotal Hb and ΔdeoxyHb
The response patterns in contralateral Δtotal Hb were concordant with those of ΔoxyHb in all lambs, except for one lamb which, when it received the 7.8 s stimulation, showed an increase in oxyHb while both total Hb and deoxyHb were reduced (light blue squares, Fig. 2 C). The changes in contralateral deoxyHb were of low amplitude with both 1.8 and 4.8 s stimulations, but with 7.8 s stimulation 6/8 lambs showed a change in deoxyHb in the opposite direction to that of the corresponding oxyHb (Fig. 2 C), and the other two lambs showed almost no change in deoxyHb.
LDF
The measurement of local cerebral perfusion using LDF was very dependent on positioning of the flow probe, and at times showed significant artefacts and was intermittently out of the measurement range, in which cases the data were excluded. When artefact‐free LDF recordings were obtained, regardless of the different patterns in the contralateral ΔoxyHb response detected by the NIRS, superficial LDF cortical perfusion was predominantly increased following median nerve stimulation; an example is shown in Fig. 3.
Figure 3. Example of the changes in oxyhaemoglobin (ΔoxyHb) and deoxyhaemoglobin (ΔdeoxyHb) recorded from the contralateral and ipsilateral hemispheres in a newborn lamb, together with laser Doppler flowmetry (LDF) measurement from the contralateral cortex and mean arterial pressure (ΔMABP) following a 3.3 Hz stimulus train lasting 4.8 s.
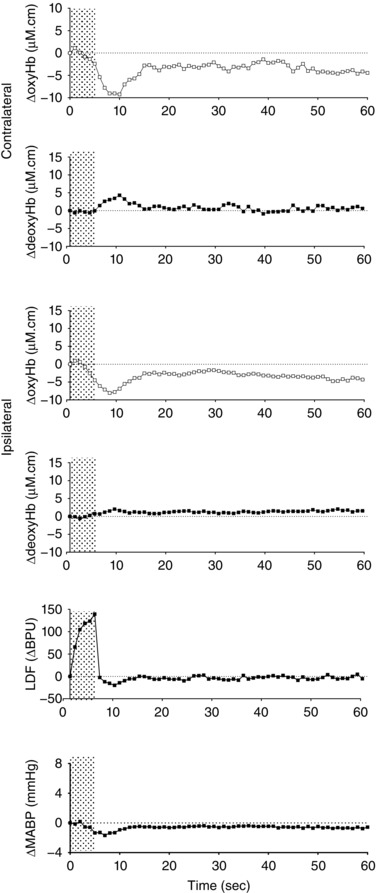
Shaded area shows the period of stimulation. Note increase in LDF measurement, while the changes in oxyHb and deoxyHb are consistent with a ‘negative’ functional response.
Changes in blood pressure and cerebral ΔHb after somatosensory stimulation
The changes in MABP following median nerve stimulation in the newborn lambs, and the peak or nadir changes in contralateral and ipsilateral oxyHb are shown for the positive and negative patterns, respectively, in Table 3.
Table 3.
Changes in physiological parameters in newborn lambs with positive or negative contralateral ΔoxyHb response, following median nerve stimulation for 1.8, 4.8 or 7.8 s (values are means ± SEM; individual values are shown where n < 3 available)
| 1.8 sa | 4.8 s | 7.8 s | |||||
|---|---|---|---|---|---|---|---|
| Negative (n = 2) | Positive (n = 3) | Negative (n = 5) | Positive (n = 3) | Negative (n = 3) | Positive (n = 5) | ||
| MABP | Time to peak (s) | 5.0, 4.0 | 7.6 ± 0.7 | 8.2 ± 1.3 | 7.0 ± 1.0 | 10.0 ± 3.5 | 9.0 ± 0.3 |
| Peak amplitude (mmHg) | 0.5, 1.5 | 1.7 ± 0.5 | 0.0 ± 0.8 | 4.2 ± 1.0† | −0.5 ± 1.1 | 5.0 ± 1.0* | |
| Contralateral ΔoxyHb | Time to peak/nadir (s) | 12.0, 3.0 | 9.3 ± 1.8 | 14.8 ± 1.4 | 8.7 ± 1.5‡ | 17.7 ± 2.3 | 10.6 ± 2.5* |
| Peak/nadir amplitude (μm cm) | −3.2, −5.3 | 3.5 ± 0.9 | −7.5 ± 0.9 | 9.2 ± 3.7* | −12.1 ± 3.9 | 8.9 ± 2.0* | |
| Ipsilateral ΔoxyHb | Time to peak/nadir (s) | 11.0, 3.0 | 9.3 ± 0.3 | 14.2 ± 2.8 | 8.3 ± 0.9 | 17.3 ± 2.6 | 9.8 ± 0.6* |
| Peak/nadir amplitude (μm cm) | −2.1, −3.3 | 4.4 ± 1.2 | −5.2 ± 1.9 | 10.3 ± 3.1* | −10.7 ± 3.6 | 10.6 ± 2.5 | |
| LDF | Time to peak (s) | 9.0 | 11.0 | 5.0 | 8.0, 6.0 | NA | 9.0 ± 0.3b |
| Peak amplitude (BPU) | 32.8 | 15.5 | 139.4 | 1.0, 2.1 | NA | 62.6 ± 31.4b | |
aWith 1.8 s stimulation, no response was obtained in 3 out of 8 lambs. b n = 3 available. † P = 0.07 vs. negative pattern; ‡ P = 0.07 vs. negative pattern; * P < 0.05 vs. Negative pattern. BPU, blood perfusion unit; ΔoxyHb, changes in oxyhaemoglobin; LDF, laser Doppler flowmetry; MABP, mean arterial blood pressure; NA, not available.
MABP
(i) Time to peak: the time taken to reach the peak MABP was similar irrespective of whether the oxyHb response pattern was positive or negative (Table 3). When a positive response pattern occurred, the time to peak MABP correlated closely with the time to peak in the contralateral ΔoxyHb, whereas when the negative response pattern occurred the peak MABP preceded the nadir contralateral ΔoxyHb (Table 3). (ii) Peak amplitude: the change in MABP was always greater for positive compared to negative responses – the difference was significant at 7.8 s of stimulation (P < 0.05; Table 3). The change in MABP was correlated significantly with the contralateral ΔoxyHb induced by the 4.8 and 7.8 s stimulations (Fig. 4 A–C).
Figure 4. Correlation of the peak changes in oxyhaemoglobin (ΔoxyHb) recorded from the contralateral hemisphere for the positive response pattern, or the nadir ΔoxyHb for the negative response pattern, with the mean arterial pressure (ΔMABP) following a 3.3 Hz stimulus trains of 1.8 (A), 4.8 (B), or 7.8 s (C) duration.
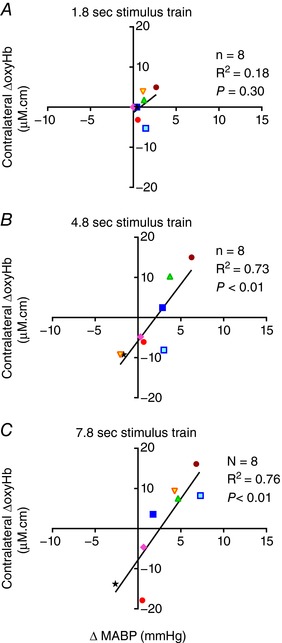
Significant positive correlations between ΔoxyHb and ΔMABP were found with the 4.8 and 7.8 s stimulus trains. [Color figure can be viewed at wileyonlinelibrary.com]
OxyHb
The rate of change in oxyHb in the contralateral hemisphere was different for the positive and negative responses, with the time to reach the peak ΔoxyHb value for the positive pattern occurring earlier than the time to the nadir oxyHb for the negative pattern, and this difference was significant with the 7.8 s stimulation (P < 0.05; Table 3). The peak changes in oxyHb in the ipsilateral cortex closely approximated those observed for the contralateral ΔoxyHb in both timing and amplitude (Table 3).
Hypercapnia
Arterial blood gases, MABP, HR, SEP and NIRS data obtained before and during the induced hypercapnia for seven newborn lambs are shown in Table 4. On increase of the , the baseline measures of both cerebral oxyHb and total Hb were increased, consistent with cerebral vasodilatation. Before hypercapnia four lambs showed a positive functional response evoked by the 7.8 s stimulation train and their response pattern remained positive during hypercapnia (Figs 5 A and 6 A). Three lambs showed the negative response pattern before hypercapnia, among which two of them became a positive pattern during hypercapnia (Figs 5 B and 6 A). Compared to the cerebral haemodynamic response before hypercapnia, six of the seven lambs showed increase in their contralateral Δtotal Hb responses after the 7.8 s stimulation during hypercapnia, indicating a higher CBF response (Fig. 6 C and D). Four of the seven lambs showed an increase in both ΔoxyHb and Δtotal Hb response, with inconsistent changes of ΔdeoxyHb (Fig. 6 A–D). Two of the seven lambs showed reduction in peak contralateral ΔoxyHb, among which one had reduced ΔdeoxyHb and Δtotal Hb, and the other had increased ΔdeoxyHb and Δtotal Hb in response to stimulation during hypercapnia (Fig. 6 D). One lamb had no change in the contralateral ΔoxyHb response during hypercapnia, but showed increased ΔdeoxyHb and Δtotal Hb responses (Fig. 6 D).
Table 4.
Newborn lambs’ arterial blood gases, mean arterial blood pressure (MABP), heart rate (HR) together with EEG and NIRS data obtained before and during the induced hypercapnia (values are means ± SEM, n = 7 lambs)
| Before hypercapnia | During hypercapnia | |
|---|---|---|
| pH | 7.38 ± 0.03 | 7.18 ± 0.02*** |
| (mmHg) | 40.4 ± 1.4 | 65.8 ± 2.3*** |
| (mmHg) | 95.2 ± 4.5 | 138.8 ± 21.2 |
| HCO3 − (mmol l−1) | 23.1 ± 1.4 | 23.9 ± 1.3 |
| BE (mmol l−1) | −1.5 ± 1.6 | −4.5 ± 1.4*** |
| Glucose (mmol l−1) | 9.7 ± 1.2 | 9.4 ± 0.9 |
| Lactate (mmol l−1) | 1.2 ± 0.1 | 0.8 ± 0.1** |
| MABP (mmHg) | 70.0 ± 5.8 | 60.2 ± 4.9** |
| HR (beats min−1) | 208.6 ± 12.0 | 218.5 ± 12.5* |
| Amplitude of SEP(N/P50) (ms) | 43.4 ± 13.1 | 41.8 ± 9.9 |
| Latency of SEPs (N/P50) (μV) | 7.9 ± 2.9 | 8.1 ± 1.6 |
| Total Hb (μm cm) | −0.4 ± 1.2 | 307.7 ± 68.1** |
| OxyHb (μm cm) | 2.7 ± 2.1 | 340.4 ± 54.8*** |
| DeoxyHb (μm cm) | −3.1 ± 2.1 | −32.7 ± 17.3 |
ΔDeoxyHb, change in deoxyhaemoglobin; ΔOxyHb, change in oxyhaemoglobin; ΔTotal Hb, changes in total haemoglobin; SEP, somatosensory evoked potential. * P < 0.05 vs. before hypercapnia; ** P < 0.01 vs. before hypercapnia; *** P < 0.001 vs. before hypercapnia.
Figure 5. Changes in oxyhaemoglobin (ΔoxyHb) and deoxyhaemoglobin (ΔdeoxyHb) recorded from the contralateral and ipsilateral hemispheres, and mean arterial pressure (ΔMABP), following a 3.3 Hz stimulus train of 7.8 s before and during hypercapnia in 7 individual fetuses, as shown by different colours.
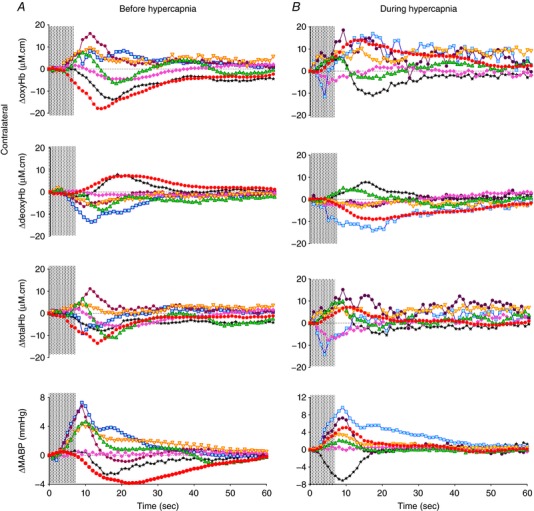
Shaded area in each graph shows the period of stimulation. [Color figure can be viewed at wileyonlinelibrary.com]
Figure 6. The peak changes in the oxyhaemoglobin (ΔoxyHb; A), deoxyhaemoglobin (ΔdeoxyHb; B) and total haemoglobin (Δtotal Hb; C) in the contralateral hemisphere for the positive response pattern, or the nadir changes for the negative response pattern, after the 7.8 s stimulus train before and during hypercapnia in 7 individual fetuses as shown by different colours.
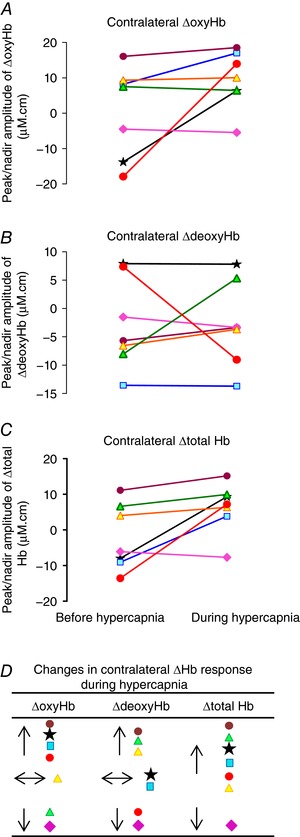
Comparing the changes before vs. during hypercapnia, P = 0.11 for ΔoxyHb, 0.99 for ΔdeoxyHb and 0.04 for Δtotal Hb. D, compared to the functional response before hypercapnia, 6 of the 7 lambs (denoted by the same coloured symbols as in A–C) showed increase in the Δtotal Hb response during hypercapnia, of which 4 lambs had increase in the ΔoxyHb response, 1 had no change and 1 had reduction in the ΔoxyHb response, with variable ΔdeoxyHb changes. One lamb showed reduction in the ΔoxyHb, ΔdeoxyHb and Δtotal Hb response during hypercapnia. [Color figure can be viewed at wileyonlinelibrary.com]
Discussion
These results demonstrate that highly variable changes in oxyHb, deoxyHb and total Hb occur in the contralateral cerebral cortex of newborn lambs in response to somatosensory stimulation. The concordant response patterns in Δtotal Hb and ΔoxyHb (with changes in deoxyHb in the opposite direction to that of the oxyHb) indicate increase in local cerebral perfusion is associated with increased oxygenation, and vice versa. With a short duration of stimulation (1.8 s), no cerebrovascular response could be evoked in 3/8 lambs, and in another two there was a negative response. This stands in contrasts with our recent findings in anaesthetized fetal sheep where the 1.8 s stimulation always produced positive responses in the contralateral cortex (Nakamura et al. 2017). It was always possible to record the SEP in all lambs from the contralateral cortex using the 1 Hz stimulation prior to stimulus trains of the three durations being delivered, so it is reasonable to assume that stimulation of the median nerve produced cortical activity with all three durations of stimulation. Longer bouts of stimulation elicited both positive and negative responses, similar to the heterogeneous patterns of functional cerebrovascular responses reported by others in neonatal human and animal studies (Meek et al. 1998; Muramoto et al. 2002; Kusaka et al. 2004; Erberich et al. 2006; Heep et al. 2009; Roche‐Labarbe et al. 2014).
With the 4.8 s stimulation, five of the eight lambs showed a negative response where both oxyHb and total Hb were decreased. These changes in CBF and oxygenation were clearly regional and localized, because superficial CBF as measured by LDF in the same contralateral somatosensory cortex predominantly increased. The negative response in oxyHb and total Hb was suggestive of decreased local perfusion rather than functional hyperaemia, possibly arising from arterial vasoconstriction as observed in some neonatal rodent studies (Kozberg et al. 2013; Zehendner et al. 2013) and in human neonates (Kusaka et al. 2004). In neonatal rodents, vasoconstriction in response to stimulation was present prior to the development of a hyperaemic response, which appeared at a later age (Kozberg et al. 2013). This vasoconstriction was proposed to be driven by innervation of the cerebral vessels (Girouard & Iadecola, 2006), and was protective in that limiting the hyperaemia would protect the immature and fragile vasculature in the developing brain from haemorrhage (Kozberg et al. 2013). Another rodent study also found that functional hyperaemia was not present during early postnatal brain development but developed gradually as cortical connectivity was established with increasing age (Kozberg et al. 2016). The lack of functional hyperaemia resulted in local tissue oxygen levels being depleted during increased activity, which would explain the negative haemodynamic response patterns as observed by us and others (Kozberg et al. 2013; Zehendner et al. 2013). The neonatal and adult sheep brain have similar cerebral fractional oxygen extraction (Jones et al. 1982). While the adult brain responds to increased neuronal activity and oxygen consumption with functional hyperaemia and increased oxygen delivery, the negative haemodynamic functional response in the neonatal brain suggests there is a potential for regional cerebral hypoxia to develop when cortical activation is prolonged.
With the 7.8 s stimulation, positive responses occurred in 5/8 lambs and the negative response occurred in the other three lambs. The positive response pattern appeared, at first glance, to be related to increase in blood presure after the stimulations.
Effect of blood pressure on cerebral oxyHb
It was notable that when positive cerebrovascular responses occurred in these newborn lambs after 4.8 and 7.8 s stimulation, they were associated with greater increases in MABP than when negative changes in oxyHb occurred. Altogether, there was a positive correlation between the changes in MABP and oxyHb in the contralateral hemisphere (Fig. 4). Moreover, the increases in MABP, oxyHb and total Hb were coincident in timing when a positive response occurred, suggesting a direct effect of systemic blood pressure on the cerebral haemodynamic response, consistent with impaired or immature cerebral autoregualation. In contrast, when the negative response occurred, the change in MABP was very small and the nadir in oxyHb occurred 6–7 s after the peak change of MABP. Similar findings were reported in a study of neonatal rats (Kozberg et al. 2013).
Interestingly, using the same experimental paradigm in fetal sheep (Nakamura et al. 2017), the correlation between cerebral ΔoxyHb response and the increase of MABP was not present in the fetus (Fig. 7). If the increase in cerebral oxyHb was driven by the increase in MABP due to poor and immature autoregulation, one would anticipate such effects to be more prominent in the fetal sheep, which was clearly not the case. In the fetus, the 7.8 s stimulation resulted in negative or biphasic ΔoxyHb responses (initial reduction followed by a rise in contralateral oxyHb) with a 6.1 ± 2.8% rise in MABP (see Nakamura et al. 2017), while a similar relative rise of MABP in the newborn lambs (7.6 ± 1.6%) was associated with positive ΔoxyHb responses. Even when a positive ΔoxyHb response was induced in fetus, the rise in MABP was of similar amplitude to that for negative/biphasic ΔoxyHb responses (Nakamura et al. 2017). Thus, we suggest firstly that the cortical processing of somatosensory stimulation that results in changes in oxyHb is different in the fetus and newborn, and secondly, that the increase in cerebral oxyHb in the newborn lambs was not solely driven by the increase in MABP.
Figure 7. Data from fetal sheep obtained using the same experimental paradigm (Nakamura et al. 2017), correlating the peak changes in oxyhaemoglobin (ΔoxyHb) recorded from the contralateral hemisphere for the positive response pattern, or the nadir ΔoxyHb for the negative response pattern, with the mean arterial pressure (ΔMABP) following a 3.3 Hz stimulus train of 1.8 (A), 4.8 (B), or 7.8 s (C) duration.
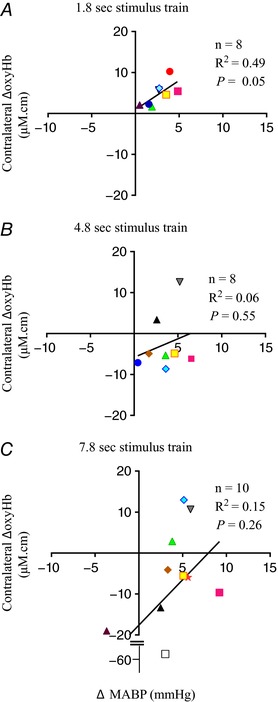
No significant correlation was found between ΔoxyHb and ΔMABP with all 3 durations of stimulation. [Color figure can be viewed at wileyonlinelibrary.com]
The increase in MABP in both the fetus and newborn is likely to be due to activation of the sympathetic nervous system from recruitment of segmental spinal reflexes, which is associated with involvement of the ascending nociceptive pathways (Sacco et al. 2013). A possible explanation for the difference between fetal and neonatal cerebral functional response is the immaturity and/or functional suppression of nocioceptive pathways in the fetus. In the human brain, while there is anatomical evidence of thalamo‐cortical projections from 24 weeks of gestation, functional nociceptive connections with cortical cells and circuits may not be formed until some time during the third trimester (Lee et al. 2005). In addition, the high levels of endogenous neuroinhibitors in the fetus, such as adenosine and pregnanolone, which are produced in the feto‐placental unit and contribute to fetal sleep states (Crossley et al. 1997; Nicol et al. 1997) may also suppress the sensory input to the central nervous system, reducing the nocioceptive response in the fetal brain.
Ipsilateral vs. contralateral changes
Although the median nerve stimulation was unilateral, a cerebrovascular response was also observed in the ipsilateral hemisphere. We suggest this is due to development of the astrocyte and neurovascular network that provides for activity in one area to influence blood flow over a larger area (Iadecola et al. 1997). In these newborn lambs, the peak ipsilateral and contralateral ΔoxyHb induced by peripheral nerve stimulation were very similar to each other (Tables 3 and 5). In contrast, the fetal sheep (Nakamura et al. 2017) showed progressively higher ipsilateral ΔoxyHb than contralateral ΔoxyHb with increasing duration of stimulations (Table 5). The difference between the ipsilateral and contralateral ΔoxyHb in the fetal brain could be due to oxygen supply in relative excess of metabolic need in the ipsilateral cortex, compared to greater oxygen consumption by the stimulated neurons in the contralateral cortex. The fetal ipsilateral ΔoxyHb may be determined largely by the vascular response and less by the increased oxygen consumption and neuronal activity that occurs in the contralateral cortex. As interhemispheric connectivity increases with age, neuronal activity to stimuli progresses from a tightly localized unilateral map to bilateral activities (Kozberg et al. 2016), which may explain the similar ipsilateral and contralateral ΔoxyHb observed in our newborn lambs. In fMRI studies using BOLD signals, brain maturation is associated with a decrease in the activated area (Erberich et al. 2006; Harris et al. 2011), presumably due to more spatial control of localized blood flow (Iadecola et al. 1997). The age‐related decrease in the BOLD activated area may also represent less excessive hyperaemia, as we have found in the newborn lamb.
Table 5.
Difference between the ipsilateral and contralateral ΔoxyHb response, following median nerve stimulation for 1.8, 4.8 or 7.8 s, in newborn lambs and fetal sheep (values are means ± SEM; individual values are shown where n < 3 available)
| Duration of stimulation | 1.8 sa | 4.8 s | 7.8 s | |||
|---|---|---|---|---|---|---|
| Newborn lamb | ||||||
| Contralateral ΔoxyHb response pattern | Negative (n = 2) | Positive (n = 3) | Negative (n = 5) | Positive (n = 3) | Negative (n = 3) | Positive (n = 5) |
| Ipsilateral − contralateral ΔoxyHb (μm cm) | 1.2 ± 1.1 (all patterns) | 1.9 ± 1.2 (all patterns) | 1.6 ± 1.3 (all patterns) | |||
| 1.1, 2.0 | 0.9 ± 1.3 | 2.4 ± 1.9 | 1.1 ± 0.6 | 1.4 ± 0.5 | 1.7 ± 2.2 | |
| Fetal sheep (Nakamura et al. 2017) | ||||||
| Contralateral ΔoxyHb response pattern | Positive (n = 8) | Negative + Biphasic (n = 6) | Positive (n = 2) | Negative + Biphasic (n = 7) | Positive (n = 3) | |
| Ipsilateral − contralateral ΔoxyHb (μm cm) | 1.6 ± 0.7 | 6.9 ± 2.6 (all patterns) | 15.1 ± 4.7 (all patterns)* | |||
| 8.2 ± 3.3 | 1.5, 4.5 | 22.6 ± 3.9† | 0.1 ± 2.3 | |||
aWith 1.8 s stimulation, no response was obtained in 3 out of 8 newborn lambs. * P < 0.05 vs. the 7.8 s group (all patterns) in newborn lambs. † P < 0.05 vs. Negative pattern of the 7.8 s stimulation in newborn lambs. ΔoxyHb, changes in oxyhaemoglobin.
Hypercapnia
The effect of hypercapnia on cerebral haemodynamic response has been investigated thoroughly in adult humans and animals, but to our knowledge this is first study to determine the specific effect of hypercapnia on the haemodynamic functional response to peripheral nerve stimulation in the newborn brain. Hypercapnia is common in newborn infants with respiratory illness, especially those born preterm. In the adult brain, fNIRS and fMRI studies show that the positive response to neuronal activation is generally diminished in hypercapnia, as shown in the rat (Jones et al. 2005), non‐human primate (Zappe et al. 2008) and human (Maggio et al. 2014). This reduced haemodynamic response may be due to decreased cerebral vasoreactivity (Cohen et al. 2002; Maggio et al. 2014) and/or altered evoked neuronal activity (Kennerley et al. 2012; Thesen et al. 2012) during hypercapnia. In contrast, in fetal sheep we reported a very different effect of hypercapnia, evoking increased Δtotal Hb and ΔoxyHb responses to neuronal stimulation (Nakamura et al. 2017). In the current study, we found that while 6/7 newborn lambs showed an increase in Δtotal Hb response indicating a higher CBF response to neuronal stimulation during hypercapnia, only 4/7 also showed a coincident increase in ΔoxyHb. The ΔdeoxyHb showed increase, decrease or no change in almost equal proportions during hypercapnia. This difference between fetuses and neonates may be due to recruitment of under‐perfused cerebral blood vessels in the fetal brain during hypercapnia. Thus, the changes observed here in newborn lambs may represent the transition from the fetal response (Nakamura et al. 2017) to the classic ‘adult’ pattern in which hypercapnia typically leads to reduced ΔoxyHb, increased ΔdeoxyHb and reduced BOLD signal following neuronal stimulation (Jones et al. 2005).
Limitations
It remains unclear how our findings would apply to the developmental changes in the human brain, as much of the methodology applied in this, and other animal models, is not applicable to infants and children. In addition, age comparisons between sheep and human are difficult to validate in terms of different aspects of brain development. Therefore, analysis of the developmental trajectory of CBF regulation from fetal to early postnatal life is probably more meaningful than absolute age comparisons. Also, further studies are clearly required to investigate the mechanisms underlying the age‐dependent changes in neurovascular responses at the cellular level. Neurovascular coupling is thought to involve multiple signalling pathways encompassing perivascular astrocytes, vasoactive chemical agents, and direct neuronal connections (Cauli & Hamel, 2010; McCaslin et al. 2011). Changes in astrocyte‐mediated processes may be of particular significance as animal studies have found marked increases in number, size and interconnectivity of these major glial cells in early life (Kaur et al. 1989; Harris et al. 2011). Further, the levels of neuronal excitation and inhibition in the brain undergo maturational changes in the mammalian central nervous system (Zehendner et al. 2013), and the contribution of excitatory and inhibitory networks to the functional coupling of local perfusion to local neural activity is likely to change.
Anaesthetics also have profound and age‐dependent influences on neural and vascular responses. Isoflurane is commonly used in electrophysiology studies due to the ease of use, even though it partially reduces neuronal excitation and cerebral metabolism, as do most volatile anaesthetics. CBF is increased by isoflurane at doses above 1.6% (Eger, 1984), and we therefore limited the inhalation of isoflurane to < 1.5% and continuously monitored the EEG to ensure absence of the burst suppresion pattern, which indicates the presence of deep anaesthesia.
The absolute level of electrical stimulation was not the same between each animal, but was always set to the minimum (2–5 mA) necessary to evoke a just‐visible muscle twitch of the limb. The stimulus amplitudes vary between animals due to differences in size of the intact nerve trunk, leakage current caused by tissue moisture, and the actual physical coupling of the cuff to the nerve. By adjusting the stimulation current according to a functional criterion, we compensated for slight differences in the coupling of stimulation cuff between animals. Similarly, in rodent studies using electrical stimulations of the forepaw/hindpaw, stimulus amplitudes were adjusted for each animal to the lowest amplitude that resulted in a forepaw/hindpaw twitch (Colonnese et al. 2008; Kozberg et al. 2016). To test the validity of this approach, fMRI showed that modulation of the stimulus amplitudes did not alter the time course or the amplitude of the BOLD response in these studies (Colonnese et al. 2008). In addition, we always used a level of stimulation that produced similar SEPs between animals and therefore was unlikely to contribute to the varability of the results.
Finally, a limitation in making comparison between this neonatal study and the previous fetal study (Nakamura et al. 2017) is that the experiments were done in animals of different post‐conceptional ages, as well as under ex utero vs. in utero conditions. Models of preterm lambs being delivered and maintained with mechanical ventilation have been established to study the impact of the fetal–neonatal transition (Polglase et al. 2012), and if this could be applied here it might allow a more relevant evaluation of the changes caused by removal of the placenta, and the change in peripheral tissue levels of oxygenation.
Conclusion
To our knowledge, this is the first study to investigate the cerebral haemodynamic functional response in the newborn lamb brain using somatosensory stimulation of different durations and during hypercapnia. The cerebral haemodynamic response in the newborn lamb showed both positive (increased total Hb and oxyHb) and negative response patterns. Positive correlations between changes in oxyHb and MABP were found in the newborn lamb but not in the fetal sheep despite similar MABP changes, which goes against the correlations being solely due to immature autoregulation in the developing brain. We have found that changes in CBF and cerebral oxygenation in response to neuronal stimulation are increased in the majority of lambs during hypercapnia, in contrast to findings in adult studies.
Additional information
Competing interests
No competing interests declared.
Author contributions
S.N.: collection, assembly, analysis and interpretation of data; financial support; drafting the article and revising it critically. D.W.: conception and design of the experiments; administrative support; provision of study materials or patients; collection and assembly of data; data analysis and interpretation; drafting the article and revising it critically. F.W.: conception and design of the experiments; financial support; administrative support; provision of study materials or patients; collection and assembly of data; data analysis and interpretation; drafting the article and revising it critically. The work was carried out in the laboratory at the Monash Medical Centre and the Ritchie Centre, Hudson Institute of Medical Research. All authors approved the final version of the manuscript and that all persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This research is supported by funding from the Victorian Government's Operational Infrastructure Support Program. S.N. is supported by the Kagawa University Faculty of Medicine School of Medicine Sanju Almini Association Sanjukai Overseas Fellowship (Japan) (#25‐2) and the Eli Lilly Fellowship of the Japan Foundation for Paediatric Research (#52), Japan Society for the Promotion Science (JSPS) Grant Fund for the Promotion of Joint International Research (16K19685, 15KK0311, 25860911) and Grant‐in‐Aid for Young Scientists (B). F.Y.W. is supported by the National Health and Medical Research Council (NHMRC, Australia)/Cerebral Palsy Alliance Career Development Fellowship (1084254). D.W.W. is supported by a Distinguished Researcher Fellowship from Cerebral Palsy Alliance‐Australia, and by The Ritchie Centre.
Acknowledgements
We would like to thank Mr Ichiyo Koyama (ADInstruments Japan Inc. Tokyo) for technical support of data recording, processing, and analysis, and also Mr Dalibor Stanojkovic and Dr Ilias Nitsos for technical assistance with the animal experiments.
References
- Abdel‐Rahman AM, Spitz M, Chang Y & Rosenberg AA (2000). Effects of combined superoxide dismutase and catalase on somatosensory evoked potentials and neuropathologic changes in asphyxiated newborn lambs. Biol Neonate 77, 115–122. [DOI] [PubMed] [Google Scholar]
- Arichi T, Fagiolo G, Varela M, Melendez‐Calderon A, Allievi A, Merchant N, Tusor N, Counsell SJ, Burdet E, Beckmann CF & Edwards AD (2012). Development of BOLD signal hemodynamic responses in the human brain. Neuroimage 63, 663–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartocci M, Winberg J, Papendieck G, Mustica T, Serra G & Lagercrantz H (2001). Cerebral hemodynamic response to unpleasant odors in the preterm newborn measured by near‐infrared spectroscopy. Pediatr Res 50, 324–330. [DOI] [PubMed] [Google Scholar]
- Bartocci M, Winberg J, Ruggiero C, Bergqvist LL, Serra G & Lagercrantz H (2000). Activation of olfactory cortex in newborn infants after odor stimulation: a functional near‐infrared spectroscopy study. Pediatr Res 48, 18–23. [DOI] [PubMed] [Google Scholar]
- Bortfeld H, Wruck E & Boas DA (2007). Assessing infants' cortical response to speech using near‐infrared spectroscopy. Neuroimage 34, 407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauli B & Hamel E (2010). Revisiting the role of neurons in neurovascular coupling. Frontiers in neuroenergetics 2, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen ER, Ugurbil K & Kim SG (2002). Effect of basal conditions on the magnitude and dynamics of the blood oxygenation level‐dependent fMRI response. J Cereb Blood Flow Metab 22, 1042–1053. [DOI] [PubMed] [Google Scholar]
- Colonnese MT, Phillips MA, Constantine‐Paton M, Kaila K & Jasanoff A (2008). Development of hemodynamic responses and functional connectivity in rat somatosensory cortex. Nat Neurosci 11, 72–79. [DOI] [PubMed] [Google Scholar]
- Cook CJ, Gluckman PD, Johnston BM & Williams C (1987). The development of the somatosensory evoked potential in the unanaesthetized fetal sheep. J Dev Physiol 9, 441–455. [PubMed] [Google Scholar]
- Crossley KJ, Nicol MB, Hirst JJ, Walker DW & Thorburn GD (1997). Suppression of arousal by progesterone in fetal sheep. Reprod Fertil Dev 9, 767–773. [DOI] [PubMed] [Google Scholar]
- Eger EI 2nd (1984). The pharmacology of isoflurane. Br J Anaesth 56 Suppl 1, 71S–99S. [PubMed] [Google Scholar]
- Erberich SG, Panigrahy A, Friedlich P, Seri I, Nelson MD & Gilles F (2006). Somatosensory lateralization in the newborn brain. Neuroimage 29, 155–161. [DOI] [PubMed] [Google Scholar]
- Fox PT & Raichle ME (1986). Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc Natl Acad Sci USA 83, 1140–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschini MA, Radhakrishnan H, Thakur K, Wu W, Ruvinskaya S, Carp S & Boas DA (2010). The effect of different anesthetics on neurovascular coupling. Neuroimage 51, 1367–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulford J, Vadeyar SH, Dodampahala SH, Ong S, Moore RJ, Baker PN, James DK & Gowland P (2004). Fetal brain activity and hemodynamic response to a vibroacoustic stimulus. Hum Brain Mapp 22, 116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girouard H & Iadecola C (2006). Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol (1985) 100, 328–335. [DOI] [PubMed] [Google Scholar]
- Gleason CA, Hamm C & Jones MD Jr (1990). Effect of acute hypoxemia on brain blood flow and oxygen metabolism in immature fetal sheep. Am J Physiol Heart Circ Physiol 258, H1064–H1069. [DOI] [PubMed] [Google Scholar]
- Gleason CA, Robinson R, Harris AP, Mayock DE & Traystman RJ (2002). Cerebrovascular effects of intravenous dopamine infusions in fetal sheep. J Appl Physiol 92, 717–724. [DOI] [PubMed] [Google Scholar]
- Harris JJ, Reynell C & Attwell D (2011). The physiology of developmental changes in BOLD functional imaging signals. Dev Cogn Neurosci 1, 199–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heep A, Scheef L, Jankowski J, Born M, Zimmermann N, Sival D, Bos A, Gieseke J, Bartmann P, Schild H & Boecker H (2009). Functional magnetic resonance imaging of the sensorimotor system in preterm infants. Pediatrics 123, 294–300. [DOI] [PubMed] [Google Scholar]
- Hykin J, Moore R, Duncan K, Clare S, Baker P, Johnson I, Bowtell R, Mansfield P & Gowland P (1999). Fetal brain activity demonstrated by functional magnetic resonance imaging. Lancet 354, 645–646. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Yang G, Ebner TJ & Chen G (1997). Local and propagated vascular responses evoked by focal synaptic activity in cerebellar cortex. J Neurophysiol 78, 651–659. [DOI] [PubMed] [Google Scholar]
- Jobsis FF (1977). Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science 198, 1264–1267. [DOI] [PubMed] [Google Scholar]
- Jones M, Berwick J, Hewson‐Stoate N, Gias C & Mayhew J (2005). The effect of hypercapnia on the neural and hemodynamic responses to somatosensory stimulation. Neuroimage 27, 609–623. [DOI] [PubMed] [Google Scholar]
- Jones MD Jr, Rosenberg AA, Simmons MA, Molteni RA, Koehler RC & Traystman RJ (1982). Oxygen delivery to the brain before and after birth. Science 216, 324–325. [DOI] [PubMed] [Google Scholar]
- Kaiser JR, Gauss CH, Pont MM & Williams DK (2006). Hypercapnia during the first 3 days of life is associated with severe intraventricular hemorrhage in very low birth weight infants. J Perinatol 26, 279–285. [DOI] [PubMed] [Google Scholar]
- Kaur C, Ling EA & Wong WC (1989). Development of the various glial cell types in the cerebral cortex of postnatal rats. Acta Anat (Basel) 136, 204–210. [DOI] [PubMed] [Google Scholar]
- Kennerley AJ, Harris S, Bruyns‐Haylett M, Boorman L, Zheng Y, Jones M & Berwick J (2012). Early and late stimulus‐evoked cortical hemodynamic responses provide insight into the neurogenic nature of neurovascular coupling. J Cereb Blood Flow Metab 32, 468–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozberg MG, Chen BR, Deleo SE, Bouchard MB & Hillman EM (2013). Resolving the transition from negative to positive blood oxygen level‐dependent responses in the developing brain. Proc Natl Acad Sci USA 110, 4380–4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozberg MG, Ma Y, Shaik MA, Kim SH & Hillman EM (2016). Rapid postnatal expansion of neural networks occurs in an environment of altered neurovascular and neurometabolic coupling. J Neurosci 36, 6704–6717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusaka T, Kawada K, Okubo K, Nagano K, Namba M, Okada H, Imai T, Isobe K & Itoh S (2004). Noninvasive optical imaging in the visual cortex in young infants. Hum Brain Mapp 22, 122–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Ralston HJ, Drey EA, Partridge JC & Rosen MA (2005). Fetal pain: a systematic multidisciplinary review of the evidence. JAMA 294, 947–954. [DOI] [PubMed] [Google Scholar]
- Maggio P, Salinet AS, Panerai RB & Robinson TG (2013). Does hypercapnia‐induced impairment of cerebral autoregulation affect neurovascular coupling? A functional TCD study. J Appl Physiol (1985) 115, 491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio P, Salinet AS, Robinson TG & Panerai RB (2014). Influence of CO2 on neurovascular coupling: interaction with dynamic cerebral autoregulation and cerebrovascular reactivity. Physiol Rep 2, e00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaslin AF, Chen BR, Radosevich AJ, Cauli B & Hillman EM (2011). In vivo 3D morphology of astrocyte‐vasculature interactions in the somatosensory cortex: implications for neurovascular coupling. J Cereb Blood Flow Metab 31, 795–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek JH, Firbank M, Elwell CE, Atkinson J, Braddick O & Wyatt JS (1998). Regional hemodynamic responses to visual stimulation in awake infants. Pediatr Res 43, 840–843. [DOI] [PubMed] [Google Scholar]
- Miller JD & Carlo WA (2007). Safety and effectiveness of permissive hypercapnia in the preterm infant. Curr Opin Pediatr 19, 142–144. [DOI] [PubMed] [Google Scholar]
- Minagawa‐Kawai Y, Mori K, Naoi N & Kojima S (2007). Neural attunement processes in infants during the acquisition of a language‐specific phonemic contrast. J Neurosci 27, 315–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramoto S, Yamada H, Sadato N, Kimura H, Konishi Y, Kimura K, Tanaka M, Kochiyama T, Yonekura Y & Ito H (2002). Age‐dependent change in metabolic response to photic stimulation of the primary visual cortex in infants: functional magnetic resonance imaging study. J Comput Assist Tomogr 26, 894–901. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Walker DW & Wong FY (2017). Cerebral haemodynamic response to somatosensory stimulation in near‐term fetal sheep. J Physiol 595, 1289–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicol MB, Hirst JJ & Walker DW (1998). Effect of pregnane steroids on electrocortical activity and somatosensory evoked potentials in fetal sheep. Neurosci Lett 253, 111–114. [DOI] [PubMed] [Google Scholar]
- Nicol MB, Hirst JJ, Walker D & Thorburn GD (1997). Effect of alteration of maternal plasma progesterone concentrations on fetal behavioural state during late gestation. J Endocrinol 152, 379–386. [DOI] [PubMed] [Google Scholar]
- Polglase GR, Miller SL, Barton SK, Baburamani AA, Wong FY, Aridas JD, Gill AW, Moss TJ, Tolcos M, Kluckow M & Hooper SB (2012). Initiation of resuscitation with high tidal volumes causes cerebral hemodynamic disturbance, brain inflammation and injury in preterm lambs. PLoS One 7, e39535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju TN (1992). Some animal models for the study of perinatal asphyxia. Biol Neonate 62, 202–214. [DOI] [PubMed] [Google Scholar]
- Richardson BS, Carmichael L, Homan J & Gagnon R (1989). Cerebral oxidative metabolism in lambs during perinatal period: relationship to electrocortical state. Am J Physiol Regul Integr Comp Physiol 257, R1251–R1257. [DOI] [PubMed] [Google Scholar]
- Richardson BS, Patrick JE & Abduljabbar H (1985). Cerebral oxidative metabolism in the fetal lamb: relationship to electrocortical state. Am J Obstet Gynecol 153, 426–431. [DOI] [PubMed] [Google Scholar]
- Roche‐Labarbe N, Fenoglio A, Radhakrishnan H, Kocienski‐Filip M, Carp SA, Dubb J, Boas DA, Grant PE & Franceschini MA (2014). Somatosensory evoked changes in cerebral oxygen consumption measured non‐invasively in premature neonates. Neuroimage 85, 279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco M, Meschi M, Regolisti G, Detrenis S, Bianchi L, Bertorelli M, Pioli S, Magnano A, Spagnoli F, Giuri PG, Fiaccadori E & Caiazza A (2013). The relationship between blood pressure and pain. J Clin Hypertens (Greenwich) 15, 600–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thesen T, Leontiev O, Song T, Dehghani N, Hagler DJ Jr, Huang M, Buxton R & Halgren E (2012). Depression of cortical activity in humans by mild hypercapnia. Hum Brain Mapp 33, 715–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf M & Greisen G (2009). Advances in near‐infrared spectroscopy to study the brain of the preterm and term neonate. Clin Perinatol 36, 807–834. [DOI] [PubMed] [Google Scholar]
- Zappe AC, Uludag K & Logothetis NK (2008). Direct measurement of oxygen extraction with fMRI using 6% CO2 inhalation. Magn Reson Imaging 26, 961–967. [DOI] [PubMed] [Google Scholar]
- Zehendner CM, Tsohataridis S, Luhmann HJ & Yang JW (2013). Developmental switch in neurovascular coupling in the immature rodent barrel cortex. PLoS One 8, e80749. [DOI] [PMC free article] [PubMed] [Google Scholar]


