Abstract
Background:
Bisphenol A (BPA) toxicity and exposure risk to humans has been the subject of considerable scientific debate; however, published occupational exposure data for BPA are limited.
Methods:
In 2013–2014, 77 workers at six US companies making BPA, BPA-based resins, or BPA-filled wax provided seven urine samples over two consecutive work days (151 worker-days, 525 samples). Participant information included industry, job, tasks, personal protective equipment used, hygiene behaviors, and canned food/beverage consumption. Total (free plus conjugated) BPA, quantified in urine by mass spectrometry, was detected in all samples.
Results:
The geometric mean (GM) creatinine-adjusted total BPA (total BPACR) concentration was 88.0 µg g−1 (range 0.78–18900 µg g−1), ~70 times higher than in US adults in 2013–2014 (1.27 µg g−1). GM total BPACR increased during Day 1 (26.6–127 µg g−1), decreased by pre-shift Day 2 (84.4 µg g−1) then increased during Day 2 to 178 µg g−1. By industry, baseline and post-baseline total BPACR was highest in BPA-filled wax manufacturing/reclaim (GM = 111 µg g−1) and lowest in phenolic resin manufacturing (GM = 6.56 µg g−1). By job, total BPACR was highest at baseline in maintenance workers (GM = 157 µg g−1) and post-baseline in those working with molten BPA-filled wax (GM = 441 µg g−1). Workers in the job of flaking a BPA-based resin had the lowest concentrations at baseline (GM = 4.81 µg g−1) and post-baseline (GM = 23.2 µg g−1). In multiple regression models, at baseline, industry significantly predicted increased total BPACR (P = 0.0248); post-baseline, handling BPA containers (P = 0.0035), taking ≥3 process/bulk samples with BPA (P = 0.0002) and wearing a Tyvek® coverall (P = 0.0042) significantly predicted increased total BPACR (after adjusting for total BPACR at baseline, time point, and body mass index).
Conclusion:
Several work-related factors, including industry, job, and certain tasks performed, were associated with increased urinary total BPACR concentrations in this group of manufacturing workers. The potential for BPA-related health effects among these workers is unknown.
Keywords: biological monitoring, bisphenol A, determinants of exposure, exposure assessment, occupational groups, reproductive health, urine analysis
Introduction
Bisphenol A (BPA) (CAS 80-05-7, 4,4′-isopropylidenediphenol) is used as a monomer in the production of polycarbonate, epoxy, and phenolic resins and as a reactant in making certain halogenated flame retardants (Kopf, 2003; Mack, 2004; Pham and Marks, 2004; Brunelle, 2014); residual BPA in these products is minimal. BPA is also used as a filler in certain investment casting waxes (Carney, 2014), where BPA can comprise up to 45% of the wax, and as a developer in thermal paper (USEPA, 2014); in both applications, BPA is unreacted. At room temperature, BPA is a white solid prill (dry sphere) or flake. BPA exposure is widespread in the USA; 92.6% of people ≥6 years of age had BPA detected in their urine (Calafat et al., 2008). Diet is thought to be the main non-occupational source of BPA exposure (NTP, 2008). BPA-coated thermal paper and certain dental materials are also possible BPA sources (Fleisch et al., 2010; Ehrlich et al., 2014).
After ingestion, BPA rapidly undergoes first-pass metabolism in the human liver to form water-soluble BPA glucuronide (BPA-G), BPA’s major metabolite, with an elimination half-life for total BPA in urine of 5.4–6.4 h (Völkel et al., 2002; Thayer et al., 2015). At oral doses between 50 and 100 µg kg−1 bw, BPA elimination in humans is essentially complete within 24 h (Völkel et al., 2002; Thayer et al., 2015). The metabolism and elimination of BPA after inhalation exposure has not been reported, and data are limited after dermal exposure. In vitro penetration and absorption of BPA into human or pig skin ranges from 9 to 13% (Kaddar et al., 2008; Mørck et al., 2010; Demierre et al., 2012), with one report of 46% in human skin explants (Zalko et al., 2011).
The toxicity of BPA in humans has been the subject of extensive research and considerable controversy. Although BPA has low acute toxicity in humans (European Union, 2008), it is weakly estrogenic (Dodds and Lawson, 1936), a finding confirmed in numerous in vitro and in vivo studies ((NTP, 2008). BPA-G, unlike free BPA, does not exhibit estrogenic activity (Snyder et al., 2000; Matthews et al., 2001). BPA exposure has been associated with health effects in animal and epidemiological studies with endocrine system disruption hypothesized to play a key role (as reviewed in WHO, 2011; Cantonwine et al., 2013; Rochester, 2013; Lakind et al., 2014; Peretz et al., 2014; Rezg et al., 2014).
Occupational exposure to BPA has been studied largely among manufacturing workers in Asia (Hanaoka et al., 2002; Xiao et al., 2005; Cha et al., 2008; He et al., 2009; Ren et al., 2012; Wang et al., 2012; Zhuang et al., 2015) and in cashiers (Ndaw et al., 2016; Thayer et al., 2016). Because of the scientific debate around BPA and the lack of published data on BPA exposure among US manufacturing workers, the National Institute for Occupational Safety and Health (NIOSH) conducted a study in 2013–2014 to quantify urinary BPA in US workers engaged in making BPA or products made with BPA. BPA air and hand wipe data will be reported separately.
Methods
Company and participant recruitment
Seventy-three companies potentially making or using BPA were identified from the 2010 (n = 66) and 2011 (n = 2) US Environmental Protection Agency (EPA) Toxic Release Inventory (USEPA, 2016a) and by referral (n = 5). Among the 73 companies, 15 did not respond; 15 no longer produced or used BPA; 37 either had few workers handling BPA, infrequent BPA use, or could not be scheduled within the study period; and 6 participated in the study. We visited participating companies to identify BPA-related jobs and invited workers performing these jobs to participate in the study. This study was approved by the NIOSH Institutional Review Board. Participants gave written informed consent and were reimbursed $70 for the time and inconvenience of providing samples.
Sample collection
Participants were sampled over two consecutive work days after having been scheduled to be off work for at least 24 h. On Day 1, participants collected pre-shift, mid-shift (±30 min of participant’s shift mid-point), end-shift, and post-shift (4–6 h after leaving work) urine samples. On Day 2, participants collected pre-shift, mid-shift, and end-shift samples for a total of seven samples (Time Points 1–7). To collect samples, participants were given an insulated bag containing a sterile, 120-ml polypropylene specimen cup (Samco™) in a ‘BPA-free’ Ziploc® or Glad® plastic bag, a large Kimwipe™ towel (Kimtech Science) pre-screened to be BPA-free, and frozen refrigerant packs for post-shift samples. Participants were instructed to wash hands with water only (to avoid potential interferences), dry hands with the provided towel, place the cup cap in the plastic bag to prevent contamination, collect the urine, write the void date and time on the cup label, and place the sample in the plastic bag.
For sample processing, study staff donned nitrile gloves (Kimberly-Clark), swirled the cup to mix the urine, measured specific gravity (SG) using a handheld refractometer (Atago® USA, Inc.) calibrated with distilled water, and aliquoted the urine into 5-ml Nalgene® polypropylene cryovials (ThermoFisher Scientific, Inc.) using sterile 5.8-ml Fisherbrand™ polyethylene transfer pipets (ThermoFisher Scientific, Inc.).
Quality control (QC) field blanks (FB) (two types) and blind duplicates of participant samples were collected. For the first FB (FB1), study staff took a kit to the bathroom used by participants, followed collection instructions and filled the specimen cup with 60 ml of Optima® LC/MS water (ThermoFisher Scientific, Inc.). For the second FB (FB2), the cup was filled with 60 ml of Optima® LC/MS water where samples were processed. FB and duplicate samples were aliquoted as described above. Cryovials were placed and shipped on dry ice, then stored at −80°C. The laboratory was blind to all participant information. The mean (±SD) duration from sample void time to freeze time was 2.5 ± 3.6 h (range 0.07–18 h), within the stability period for BPA-G in urine at room temperature (Waechter et al., 2007; Ye et al., 2007).
Sample analysis
We quantified urinary concentrations of free and total (free plus conjugated) BPA by online solid phase extraction-high performance liquid chromatography-isotope dilution tandem mass spectrometry (Zhou et al., 2014). Each analytical run included calibration standards, reagent blanks, and high and low concentration QC urine materials. The limit of detection (LOD) was 0.1 µg l−1. The mean (±SD) relative difference in the blind duplicates (n = 26) was 6.7 ± 4.7% (total BPA) and 5.9 ± 6.7% (free BPA). The mean (±SD) coefficient of variation (CV) for total BPA in QC materials (n = 83 each) was 6.4 ± 2.1% (QC low) and 3.7 ± 0.9% (QC high). For FB1 (n = 17), we detected total and free BPA in one (but different) blank each; for FB2 (n = 26), we detected total BPA in two blanks. Blank concentrations were at or near the LOD; therefore, we did not FB-correct BPA concentrations. Urine samples were analyzed within 1–5 months of collection, within BPA-G’s stability period when frozen (Ye et al., 2007).
We measured urinary creatinine using a Vitros® 250 Chemistry Analyzer (Ortho-Clinical Diagnostics). Each run included calibration standards and high and low concentration pooled urine QC. The lower reportable limit was 1.7 mg dl−1. The mean (±SD) relative difference for blind splits was 4.2 ± 7.0% (n = 26). The mean (±SD) CV across five runs was 2.7 ± 1.7% (QC low) and 3.2 ± 2.2% (QC high). To adjust for urine dilution, BPA concentrations were divided by creatinine (units = µg g−1 creatinine) or multiplied by (1.024 − 1)/(SG − 1) (units = µg l−1) (Elkins et al., 1974).
Other information collected
For each participant, we collected information on sex, age, race/ethnicity, self-reported weight and height, job, shift duration, current smoking (yes/no), hours since last worked, and jobs worked during days off. We asked participants about the number of canned beverages and canned foods consumed in the past 24 h, dental procedures in the past 3 days (Day 1) and past 24 h (Day 2), clothing and personal protective equipment worn during work, BPA-related tasks performed, hand washing frequency during work, BPA spills, hand-to-mouth behaviors during work, changing clothes at shift end, and showering/bathing between Day 1 and Day 2.
We grouped companies into five industry categories: (i) phenolic resin manufacturing (Companies 1 and 2), (ii) BPA and polycarbonate manufacturing (Company 3), (iii) BPA-filled wax manufacturing/reclaim (Company 4), (iv) BPA manufacturing (Company 5), and (v) BPA-filled wax manufacturing/investment casting (Company 6). We assigned each worker’s job into one of seven job categories: (i) making BPA, (ii) kettle/reaction/field operator making a BPA-based resin (hereafter referred to as ‘kettle operator’), (iii) operator flaking a BPA-based resin, (iv) maintenance work in a BPA or resin manufacturing area, (v) making BPA-filled wax, (vi) working with solid BPA-filled wax (e.g. wax injection, pattern and mold assembly), and (viii) working with molten BPA-filled wax (e.g. wax reclaim, melt/burnout of BPA-filled wax from shells/molds). While we attempted to create job categories having similar tasks, some task variation occurred within jobs, usually because we did not have sufficient sample size to create a separate job(s).
Statistical analysis
We detected total BPA in all urine samples; free BPA in 71% of them. We assigned LOD/2 to free-BPA concentrations <LOD (Hornung and Reed, 1990). We used the ratio of free to total BPA to indicate possible exogenous BPA contamination. We excluded one participant from analyses who had two samples with >20% free BPA, a percentage used previously to suggest BPA contamination (Guidry et al., 2015; Thayer et al., 2016).
BPA concentrations were skewed to the right; therefore, a natural log transformation was applied. We computed summary statistics for total and free BPA, and for the ratio of free to total BPA. We split the data into two groups, baseline (Time Point 1) and post-baseline (Time Points 2–7). For both groups, we compared geometric means (GM) of creatinine-adjusted total BPA (total BPACR) by industry and by job using the PROC MIXED procedure in SAS; for post-baseline samples, we used a first-order autoregressive covariance structure [AR(1)]. We used Tukey’s method to adjust P-values for multiple comparisons.
We conducted separate linear regression modeling for baseline and post-baseline samples using the natural log of total BPACR [ln(total BPACR)] as the dependent variable. For the baseline analysis, we initially examined one-at-a-time the effect of age, body mass index (BMI), total hours off work before collecting the first sample, number of canned beverages and canned foods consumed in the past 24 h (separately and combined), current smoking, job, and industry on ln(total BPACR). Covariates with a P-value ≤ 0.2 were evaluated in a multiple linear regression model.
For the post-baseline analysis, we evaluated 22 covariates as potential predictors of total BPACR. These covariates included job, industry, personal protective equipment and clothing worn (four covariates), hygiene behaviors (six covariates), work tasks (seven covariates), age, number of hours away from work before collecting the first sample, and current smoking. Where covariate responses could vary between Day 1 and Day 2, responses for Day 1 were assigned to Time Points 2–5 and responses for Day 2 to Time Points 6 and 7. We initially examined each covariate one-at-a-time after adjusting for time point, ln(total BPACR) at baseline, and BMI. Covariates with a P-value ≤ 0.2 were included in a stepwise forward selection regression model with worker treated as a random effect, covariates as fixed effects and an AR(1) covariance structure. Covariates were entered into the model until all remaining covariates had P-values > 0.05.
In separate linear regression models, we examined the effect of BMI on ln(total BPACR) at Time Points 2–7 (separately) after adjusting for age and ln(total BPACR) at baseline. To test for a difference in total BPACR between Day 1 and Day 2, we used each person’s averages of ln(total BPACR) at Time Points 2 and 3 and 6 and 7 in a mixed model with person as a random effect. These four time points were selected to obtain comparable data between the 2 days.
Baseline and post-baseline regression models were rerun with ln(creatinine) as an independent variable instead of correcting BPA concentrations for creatinine in the dependent variable. Statistical analyses were performed in SAS v. 9.3 (SAS Institute, Inc.). Significance testing was done at α = 0.05.
Results
Participants
Of 199 eligible workers at six companies (average 33 workers/company, range 25–55), 125 consented to participate (average consent rate of 63%, range 51–75%/company). We were able to schedule 78 (62%) of consenting workers for sampling; 77 remained after excluding one participant with possible BPA sample contamination. Two days of sampling were completed for 74 workers and 1 day for 3 workers for a total of 151 worker-days. All but one of the 77 workers were male, and 89.6% were white (Table 1). Median age was 44 years (range 20–63 years). Median BMI was 29.8 kg m−2 (range 21.0–44.3 kg m−2).
Table 1.
Characteristics of study participants, N = 77.
| Characteristic | Frequency (%) |
|---|---|
| Sex | |
| Male | 76 (98.7) |
| Female | 1 (1.3) |
| Age, years | AM ± SD = 43.5 ± 11.0; median = 44.0; range = 20–63 |
| Race | |
| White | 69 (89.6) |
| Black | 6 (7.8) |
| More than one race | 2 (2.6) |
| Ethnicity | |
| Hispanic | 2 (2.6) |
| Not Hispanic | 75 (97.4) |
| BMI, kg m−2 | AM ± SD = 30.4 ± 5.6; median = 29.8; range = 21.0–44.3 |
| Current smoker | |
| No | 52 (67.5) |
| Yes | 25 (32.5) |
| Shift type | |
| Fixed | 45 (58.4) |
| Rotating | 32 (41.6) |
| Company | |
| 1 (phenolic resin mfg) | 15 (19.5) |
| 2 (phenolic resin mfg) | 13 (16.9) |
| 3 (BPA and PC resin mfg) | 18 (23.4) |
| 4 (BPA-filled wax mfg and wax reclaim) | 14 (18.2) |
| 5 (BPA mfg) | 7 (9.1) |
| 6 (BPA-filled wax mfg, casting patterns/molds, wax burnout) | 10 (13.0) |
| Industry | |
| Phenolic resin mfg (Companies 1 and 2) | 28 (36.4) |
| BPA and PC resin mfg (Company 3) | 18 (23.4) |
| BPA-filled wax mfg and wax reclaim (Company 4) | 14 (18.2) |
| BPA mfg (Company 5) | 7 (9.1) |
| BPA-filled wax mfg, casting patterns/molds, wax melt/burnout (Company 6) | 10 (13.0) |
| Job | |
| Flaker operator—resins | 12 (15.6) |
| Make/load BPA | 12 (15.6) |
| Kettle operator—resin mfg (phenolic or PC) | 22 (28.6) |
| Maintenance—BPA and PC resin mfg | 7 (9.1) |
| Molten BPA-filled wax work—reclaim, melt/burnout | 6 (7.8) |
| Make BPA-filled wax | 14 (18.2) |
| Solid BPA-filled wax work: wax patterns, mold assembly, lab QC | 4 (5.2) |
| Total hours off work before collecting first urine sample | AM ± SD = 69.6 ± 44.7; median = 62.5; range = 11.5–252 |
| <24 | 12 (15.6) |
| 24 to <48 | 10 (13.0) |
| 48 to <72 | 24 (31.2) |
| 72 to <96 | 13 (16.9) |
| 96+ | 18 (23.4) |
| 24 h prior to first urine sample, number of canned: | |
| Beverages | AM ± SD = 1.2 ± 1.8; median = 0; range = 0–8 |
| Food | AM ± SD = 0.29 ± 0.58; median = 0; range = 0–3 |
| Beverages and food | |
| None | 31 (40.3) |
| 1–2 cans | 28 (36.4) |
| >2 cans | 18 (23.4) |
AM, arithmetic mean; mfg, manufacturing; PC, polycarbonate.
Companies
Companies 1 and 2 added BPA and other ingredients to kettles, solidified molten resin, and converted it into a flake product. Participant jobs were kettle and flaker operators. Company 3 made BPA from acetone and phenol, then reacted BPA with phosgene to make polycarbonate resin. Participant jobs included operators, shift leads, and maintenance. Company 4 added BPA and other ingredients to kettles, then solidified molten wax into pastilles, billets, or slabs. Company 4 also reclaimed the wax component from used wax using large hot boxes at ~100°C to melt the wax, followed by removal of water and non-wax solids. Participant jobs included warehouse, wax preparation, blending and packaging, wax reclaim, and QC. Company 5 made BPA from acetone and phenol and then transferred BPA to the epoxy resin manufacturing unit via a closed system. Participant jobs included BPA operators, flakers, and loaders. Company 6 added BPA and other ingredients to kettles, solidified molten wax into pastilles, and used the wax in investment casting. Participant jobs included making wax, engineer, shift lead, lift-truck driver, wax injection, wax pattern/mold assembly, and wax removal from shells/molds in heated Boilerclaves® at ~170°C followed by burnout in ovens at ~1000°C. All companies adding BPA to reaction or mixing kettles used a mix of manual/partly manual methods (e.g. emptying bags or bulk sacks into addition hoppers) and automated methods (e.g. transporting BPA through enclosed systems). Specific addition methods and BPA form (prill or flake) were generally proprietary.
Urinary BPA concentrations
The GM concentration of total BPACR in 525 samples over seven time points was 88.0 µg g−1, ~70 times higher than in adults ≥20 years in the 2013–2014 US National Health and Nutrition Examination Survey (NHANES) (GM = 1.27 µg g−1) (NHANES, 2016) (Table 2). At baseline, the GM total BPACR (26.6 µg g−1) was 20 times higher for adults in NHANES 2013–2014.
Table 2.
Creatinine-adjusted total- and free-BPA concentrations, and percent free BPA by collection time point.
| Day 1 | Day 2 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time point | All | Pre-shift 1 | Mid-shift 2 | End-shift 3 | Post-shift 4 | Pre-shift 5 | Mid-shift 6 | End-shift 7 | ||||||||
| Total BPA, µg g−1a | ||||||||||||||||
| n | 525 | 77 | 77 | 77 | 74 | 74 | 74 | 72 | ||||||||
| No. <LOD | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||
| AM (SD) | 481 (1380) | 115 (252) | 315 (839) | 608 (1710) | 601 (1330) | 413 (965) | 527 (1200) | 812 (2330) | ||||||||
| GM (GSD) | 88.0 (6.64) | 26.6b,c,d,e,f,g (5.74) | 60.7b,h,i,j (6.18) | 115c,h,k (6.31) | 127d,l (6.25) | 84.4e,k,l,m,n (6.53) | 125f,i,m,o (5.56) | 178g,j,n,o (6.20) | ||||||||
| Median | 98.7 | 20.7 | 57.1 | 132 | 135 | 98.2 | 112 | 167 | ||||||||
| 25th, 75th percentiles | 20.3, 273 | 6.56, 103 | 16.7, 204 | 33.4, 290 | 37.6, 335 | 20.5, 231 | 38.0, 392 | 63.3, 668 | ||||||||
| Range | 0.78–18900 | 0.78–1580 | 1.64–6300 | 1.87–13100 | 2.51–7780 | 1.15–5790 | 5.54–8610 | 3.02–18900 | ||||||||
| Free BPA, µg g−1 | ||||||||||||||||
| n | 525 | 77 | 77 | 77 | 74 | 74 | 74 | 72 | ||||||||
| No. <LOD (%) | 153 (29.1) | 30 (39.0) | 21 (27.3) | 20 (26.0) | 25 (33.8) | 21 (28.4) | 19 (25.7) | 17 (23.6) | ||||||||
| AM (SD) | 2.70 (7.08) | 0.72 (1.41) | 1.41 (2.53) | 3.85 (10.5) | 3.34 (8.19) | 2.78 (7.88) | 2.68 (5.98) | 4.24 (7.97) | ||||||||
| GM (GSD) | 0.43 (7.13) | 0.16 (5.72) | 0.36 (5.93) | 0.55 (7.62) | 0.50 (7.06) | 0.40 (7.09) | 0.57 (6.42) | 0.76 (8.18) | ||||||||
| Median | 0.44 | 0.096 | 0.46 | 0.49 | 0.42 | 0.52 | 0.45 | 0.72 | ||||||||
| 25th, 75th percentiles | 0.084, 1.62 | ND, 0.71 | ND, 1.16 | ND, 1.95 | ND, 1.58 | ND, 1.40 | ND, 2.70 | 0.13, 4.93 | ||||||||
| Range | ND–62.9 | ND–8.74 | ND–11.7 | ND–62.9 | ND–42.3 | ND–50.5 | ND–42.0 | ND–51.1 | ||||||||
| % Free BPAp | ||||||||||||||||
| n | 525 | 77 | 77 | 77 | 74 | 74 | 74 | 72 | ||||||||
| AM (SD) | 0.79 (1.02) | 0.83 (0.70) | 0.91 (1.32) | 0.92 (1.56) | 0.60 (0.64) | 0.76 (0.84) | 0.74 (0.86) | 0.73 (0.88) | ||||||||
| GM (GSD) | 0.49 (2.76) | 0.61 (2.26) | 0.59 (2.45) | 0.48 (3.10) | 0.39 (2.65) | 0.48 (2.87) | 0.46 (2.79) | 0.43 (3.11) | ||||||||
| Median | 0.51 | 0.65 | 0.62 | 0.46 | 0.45 | 0.52 | 0.46 | 0.48 | ||||||||
| 25th, 75th percentiles | 0.28, 0.90 | 0.38, 1.04 | 0.36, 1.00 | 0.30, 9.15 | 0.21, 0.76 | 0.31, 0.93 | 0.25, 0.81 | 0.25, 0.76 | ||||||||
| Range | 0.005–10.8 | 0.047–3.60 | 0.08–10.8 | 0.024–9.56 | 0.031–3.65 | 0.029–5.86 | 0.021–4.55 | 0.005–5.45 | ||||||||
LOD = 0.1 µg l−1; LOD/2 used for non-detects. AM, arithmetic mean; ND, not detected.
aDifferences in GMs between time points were evaluated in a mixed model with time point as a fixed effect and a first-order autoregressive covariance structure. N = 525. Values with the same letter are significantly different. Tukey’s method used to adjust P-values for multiple comparisons.
b,c,d,e,f,g,h,j,l,m,n P < 0.0001; iP = 0.0006; kP = 0.0244; oP = 0.0033.
p% Free BPA = (free-BPA concentration/total-BPA concentration) × 100.
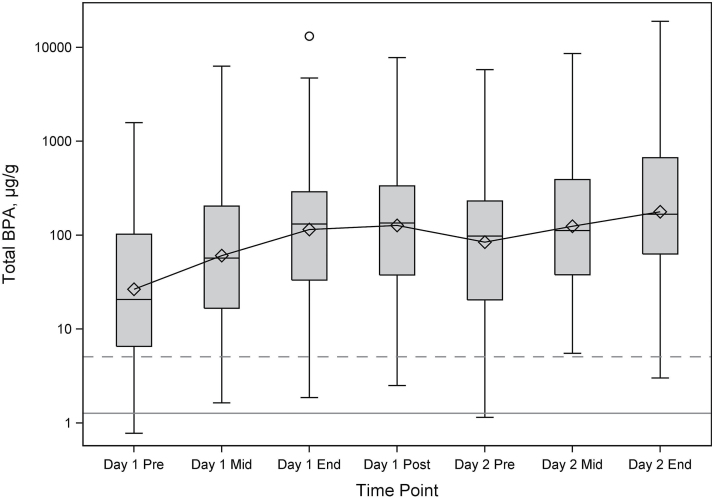
On average, total BPACR increased from pre-shift to post-shift on Day 1 (pre-shift GM = 26.6 µg g−1, mid-shift GM = 60.7 µg g−1, end-shift GM = 115 µg g−1, post-shift GM = 127 µg g−1), decreased between post-shift Day 1 and pre-shift Day 2 (GM = 84.4 µg g−1) without returning to Day 1 pre-shift levels, and then increased during Day 2 (mid-shift GM = 125 µg g−1, end-shift GM = 178 µg g−1) (Table 2, Fig. 1). Pre-shift Day 1 GM total BPACR was significantly lower than each of the other time points (P < 0.0001 each, Table 2). Differences in total BPACR between many other time points were also statistically significant (Table 2). Although total BPACR post-shift Day 1 (GM = 127 µg g−1) was higher than end-shift Day 1 (GM = 115 µg g−1), the difference was not statistically significant. The highest concentration measured (18900 µg g−1) was more than three orders of magnitude higher than the 95th percentile of adults from NHANES 2013–2014 (5.09 µg g−1) (NHANES, 2016). We averaged total BPACR for Time Points 2–5 for 74 participants with all four samples to obtain an approximate ‘24-h’ total BPACR concentration for Day 1 (GM = 96.6 µg g−1, range 3.05–7890 µg g−1).
Figure 1.
Plot of total BPA (µg g−1) by urine collection time point (Day 1 pre-shift, mid-shift, end-shift, and post-shift; Day 2 pre-shift, mid-shift, and end-shift). The box represents the interquartile range, and the diamond represents the GM. The solid horizontal line is the GM (1.27 µg g−1), and the dashed horizontal line is the 95th percentile (5.09 µg g−1) for total BPA from NHANES 2013–2014, adults 20 years and older. N = 525 samples on 151 worker-days (77 workers).
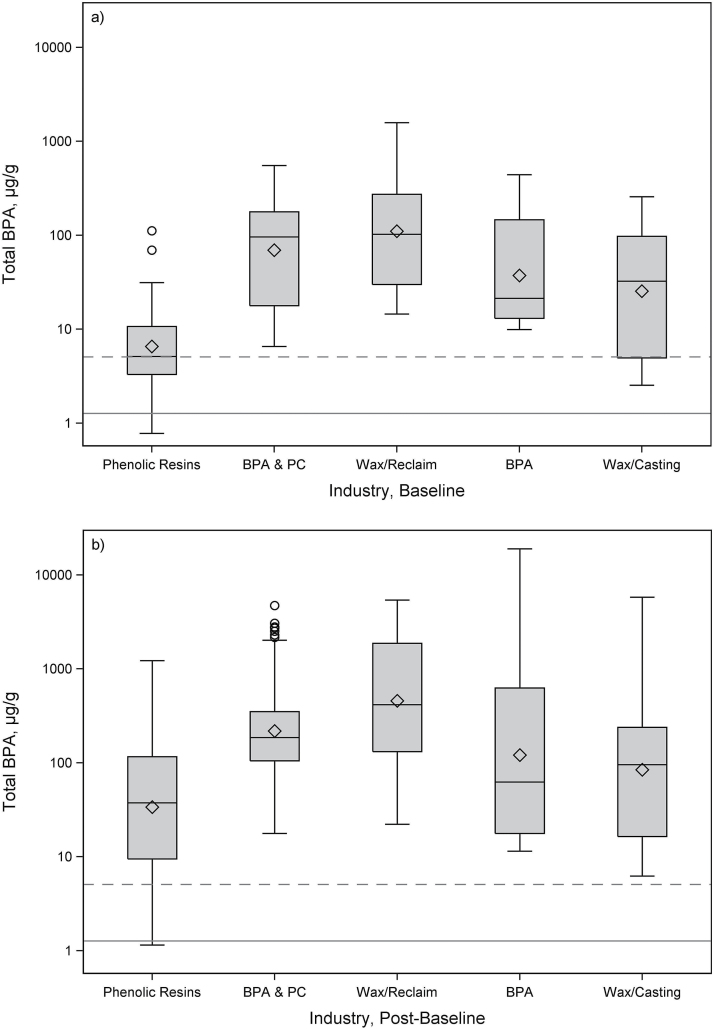
Total BPACR by industry and job are presented in Table 3; Figs 2 and 3; and Supplementary Figs S1 and S2, available at Annals of Work Exposures and Health online, and by time point within industry and job in Supplementary Table S1, available at Annals of Work Exposures and Health online. All industries and jobs had GM total BPACR concentrations significantly higher than adults in NHANES 2013–2014 (Supplementary Table S2, available at Annals of Work Exposures and Health online). By industry, at baseline, total BPACR was highest in BPA-filled wax manufacturing/reclaim (GM = 111 µg g−1), followed by BPA/polycarbonate manufacturing (GM = 69.4 µg g−1) and BPA manufacturing (GM = 37.4 µg g−1), and lowest in phenolic resin manufacturing (GM = 6.56 µg g−1). Compared to phenolic resin manufacturing, total BPACR at baseline was significantly higher in BPA-filled wax manufacturing/reclaim (P < 0.0001), BPA/polycarbonate manufacturing (P < 0.0001), and BPA manufacturing (P = 0.0257). Post-baseline, total BPACR was also highest in BPA-filled wax manufacturing/reclaim (GM = 121 µg g−1), followed by BPA/polycarbonate manufacturing (GM = 218 µg g−1) and BPA manufacturing (GM = 121 µg g−1), and lowest in phenolic resin manufacturing (GM = 33.8 µg g−1). Compared to phenolic resin manufacturing, total BPACR post-baseline was significantly higher in BPA-filled wax manufacturing/reclaim (P < 0.0001) and BPA/polycarbonate manufacturing (P = 0.0003).
Table 3.
Creatinine-adjusted total BPA by industry and job (a) for all urine samples, N = 525, (b) for baseline (Day 1 pre-shift) samples, n = 77, and (c) post-baseline (Day 1 mid-shift to Day 2 end-shift) samples, n = 448. The 525 samples represent 77 workers and 151 worker-days.
| Group | All samples (N = 525) | Baselinea | Post-baselineb | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time Point 1 | Time Points 2–7 | |||||||||||||||||
| n = 77 | n = 448 | |||||||||||||||||
| n | GM (GSD) | Range | n | GM (GSD) | Range | n | GM (GSD) | Range | ||||||||||
| µg g−1 | µg g−1 | µg g−1 | µg g−1 | µg g−1 | µg g−1 | |||||||||||||
| Industryc | ||||||||||||||||||
| Phenolic resin mfg | 186 | 26.4 (4.91) | 0.78–1230 | 28 | 6.56d,e,f (2.90) | 0.78–112 | 158 | 33.8n,o (4.68) | 1.15–1230 | |||||||||
| BPA and PC resin mfg | 122 | 184 (3.72) | 6.56–4720 | 18 | 69.4d (4.12) | 6.56–553 | 104 | 218n (3.41) | 17.8–4720 | |||||||||
| BPA-filled wax mfg/reclaim | 98 | 373 (4.70) | 14.5–5400 | 14 | 111e (4.66) | 14.5–1580 | 84 | 457o,p (4.31) | 22.2–5400 | |||||||||
| BPA mfg | 49 | 102 (8.71) | 9.94–18900 | 7 | 37.4f (4.01) | 9.94–441 | 42 | 121 (9.36) | 11.5–18900 | |||||||||
| BPA-filled wax mfg, casting patterns/molds, wax melt/burnout | 70 | 71.2 (5.44) | 2.54–5790 | 10 | 25.4 (5.14) | 2.54–257 | 60 | 84.5p (5.23) | 6.24–5790 | |||||||||
| Jobc | ||||||||||||||||||
| Flaker operator—resins | 80 | 18.3 (4.47) | 0.78–521 | 12 | 4.81g,k,l,m (2.07) | 0.78–11.0 | 68 | 23.2q,r,s (4.38) | 1.32–521 | |||||||||
| Make/load BPA | 80 | 156 (8.83) | 6.56–18900 | 12 | 49.1g (5.38) | 6.56–553 | 68 | 192q (9.04) | 11.5–18900 | |||||||||
| Kettle operator—resin mfg | 148 | 53.0 (5.19) | 1.15–2720 | 22 | 11.3h,i,j (3.42) | 1.73–112 | 126 | 69.5 (4.77) | 1.15–2720 | |||||||||
| Maintenance—BPA and PC resin mfg | 49 | 156 (1.61) | 57.1–453 | 7 | 157h,k (1.79) | 89.8–453 | 42 | 156 (1.59) | 57.1–348 | |||||||||
| Molten BPA-filled wax work: reclaim, melt/burnout | 42 | 354 (10.6) | 2.54–5400 | 6 | 94.9i,l (18.1) | 2.54–1580 | 36 | 441r (9.30) | 8.78–5400 | |||||||||
| Make BPA-filled wax | 98 | 208 (4.46) | 3.29–5790 | 14 | 63.0j,m (3.46) | 3.29–273 | 84 | 254s (4.25) | 6.24–5790 | |||||||||
| Solid BPA-filled wax work: wax patterns, mold assembly, lab QC | 28 | 49.4 (2.84) | 10.9–351 | 4 | 25.2 (1.94) | 11.3–55.4 | 24 | 55.3 (2.90) | 10.9–351 | |||||||||
mfg, manufacturing; PC, polycarbonate.
aDay 1 pre-shift sample.
bDay 1 mid-shift through Day 2 end-shift samples.
cDifferences between GMs between industries or between jobs were using the PROC MIXED procedure, with a first-order autoregressive covariance structure used for Time Points 2–7. Values with the same letter are significantly different. Tukey’s method was used to adjust P-values for multiple comparisons.
d,e,k,o P < 0.0001; fP = 0.0257; g,sP = 0.0019; hP = 0.0008; iP = 0.0219; jP = 0.0092; lP = 0.0010; mP = 0.0002; nP = 0.0003; pP = 0.0387; qP = 0.0130; rP = 0.0027.
Figure 2.
Box plots of total BPA (µg g−1) by industry for (a) baseline (n = 77) and (b) post-baseline (n = 448) samples. The box represents the interquartile range, and the diamond represents the GM. Solid horizontal line is the GM (1.27 µg g−1); dashed horizontal line is the 95th percentile (5.09 µg g−1) for total BPA from NHANES 2013–2014, adults 20 years and older. PC, polycarbonate.
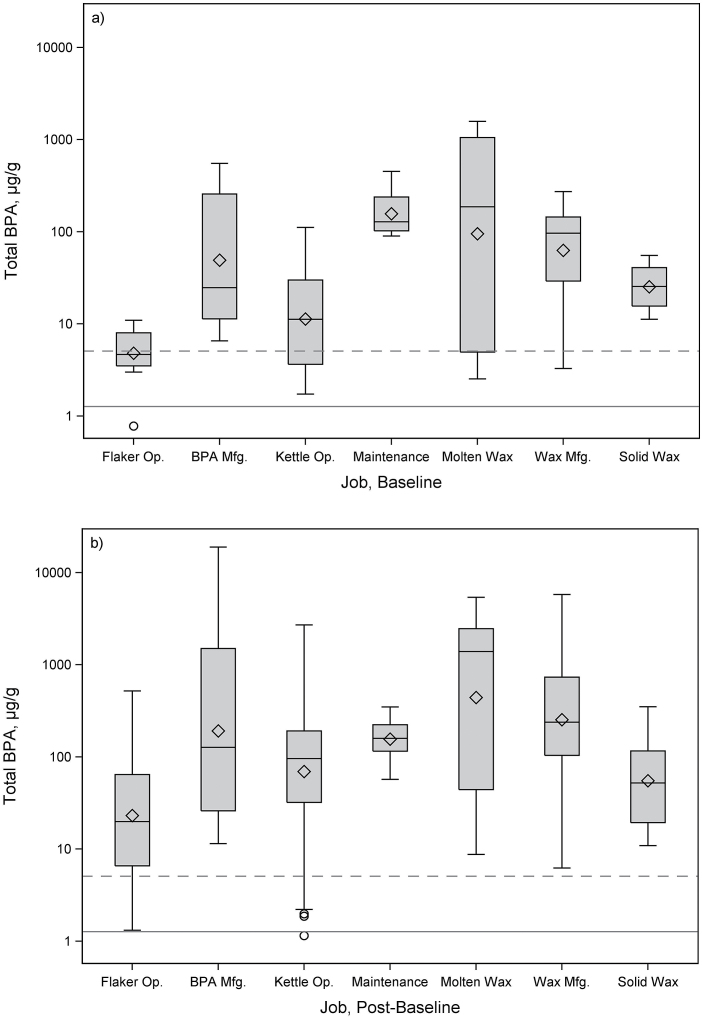
Figure 3.
Box plots of total BPA (µg g−1) by job for (a) baseline (n = 77) and (b) post-baseline (n = 448) samples. The box represents the interquartile range, and the diamond represents the GM. Solid horizontal line is the GM (1.27 µg g−1); dashed horizontal line is the 95th percentile (5.09 µg g−1) for total BPA from NHANES 2013–2014, adults 20 years and older. Mfg, manufacturing; Op., operator.
By job, total BPACR at baseline was highest for maintenance (all at Company 3, GM = 157 µg g−1), followed by working with molten BPA-filled wax (GM = 94.9 µg g−1), and lowest for flaking a BPA-based resin (GM = 4.81 µg g−1). Total BPACR at baseline in maintenance was 30 times higher than flaking a BPA-based resin (P < 0.0001). Total BPACR post-baseline was highest for the job of working with molten BPA-filled wax (GM = 441 µg g−1), followed by making BPA-filled wax (GM = 254 µg g−1), and making BPA (GM = 192 µg g−1), while lowest for flaking a BPA-based resin (GM = 23.2 µg g−1). Compared to the BPA-based resin flaking job, total BPACR post-baseline was significantly higher in those working with molten BPA-filled wax (P = 0.0027), in those making BPA-filled wax (P = 0.0019), and in those making BPA (P = 0.013). The maintenance job showed little difference in total BPACR concentrations at baseline (GM = 157 µg g−1) and post-baseline (GM = 156 µg g−1); all other jobs showed an increase, although not always significant. Total BPACR in the maintenance job also changed minimally across time points as compared to other jobs (Supplementary Table S1, available at Annals of Work Exposures and Health online).
For free BPACR, the GM was 0.43 µg g−1 (range <LOD–62.9 µg g−1, n = 525), with GMs for each of the seven time points <1 µg g−1 (Table 2). For percent free BPA, the GM was 0.49 (75th percentile = 0.9%) (Table 2). SG-adjusted and volume-based (unadjusted) total- and free-BPA concentrations are summarized in Supplementary Tables S3 and S4, available at Annals of Work Exposures and Health online.
Covariates
Covariate responses are summarized in Tables 1 and 4. Approximately 84% of the participants were off work ≥24 h before collecting the first sample; 72% for ≥48 h (range 11.5–252 h). Twelve participants (15.6%) were off work <24 h due to schedule changes, 7 of these worked in maintenance at Company 3. On 133 (88%) out of 151 days, participants wore either chemical-resistant gloves (66 days) or fabric/leather gloves (67 days). Respirator use was less common, 29 days (19.2%). Tyvek® coveralls were worn on 19 days (12.6%); 15 of these days at Company 1. Among hand-to-mouth activities queried (Table 4), eating during work (excluding lunch and breaks) was most frequent (91 days, 60.3%). Participants reported washing their hands during work one to four times on 43 days (28.4%), five to seven times on 68 days (45%), and more than seven times on 40 days (26.5%). None of the participants had dental work performed or worked at jobs associated with BPA exposure during their time off before collecting samples. All but three participants showered/bathed between Day 1 and Day 2.
Table 4.
Work-related covariates. N = 77 workers, 151 worker-days, 448 urine samples.
| Covariate | Category | i worker-days | n samples | |||
|---|---|---|---|---|---|---|
| PPE/clothing | ||||||
| Wore gloves | No | 18 | 54 | |||
| Fabric or leather | 67 | 202 | ||||
| Chemical resistant | 66 | 192 | ||||
| Wore a respirator | No | 122 | 358 | |||
| Yes | 29 | 90 | ||||
| Wore a Tyvek® coverall | No | 132 | 394 | |||
| Yes | 19 | 54 | ||||
| Type of shirt worn | Long sleeve | 106 | 317 | |||
| Short sleeve | 45 | 131 | ||||
| Hygiene | ||||||
| Smoked during work shift | No | 129 | 382 | |||
| Yes | 22 | 66 | ||||
| Ate food during work shift | No | 60 | 180 | |||
| Yes | 91 | 268 | ||||
| Chewed gum during work shift | No | 120 | 351 | |||
| Yes | 31 | 97 | ||||
| Chewed tobacco during work shift | No | 124 | 368 | |||
| Yes | 27 | 80 | ||||
| Number of times washed hands during work shift | 1–4 | 43 | 119 | |||
| 5–7 | 68 | 207 | ||||
| >7 | 40 | 122 | ||||
| Changed clothes/uniform before leaving work | No | 68 | 203 | |||
| Yes | 83 | 245 | ||||
| Work activities | ||||||
| Actual shift length worked, ha | ≤8 | 30 | 81 | |||
| >8 and <12 | 43 | 132 | ||||
| ≥12 | 78 | 235 | ||||
| Percent of shift in production areas (versus offices/control rooms)b | <50 | 45 | 130 | |||
| ≥50 | 105 | 318 | ||||
| Handled bulk sacks, bags, drums, or buckets of BPA | No | 133 | 394 | |||
| Yes | 18 | 54 | ||||
| Handled empty bulk sacks, bags, or drum liners of BPA | No | 136 | 402 | |||
| Yes | 15 | 46 | ||||
| Number of process/bulk samples taken containing BPAc | ≤3 | 140 | 418 | |||
| >3 | 11 | 30 | ||||
| Spilled BPA | No | 141 | 420 | |||
| Yes | 10 | 28 | ||||
| Cleaned up a spill of BPA | No | 139 | 412 | |||
| Yes | 12 | 36 | ||||
aTreated as ordinal in regression models.
bCompanies 1, 2, 3, and 5 had control rooms for monitoring operations.
cIncludes samples of BPA reaction mixtures from kettles, raw BPA, or BPA-based product.
We created several cross-company variables (Table 4). On 80% of the days, participants worked >8 h; on 70% of the days participants spent ≥50% of their shift in production areas versus control rooms/offices. On 18 days (12%), participants handled one or more containers of BPA (bulk sacks, bags, drums, or buckets). On 15 days (9.9%), participants handled one or more empty BPA containers. Taking process or bulk samples was dichotomized into less than three samples (140 days, 92.7%) or more than three samples (11 days, 7.3%). Because of small numbers, we could not examine process sample types separately. Participants spilled BPA on 10 days (6.6%) or cleaned up a BPA spill on 12 days (8.0%).
Exposure modeling
In univariate analyses where ln(total BPACR) at baseline was the dependent variable, job (P < 0.0001), industry (P < 0.0001), total hours off work before collecting the first sample (P = 0.0269), and current smoker (P = 0.0813) had P-values ≤ 0.2 (Table 5). When these covariates were included in a multiple regression model, only industry remained significant (P = 0.0248, data not shown).
Table 5.
Results of univariate linear regression models for creatinine-adjusted total BPA (µg g−1) at baseline (n = 77) and post-baseline (n = 448).
| Model | β (SE) | P-value |
|---|---|---|
| Baseline: Dependent variable: ln(total BPACR) at Time Point 1, n = 77a | ||
| Industry | <0.0001 | |
| Phenolic resin mfg | Ref. | |
| BPA and PC resin mfg | 2.359 (0.408) | <0.0001 |
| BPA-filled wax mfg/reclaim | 2.826 (0.442) | <0.0001 |
| BPA mfg | 1.740 (0.570) | 0.0032 |
| BPA-filled wax mfg, casting patterns/molds, wax melt/burnout | 1.352 (0.497) | 0.0082 |
| Job | <0.0001 | |
| Flaker operator—resins | Ref. | |
| Make/load BPA | 2.324 (0.565) | 0.0001 |
| Kettle Operator—resin mfg | 0.855 (0.497) | 0.0895 |
| Maintenance—BPA and PC resin mfg | 3.487 (0.658) | <0.0001 |
| Molten BPA-filled wax work: reclaim, melt/burnout | 2.983 (0.692) | <0.0001 |
| Make BPA-filled wax | 2.572 (0.544) | <0.0001 |
| Solid BPA-filled wax work: wax patterns, mold assembly, lab QC | 1.658 (0.799) | 0.0416 |
| Total hours off work before collecting first urine sample | −0.00986 (0.00437) | 0.0269 |
| Current smoker (yes/no = Ref.) | 0.741 (0.419) | 0.0813 |
| bBMI, kg m−2 | −0.0439 (0.0355) | 0.2207 |
| Number of canned foods consumed past 24 h | 0.304 (0.345) | 0.3818 |
| Age, years | −0.00466 (0.0184) | 0.8009 |
| Number of cans (food or beverage) consumed past 24 h | 0.8876 | |
| None | Ref. | |
| 1–2 | 0.197 (0.461) | 0.6698 |
| >2 | 0.206 (0.524) | 0.6957 |
| Number of canned beverages consumed past 24 h | 0.0119 (0.113) | 0.9165 |
| Post-baseline: Dependent variable: ln(total BPACR) at Time Points 2–7, n = 448 | ||
| Handled bulk sacks, bags, drums, or buckets of BPA (yes/no = Ref.) | 0.935 (0.180) | <0.0001 |
| Wore a Tyvek® coverall (yes/no = Ref.) | 1.044 (0.205) | <0.0001 |
| Wore a respirator (yes/no = Ref.) | 0.710 (0.158) | <0.0001 |
| Handled empty bulk sacks, bags or drum liners of BPA (yes/no = Ref.) | 0.781 (0.179) | <0.0001 |
| Number of process/bulk samples taken containing BPA (>3, ≤3 = Ref.) | 0.988 (0.233) | <0.0001 |
| Actual shift length worked (treated as ordinal), h | 0.285 (0.985) | 0.0040 |
| Glove worn | 0.0119 | |
| None | Ref. | |
| Leather or fabric | 0.0472 (0.258) | 0.8553 |
| Chemical resistant | 0.517 (0.259) | 0.0470 |
| Job | 0.0419 | |
| Flaker operator—resins | Ref. | |
| Make/load BPA | −0.133 (0.454) | 0.7706 |
| Kettle operator—resin mfg | 0.172 (0.368) | 0.6418 |
| Maintenance—BPA and PC resin mfg | −1.230 (0.548) | 0.0277 |
| Molten BPA-filled wax work: reclaim, melt/burnout | 0.273 (0.554) | 0.6171 |
| Make BPA-filled wax | 0.143 (0.435) | 0.7433 |
| Solid BPA-filled wax work; wax patterns, mold assembly, lab QC | −0.678 (0.573) | 0.2403 |
| Percent of shift in production areas versus offices/control rooms (≥50%, <50% = Ref.) | 0.360 (0.194) | 0.0644 |
| Total hours off work before collecting first urine sample | 0.00361 (0.00269) | 0.1838 |
| Age, years | −0.0132 (0.0104) | 0.2097 |
| Number of times washed hands during work shift | 0.2597 | |
| 1–4 | Ref. | |
| 5–7 | −0.102 (0.149) | 0.4952 |
| >7 | 0.151 (0.194) | 0.4359 |
| Smoked during work shift (yes/no = Ref.) | 0.263 (0.253) | 0.2987 |
| Current smoker (yes/no = Ref.) | 0.246 (0.249) | 0.3260 |
| Industry | 0.3962 | |
| Phenolic resin mfg | Ref. | |
| BPA and PC resin mfg | −0.0646 (0.376) | 0.8642 |
| BPA-filled wax mfg/reclaim | 0.586 (0.409) | 0.1560 |
| BPA mfg | 0.00451 (0.449) | 0.9920 |
| BPA-filled wax mfg, casting patterns/molds, wax melt/burnout | −0.153 (0.387) | 0.6937 |
| Spilled BPA (yes/no = Ref.) | 0.164 (0.244) | 0.5008 |
| Changed clothes/uniform before leaving work (yes/no = Ref.) | 0.128 (0.202) | 0.5259 |
| Chewed tobacco during work shift (yes/no = Ref.) | 0.0929 (0.298) | 0.7561 |
| Chewed gum during work shift (yes/no = Ref.) | 0.0608 (0.189) | 0.7477 |
| Type of shirt (short/long = Ref.) | 0.0432 (0.217) | 0.8426 |
| Cleaned up a BPA spill (yes/no = Ref.) | −0.0106 (0.239) | 0.9645 |
| Ate food during work shift (yes/no = Ref.) | −0.000265 (0.137) | 0.9985 |
BMI, body mass index; Mfg, manufacturing; PC, polycarbonate; QC, quality control; Ref., referent group.
aWhen covariates with a univariate P-value ≤ 0.2 (current smoker, hours off, job, and industry) were included in a model, only industry remained significant, P = 0.0248.
bWhen BMI adjusted for age, P = 0.2243.
We had an a priori interest in the relationship between BMI and total BPACR. After adjusting for age and ln(total BPACR) at baseline, we observed significant positive associations between BMI and ln(total BPACR) at Time Points 3 (P = 0.0448), 4 (P = 0.0173), 5 (P = 0.0045), and 7 (P = 0.0075), and borderline significance at Time Point 6 (P = 0.0610) (Supplementary Table S5, available at Annals of Work Exposures and Health online). Therefore, we adjusted for BMI in post-baseline models.
When ln(total BPACR) post-baseline was the dependent variable, 10 covariates with P-values ≤ 0.2 in univariate analyses (Table 5) were included in a stepwise forward selection model (Table 6). After adjusting for ln(total BPACR) at baseline, time point and BMI, total BPACR was positively associated with handling containers of BPA (1.8 times, P = 0.0035), taking more than three process/bulk samples (2.33 times, P = 0.0002) and wearing a Tyvek® coverall (1.93-times, P = 0.0042). Stepwise results for SG-adjusted total BPA were generally similar (Supplementary Table S6, available at Annals of Work Exposures and Health online).
Table 6.
Results of stepwise forward selection regression model for creatinine-adjusted total BPA (µg g−1) at Time Points 2–7. n = 448 samples (151 worker-days, 77 workers).
| Dependent variable: ln(total BPACR)a | Β (SE) | P-value | Factorb | |||
|---|---|---|---|---|---|---|
| Intercept | −0.718 (0.681) | 0.2952 | ||||
| ln(total BPACR) at Time Point 1 (baseline) | 0.852 (0.0634) | <0.0001 | 2.34c | |||
| Time point | <0.0001 | |||||
| 2 (mid-shift Day 1) | Ref. | |||||
| 3 (end-shift Day 1) | 0.637 (0.0707) | <0.0001 | 1.89d | |||
| 4 (post-shift Day 1) | 0.664 (0.0972) | <0.0001 | 1.94 | |||
| 5 (pre-shift Day 2) | 0.256 (0.115) | 0.0262 | 1.29 | |||
| 6 (mid-shift Day 2) | 0.598 (0.128) | <0.0001 | 1.82 | |||
| 7 (end-shift Day 2) | 0.917 (0.139) | <0.0001 | 2.50 | |||
| BMI, kg m−2 | 0.0603 (0.0198) | 0.0032 | 1.06 | |||
| Handled bulk sacks, bags, drums, or buckets of BPA | ||||||
| No | Ref. | |||||
| Yes | 0.590 (0.201) | 0.0035 | 1.80e | |||
| Number of process/bulk samples taken containing BPA | ||||||
| ≤3 | Ref. | |||||
| >3 | 0.847 (0.226) | 0.0002 | 2.33 | |||
| Wore a Tyvek® coverall | ||||||
| No | Ref. | |||||
| Yes | 0.659 (0.229) | 0.0042 | 1.93 | |||
Ref., referent group.
aMixed model with a first-order autoregressive covariance structure. Results adjusted for ln(total BPACR) at Time Point 1 (baseline), time point, and BMI. Remaining covariates presented in order of entry into the model. Estimated lag-one autocorrelation coefficient (rho) = 0.8499.
beβ.
cA 2.34 times increase in total BPACR when total BPACR at Time Point 1 (baseline) increases by a factor of e.
dTotal BPACR is increased 1.89 times at Time Point 3 (end-shift Day 1) as compared to Time Point 2 (mid-shift Day 1). Time Points 4, 5, 6, and 7 are also compared to Time Point 2.
eParticipants who reported ‘Yes’ had a 1.8 times increase in total BPACR as compared to those who reported ‘No’.
We found a significant 64% increase in total BPACR from Day 1 to Day 2 when comparing the average of Time Points 6 and 7 (GM = 152 µg g−1) to the average of Time Points 2 and 3 (GM = 92.5 µg g−1) (P < 0.0001, n = 72). We did not find a significant interaction between day and industry (P = 0.28) or between day and job (P = 0.60), although total BPACR increased from Day 1 to Day 2 in all industries (1.3–2.0 times) and jobs (1.2–1.9 times) except maintenance (1.0 times) (data not shown).
Regression models that included ln(creatinine) as a covariate gave similar results, including coefficients, standard errors, and P-values (Supplementary Tables S7–S9, available at Annals of Work Exposures and Health online).
BPA intake estimation
Absent human data on BPA elimination following inhalation or dermal exposures, we applied a simplified approach to estimate a participant’s 24-h BPA intake on Day 1 [equation (1)]. Specifically, we used the average total BPACR concentrations at Time Points 2–5, the average creatinine concentration at the four times, imputed values for 24-h urine volume representing the mean (1.2 l) and range (0.6–2.0 l) for adults (Wallach, 2007), and self-reported body weight. The GM (range) estimated BPA intake for Day 1 was 0.88 µg kg−1 day (0.035–73.9 µg kg−1 day, n = 74) for a urine volume of 0.6 l, 1.77 µg kg−1 day (0.069–148 µg kg−1 day) for 1.2 l, and 2.95 µg kg−1 day (0.12–246 µg kg−1 day) for 2.0 l (Supplementary Table S10, available at Annals of Work Exposures and Health online). Based on these estimates, 1.4% of the participants at a urine volume of 0.6 l, 2.7% at 1.2 l, and 8.1% at 2.0 l exceeded the US EPA oral Reference Dose for BPA of 50 µg kg−1 day (USEPA, 2016b). Exceedance fractions were higher when comparing estimates to the European Food Safety Authority temporary Tolerable Daily Intake of 4 µg kg−1 day (EFSA, 2015), 20.3% at a urine volume of 0.6 l; 28.4% at 1.2 l, and 41.6% at 2.0 l. Single-day estimates may not represent a worker’s lifetime exposure. Also, different approaches were used by EFSA (forward modeling of external exposure), EPA [lowest observable adverse effect level], and our study (backward modeling from biomonitoring data) to derive reference levels and intake estimates.
| (1) |
Discussion
This study is the first broad investigation of BPA exposure among US manufacturing workers. GM total BPACR concentrations across seven collection time points were 20–140 times higher than NHANES 2013–2014 for adults in the USA (NHANES, 2016) suggesting that BPA exposure among participants was mostly occupational. The generally increasing trend in total BPACR concentrations across 2 days was consistent with workplace exposure. Tasks involving BPA such as handling BPA containers or taking process/bulk samples containing BPA were positively associated with total BPACR post-baseline. We did not find evidence that canned food or canned beverage consumption contributed significantly to total BPACR in these workers. Any dietary contribution was likely overshadowed by the occupational contribution.
Post-baseline, the highest exposed job was working with molten BPA-filled wax. BPA exposure routes for these workers are unclear. While hot boxes used to melt wax for reclamation and Boilerclaves® used to melt wax out of shells/molds were closed during operation, workers opened hot box and Boilerclave® doors at cycle completion. Reclaim workers may have had dermal contact with used wax or with solid non-wax residues; however, for Boilerclave® workers, wax patterns were encased in a ceramic shell and dermal contact with the wax seemed unlikely. At room temperature, BPA has a low vapor pressure (3.96 × 10−7 to 8.7 × 10−10 mm Hg) and a high boiling point (398°C at 760 mm Hg) (Staples et al., 1998). Therefore, vapor phase exposure to BPA would not be expected at normal temperature and pressure (20°C, 100 kPa). We are not aware of experimental data on the potential for vapor formation at temperatures ≥ 100°C. The job that appeared to have the most dermal contact with BPA-filled wax, making wax patterns and mold assemblies, had the second lowest post-baseline total BPACR concentration, although only four workers performed this job. Chemical-resistant glove use did not appear to explain higher BPA concentrations in molten wax workers as compared to solid wax workers; chemical-resistant gloves were worn more frequently in the molten wax job (nitrile gloves on 6 out of 12 days), than in the solid wax job (rubber gloves on 1 out of 8 days). Respirators were not worn on any days for either job. The job of flaking a BPA-based resin had the lowest post-baseline total BPACR concentration, consistent with BPA having been largely consumed in making the resin.
Reasons for high baseline total BPACR concentrations, even with >70% of the participants off work ≥48 h before collecting their first sample are unclear. Possibilities include insufficient elimination time for some workers, a longer-than-expected elimination half-life, unaccounted for BPA exposure away from work, or BPA storage in the body. In univariate analyses, we saw a significant inverse relationship between total BPACR at baseline and total hours off work before the baseline sample (Table 5 and Supplementary Fig. S3, available at Annals of Work Exposures and Health online), although the effect did not persist in the multiple regression model. While no workers reported having a job during their time off likely involving BPA exposure, we cannot rule out off-job exposure from take-home BPA residues on clothes, in vehicles or in homes.
Workers in our study were most likely exposed repeatedly to BPA via inhalation or dermal contact over months or years whereas elimination half-life estimates in humans are based on single oral doses. Teeguarden et al. (2015) found no evidence of a BPA depot in humans after ingesting a single dose of BPA; however, in an analysis of >1400 NHANES 2003–2004 participants, urinary BPA concentrations did not decline rapidly with fasting time and the estimated ‘population’ half-life was 43 h (Stahlhut et al., 2009). Our results, Stahlhut et al. (2009), and those of another fasting study (Christensen et al., 2012) raise the question of whether unaccounted for sources of BPA or accumulation and slow release of BPA from tissues, including after dermal exposure, could influence urinary BPA concentrations. BPA is moderately lipophilic (Staples et al., 1998). In in vitro studies, human adipose tissue had the highest BPA concentration (Csanády et al., 2002; Geens et al., 2012). Thus, BPA storage in fat under certain conditions may be biologically plausible.
The positive association of wearing a Tyvek® coverall with total BPACR was unexpected for an item intended to prevent dermal exposure. Wearing Tyvek® may represent some unmeasured aspect of handling BPA, e.g. on 12 out 19 days that Tyvek® was worn participants handled sacks, bags, or buckets of BPA or worked closely with someone who performed these tasks. Or perhaps when removing Tyvek®, workers were exposed to resuspended BPA in the air or had skin contact with BPA residues on the garment.
The He et al. (2009) study of workers in BPA and epoxy resin manufacturing plants in China is most comparable to our study. Pre-shift total BPACR concentrations in the Chinese workers (median = 84.6 µg g−1) and in our US workers (Day 1 pre-shift GM = 26.6 µg g−1) were higher than NHANES 2013–2014 participants (GM = 1.27 µg g−1) indicating that total BPACR had not dropped to background levels before re-exposure at work. He et al. (2009) did not report the timing of sample collection in relation to time off work. Our Day 2 pre-shift concentrations (GM = 84.4 µg g−1) were similar to He et al. (2009) at pre-shift. Compared to end-shift concentrations in the Chinese workers (median = 111 µg g−1), our Day 1 end-shift concentrations were similar (GM = 115 µg g−1), but our Day 2 concentrations (GM = 178 µg g−1) were 60% higher. In a cross-sectional study of these BPA-exposed male Chinese workers, changes in self-reported sexual dysfunction, reproductive hormone levels, and semen quality were reported (Li et al., 2010a, 2010b, 2011; Zhou et al., 2013; Liu et al., 2015). GM total BPACR concentrations for workers in our study were comparable to (pre-shift and end-shift Day 1) or higher than (end-shift Day 2) concentrations reported for the Chinese workers. Endpoints in the Chinese study have not been evaluated in another similarly-exposed occupational group.
A few other studies have been conducted among BPA-exposed workers. Cashiers handling BPA-coated thermal paper receipts in the USA had pre-shift total BPACR concentrations 14 times lower than in our study and end-shift concentrations 42 times (Day 1) and 64 times (Day 2) lower than we found (Thayer et al., 2016), while cashiers in France (Ndaw et al., 2016) had overall total BPACR concentrations more than 10 times lower than in our study. Two small studies of Chinese workers making and packaging BPA-based epoxy resins (Ren et al., 2012; Wang et al., 2012) generally reported total BPACR concentrations several times lower than we found. Total BPACR concentrations in epoxy resin painters were also much lower than in our study, consistent with little unreacted BPA remaining in paint (Hanaoka et al., 2002; Cha et al., 2008).
Occupational exposure limits have not been established for BPA in urine. Germany has a biological guidance value (BGV) of 80000 µg l−1 for urinary total BPA based on the German maximum workplace BPA air concentration of 5 mg m−3, inhalable fraction (DFG, 2013). Our median concentration (108 µg l−1) was 0.14% of this guidance value, 25th percentile (25.4 µg l−1) 0.03%, 75th percentile (379 µg l−1) 0.47%, and maximum (32900 µg l−1) 41% (Supplementary Table S3, available at Annals of Work Exposures and Health online). The Scientific Committee on Occupational Exposure Limits of the European Commission has recommended a BGV intended to identify occupational from non-occupational exposure of 7 µg l−1 for total urinary BPA based on the 95th percentile for total BPA in German adults 20–29 years of age (EC, 2014). In our study, total BPA exceeded 7 µg l−1 in 92% of 525 samples (range 1.1–32900 µg l−1).
Study strengths include a variety of BPA-related jobs and industries, multiple samples per worker to capture BPA elimination over time, worker-specific information on possible exposure determinants, and information on number of hours off work and canned food/beverage consumption prior to baseline sampling. The overall low free-BPA percentage indicated that urine biomonitoring for BPA can be conducted reliably in workplaces handling raw BPA.
Some limitations should also be noted. Although study companies included major producers and users of BPA, the companies may not represent all BPA producers/users. Worker participation was voluntary, so we may not have captured the full distribution of BPA exposures for jobs at each company. Sample size may have limited our ability to identify some exposure determinants and the exposure determinants we identified reflected tasks and conditions on the days we sampled. Because biomonitoring captures exposure by all routes, we could not determine the relative importance of each route from these data alone. Finally, our BPA 24-h intake estimates were compared to reference intakes based on elimination assumptions following oral exposure, assumptions that may not apply to inhalation or dermal exposure.
Conclusion
US workers manufacturing BPA or making products with BPA had urinary total BPACR concentrations averaging ~70 times higher than US adults in NHANES 2013–2014. Total BPACR concentrations in the US manufacturing workers were also 10–60 times higher than in cashiers handling BPA-coated thermal paper. Determinants of increased BPA exposure included total BPACR concentration at baseline, collection time point, BMI, handling containers of raw BPA, and taking more than three process samples containing BPA. Total BPACR concentrations were especially elevated among workers in jobs/industries handling molten BPA-filled wax, a group not previously studied. Because reproductive health effects were reported in a cross-sectional study of manufacturing workers in China who had, on average, to urinary total BPACR concentrations similar to or above concentrations in our study, additional investigation among US workers is warranted.
Supplementary Data
Supplementary data are available here.
Funding
This study was supported in part by an interagency agreement between the National Institute for Occupational Safety and Health and the National Institute of Environmental Health Sciences (AES12009) as a collaborative National Toxicology Program research activity. The authors declare they have no conflict of interest.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention. Mention of any company or product does not constitute endorsement by the Centers for Disease Control and Prevention.
Supplementary Material
Acknowledgements
We gratefully acknowledge the contributions of Donna Olsen to recruitment efforts; Donald Fleming, Kevin L. Dunn, Belinda Johnson, Deborah Sammons, Kenneth Sparks, Donald Booher, Kevin Moore, and Jonathan Slone to field support and data preparation; James Kesner, Xiaoliu Zhou, Josh Kramer, Prabha Dwivedi, and Tao Jia to laboratory support; and Cheryl Estill and Steven Schrader to protocol development. We especially appreciate the contributions of participating workers, companies and unions.
References
- Brunelle DJ. (2014) Polycarbonates. Kirk-Othmer encyclodpedia of chemical technology. John Wiley & Sons; pp. 1–30. Available at http://onlinelibrary.wiley.com/doi/10.1002/0471238961.1615122502182114.a01.pub3/abstract. Accessed 14 December 2016. [Google Scholar]
- Calafat AM, Ye X, Wong LY, et al. (2008) Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003-2004. Environ Health Perspect; 116: 39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantonwine DE, Hauser R, Meeker JD. (2013) Bisphenol A and human reproductive health. Expert Rev Obstet Gynecol; 8: 329–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney C. (2014) Advanced casting methodologies: investment casting, centrifugal casting, squeeze casting, metal spinning, and batch casting. In Hashmi S, editor. Comprehensive materials processing. Vol. 5, Chapter 5.03. Amsterdam, The Netherlands: Elsevier; pp. 39–47. [Google Scholar]
- Cha BS, Koh SB, Park JH, et al. (2008) Influence of occupational exposure to bisphenol A on the sex hormones of male epoxy resin painters. Mol Cell Toxicol; 4: 203–34. [Google Scholar]
- Christensen KL, Lorber M, Koslitz S, et al. (2012) The contribution of diet to total bisphenol A body burden in humans: results of a 48 hour fasting study. Environ Int; 50: 7–14. [DOI] [PubMed] [Google Scholar]
- Csanády GA, Oberste-Frielinghaus HR, Semder B, et al. (2002) Distribution and unspecific protein binding of the xenoestrogens bisphenol A and daidzein. Arch Toxicol; 76: 299–305. [DOI] [PubMed] [Google Scholar]
- Demierre AL, Peter R, Oberli A, et al. (2012) Dermal penetration of bisphenol A in human skin contributes marginally to total exposure. Toxicol Lett; 213: 305–8. [DOI] [PubMed] [Google Scholar]
- DFG, Deutsche Forschungsgemeinshaft (2013) MAK- und BAT-Werte-Liste 2013 Senatskommission zur Prüfung Gesundheitsschädlicher Arbeitsstoffe. Weinheim, Germany: Wiley-VCH Verlag GmbH&Co; Available at http://onlinelibrary.wiley.com/doi/10.1002/9783527675135.oth1/pdf Accessed 14 December 2016. [Google Scholar]
- Dodds EC, Lawson W. (1936) Synthetic oestrogenic agents without the phenanthrene nucleus. Nature; 137: 996. [Google Scholar]
- EC, European Commission (2014) Recommendation from the Scientific Committee on Occupational Exposure Limits for Bisphenol-A. SCOEL/SUM/113. Brussels, Belgium: European Commission. [Google Scholar]
- EFSA, European Food Safety Authority (2015) Scientific opinion on the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs: executive summary. EFSA Journal; 13: 3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich S, Calafat AM, Humblet O, et al. (2014) Handling of thermal receipts as a source of exposure to bisphenol A. JAMA; 311: 859–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkins HB, Pagnotto LD, Smith HL. (1974) Concentration adjustments in urinalysis. Am Ind Hyg Assoc J; 35: 559–65. [DOI] [PubMed] [Google Scholar]
- European Union (2008) Risk assessment report: 4,4’-isopropylidenediphenol (bisphenol-A), part 2, human health. Ispara, Italy: European Commission, Joint Research Centre, Institute for Health and Consumer Protection; p. 69. [Google Scholar]
- Fleisch AF, Sheffield PE, Chinn C, et al. (2010) Bisphenol A and related compounds in dental materials. Pediatrics; 126: 760–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geens T, Neels H, Covaci A. (2012) Distribution of bisphenol-A, triclosan and n-nonylphenol in human adipose tissue, liver and brain. Chemosphere; 87: 796–802. [DOI] [PubMed] [Google Scholar]
- Guidry VT, Longnecker MP, Aase H, et al. (2015) Measurement of total and free urinary phenol and paraben concentrations over the course of pregnancy: assessing reliability and contamination of specimens in the Norwegian Mother and Child Cohort Study. Environ Health Perspect; 123: 705–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanaoka T, Kawamura N, Hara K, et al. (2002) Urinary bisphenol A and plasma hormone concentrations in male workers exposed to bisphenol A diglycidyl ether and mixed organic solvents. Occup Environ Med; 59: 625–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Miao M, Wu C, et al. (2009) Occupational exposure levels of bisphenol A among Chinese workers. J Occup Health; 51: 432–6. [DOI] [PubMed] [Google Scholar]
- Hornung RW, Reed LD. (1990) Estimation of average concentration in the presence of non-detectable values. App Occup Environ Hyg; 5: 46–51. [Google Scholar]
- Kaddar N, Harthé C, Déchaud H, et al. (2008) Cutaneous penetration of bisphenol A in pig skin. J Toxicol Environ Health A; 71: 471–3. [DOI] [PubMed] [Google Scholar]
- Kopf PW. (2003) Phenolic resins. Kirk-Othmer encyclopedia of chemical technology. John Wiley & Sons; pp. 1–54. Available at http://onlinelibrary.wiley.com/doi/10.1002/0471238961.1608051411151606.a01.pub2/abstract. Accessed 14 December 2016. [Google Scholar]
- Lakind JS, Goodman M, Mattison DR. (2014) Bisphenol A and indicators of obesity, glucose metabolism/type 2 diabetes and cardiovascular disease: a systematic review of epidemiologic research. Crit Rev Toxicol; 44: 121–50. [DOI] [PubMed] [Google Scholar]
- Li DK, Zhou Z, Miao M, et al. (2010. a) Relationship between urine bisphenol-A level and declining male sexual function. J Androl; 31: 500–6. [DOI] [PubMed] [Google Scholar]
- Li DK, Zhou Z, Miao M, et al. (2011) Urine bisphenol-A (BPA) level in relation to semen quality. Fertil Steril; 95: 625–30.e1–4. [DOI] [PubMed] [Google Scholar]
- Li DK, Zhou Z, Qing D, et al. (2010. b) Occupational exposure to bisphenol-A (BPA) and the risk of self-reported male sexual dysfunction. Hum Reprod; 25: 519–27. [DOI] [PubMed] [Google Scholar]
- Liu X, Miao M, Zhou Z, et al. (2015) Exposure to bisphenol-A and reproductive hormones among male adults. Environ Toxicol Pharmacol; 39: 934–41. [DOI] [PubMed] [Google Scholar]
- Mack AG. (2004) Flame retardants, halogenated. Kirk-Othmer encyclopedia of chemical technology. John Wiley & Sons; pp. 1–30. Available at http://onlinelibrary.wiley.com/doi/10.1002/0471238961.0801121516052020.a01.pub2/abstract. Accessed 14 December 2016. [Google Scholar]
- Matthews JB, Twomey K, Zacharewski TR. (2001) In vitro and in vivo interactions of bisphenol A and its metabolite, bisphenol A glucuronide, with estrogen receptors α and β. Chem Res Toxicol; 14: 149–57. [DOI] [PubMed] [Google Scholar]
- Mørck TJ, Sorda G, Bechi N, et al. (2010) Placental transport and in vitro effects of Bisphenol A. Reprod Toxicol; 30: 131–7. [DOI] [PubMed] [Google Scholar]
- Ndaw S, Remy A, Jargot D, et al. (2016) Occupational exposure of cashiers to Bisphenol A via thermal paper: urinary biomonitoring study. Int Arch Occup Environ Health; 89: 935–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NHANES (2016) National Health and Nutrition Examination Survey. 2013–2014 data documentation, codebook, and frequencies. Personal care and consumer product chemicals and metabolites (EPHPP_H) Available at https://wwwn.cdc.gov/Nchs/Nhanes/2013-2014/EPHPP_H.htm Accessed 14 December 2016.
- NTP, National Toxicology Program (2008) NTP-CERHR monograph on the potential human reproductive and developmental effects of bisphenol A NIH Pub. No. 08-5994. Available at http://ntp.niehs.nih.gov/ntp/ohat/bisphenol/bisphenol.pdf Accessed 14 December 2016.
- Pham HQ, Marks MJ. (2004) Epoxy resins. Kirk-Othmer encyclopedia of chemical technology. John Wiley & Sons; pp. 1–125. Available at http://onlinelibrary.wiley.com/doi/10.1002/0471238961.0516152407011414.a01.pub2/abstract. Accessed 14 December 2016. [Google Scholar]
- Peretz J, Vrooman L, Ricke WA, et al. (2014) Bisphenol A and reproductive health: update of experimental and human evidence, 2007–2013. Environ Health Perspect; 122: 775–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren D-S, Wu C-H, Chang X-L, et al. (2012) Exposure levels of bisphenol A among workers in a resin factory. J Environ Occup Med; 29: 269–273 [in Chinese]. [Google Scholar]
- Rezg R, El-Fazaa S, Gharbi N, et al. (2014) Bisphenol A and human chronic diseases: current evidences, possible mechanisms, and future perspectives. Environ Int; 64: 83–90. [DOI] [PubMed] [Google Scholar]
- Rochester JR. (2013) Bisphenol A and human health: a review of the literature. Reprod Toxicol; 42: 132–55. [DOI] [PubMed] [Google Scholar]
- Snyder RW, Maness SC, Gaido KW, et al. (2000) Metabolism and disposition of bisphenol A in female rats. Toxicol Appl Pharmacol; 168: 225–34. [DOI] [PubMed] [Google Scholar]
- Stahlhut RW, Welshons WV, Swan SH. (2009) Bisphenol A data in NHANES suggest longer than expected half-life, substantial nonfood exposure, or both. Environ Health Perspect; 117: 784–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staples CA, Dorn PB, Klecka GM, et al. (1998) A review of the environmental fate, effects, and exposures of bisphenol A. Chemosphere; 36: 2149–73. [DOI] [PubMed] [Google Scholar]
- Teeguarden JG, Twaddle NC, Churchwell MI, et al. (2015) 24-hour human urine and serum profiles of bisphenol A: evidence against sublingual absorption following ingestion in soup. Toxicol Appl Pharmacol; 288: 131–42. [DOI] [PubMed] [Google Scholar]
- Thayer KA, Doerge DR, Hunt D, et al. (2015) Pharmacokinetics of bisphenol A in humans following a single oral administration. Environ Int; 83: 107–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer KA, Taylor KW, Garantziotis S, et al. (2016) Bisphenol A, bisphenol S, and 4-hydroxyphenyl 4-isoprooxyphenylsulfone (BPSIP) in urine and blood of cashiers. Environ Health Perspect; 124: 437–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USEPA, United States Environmental Protection Agency (2014) Bisphenol A alternatives in thermal paper Final report. Available at http://www2.epa.gov/sites/production/files/2014-05/documents/bpa_final.pdf Accessed 14 December 2016.
- USEPA, United States Environmental Protection Agency (2016. a) Toxic Release Inventory, TRI Explorer Release reports. Available at http://iaspub.epa.gov/triexplorer/tri_release.chemical Accessed 14 December 2016.
- USEPA, United States Environmental Protection Agency (2016. b) Integrated risk information system. Bisphenol A Available at http://cfpub.epa.gov/ncea/iris/index.cfm?fuseaction=iris.showQuickview&substance_nmbr=0356 Accessed 14 December 2016.
- Völkel W, Colnot T, Csanády GA, et al. (2002) Metabolism and kinetics of bisphenol a in humans at low doses following oral administration. Chem Res Toxicol; 15: 1281–7. [DOI] [PubMed] [Google Scholar]
- Waechter J, Thornton C, Markham D, et al. (2007) Factors affecting the accuracy of bisphenol a and bisphenol a-monoglucuronide estimates in Mammalian tissues and urine samples. Toxicol Mech Methods; 17: 13–24. [DOI] [PubMed] [Google Scholar]
- Wallach J. (2007) Urine. Interpretation of diagnostic tests. 8th edn. Chapter 4. Philadelphia, PA: Lippincott Williams & Wilkins; p. 90. [Google Scholar]
- Wang F, Hua J, Chen M, et al. (2012) High urinary bisphenol A concentrations in workers and possible laboratory abnormalities. Occup Environ Med; 69: 679–84. [DOI] [PubMed] [Google Scholar]
- WHO, World Health Organization. (2011) Toxicological and Health Aspects of Bisphenol A. Report of Joint FAO/WHO Expert Meeting, 2–5 November 2010 and Report of Stakeholder Meeting on Bisphenol A, 1 November 2010, Ottawa, Canada. Section 6.2 Epidemiological Studies, pp. 31–34. Geneva, Switzerland. Available at http://apps.who.int/iris/bitstream/10665/44624/1/97892141564274_eng.pdf?ua=1. Accessed 14 December 2016. [Google Scholar]
- Xiao G-B, Shi J-L, He G-H, et al. (2005) Investigation into serum BPA and sex hormone level of workers in epoxy resin manufacture. J Environ Occup Med; 22: 295–8 [in Chinese]. [Google Scholar]
- Ye X, Bishop AM, Reidy JA, et al. (2007) Temporal stability of the conjugated species of bisphenol A, parabens, and other environmental phenols in human urine. J Expo Sci Environ Epidemiol; 17: 567–72. [DOI] [PubMed] [Google Scholar]
- Zalko D, Jacques C, Duplan H, et al. (2011) Viable skin efficiently absorbs and metabolizes bisphenol A. Chemosphere; 82: 424–30. [DOI] [PubMed] [Google Scholar]
- Zhou X, Kramer JP, Calafat AM, et al. (2014) Automated on-line column-switching high performance liquid chromatography isotope dilution tandem mass spectrometry method for the quantification of bisphenol A, bisphenol F, bisphenol S, and 11 other phenols in urine. J Chromatogr B Analyt Technol Biomed Life Sci; 944: 152–6. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Miao M, Ran M, et al. (2013) Serum bisphenol-A concentration and sex hormone levels in men. Fertil Steril; 100: 478–82. [DOI] [PubMed] [Google Scholar]
- Zhuang W, Wu K, Wang Y, et al. (2015) Association of serum bisphenol-A concentration and male reproductive function among exposed workers. Arch Environ Contam Toxicol; 68: 38–45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.