Abstract
Objectives
To examine contemporary trends in end-of-life cancer care and geographic variation of end-of-life care aggressiveness among Medicare beneficiaries.
Materials and Methods
Using the Surveillance, Epidemiology, and End Results—Medicare data, we identified 132,051 beneficiaries who died as a result of cancer in 2006–2011. Aggressiveness of end-of-life care was measured by chemotherapy received within 14 days of death, >1 emergency department (ED) visit within 30 days of death, >1 hospitalization within 30 days of death, ≥1 intensive care unit (ICU) admission within 30 days of death, in-hospital death, or hospice enrollment ≤3 days before death. Using hierarchical generalized linear models, we assessed potentially aggressive end-of-life care adjusting for patient demographics, tumor characteristics, and hospital referral region (HRR)-level market factors.
Results
The proportion of beneficiaries receiving at least one potentially aggressive end-of-life intervention increased from 48.6% in 2006 to 50.5% in 2011 (P < .001). From 2006 to 2011, increases were apparent in repeated hospitalization (14.1% vs. 14.8%; P = .01), repeated ED visits (34.3% vs. 36.6%; P < .001), ICU admissions (16.2% vs. 21.3%; P < .001), and late hospice enrollment (11.2% vs. 12.9%; P < .001), whereas in-hospital death declined (23.5% vs. 20.9%; P < .001). End-of-life chemotherapy use (4.4% vs. 4.5%) did not change significantly over time (P = .12). The use of potentially aggressive end-of-life care varied substantially across HRRs, ranging from 40.3% to 58.3%. Few HRRs had a decrease in aggressive end-of-life care during the study period.
Conclusions
Despite growing focus on providing appropriate end-of-life care, there has not been an improvement in aggressive end-of-life cancer care in the Medicare program.
Keywords: End-of-life care, Intensity, Geographic variation
1. Introduction
Overly aggressive care at the end of life is not consistent with patient preferences,1–3 incurs substantial costs, and is not associated with better outcomes.4–6 Since 1999, the Institute of Medicine (IOM) has released several reports calling for improvement of end-of-life cancer care.7,8 Efforts have identified concerning end-of-life care patterns, such as very late chemotherapy use, very short hospice enrollment, and repeated hospitalization during patients’ last month of life.9 Such aggressive care patterns have been used by oncologists to indicate poor end-of-life care quality.10,11 While palliative care has been embraced by the medical community, the recent IOM report Dying in America highlights continued deficiencies in promoting palliative care.12 To improve end-of-life care, several organizations, including the American Society of Clinical Oncology, have been working to improve care delivery, clinician– patient communication, and advance care planning.13,14
Available literature examining end-of-life cancer care among older individuals with cancer in the United States is outdated,15,16 limited in scope,17 or lacks important clinical detail.18–20 For instance, one study of Medicare beneficiaries with ovarian cancer found that intensity of hospital-based end-of-life care increased between 1997 and 2007.17 The other analyses of Medicare beneficiaries lacked clinical detail regarding cancer characteristics, either focusing on patients with cancer who had been hospitalized during the last 6 months of life18 or comparing general end-of-life care patterns across decedents who died from cancer or other causes.20 These analyses highlight the need for more comprehensive, updated information regarding end-of-life cancer care in the United States in order to assess progress after over a decade of efforts to improve care, and identify opportunities for improvement.15
To address this knowledge gap, we examined the trends in the aggressiveness of end-of-life cancer care over time in a population-based cohort of Medicare beneficiaries who had died after a cancer diagnosis. We also evaluated the trends of geographic variation of end-of-life care aggressiveness and identified the geographic regions that experienced a greater improvement in end-of-life cancer care than others. We assessed the associations of end-of-life care aggressiveness with patient characteristics and the availability of related healthcare resources. Findings from this study can not only provide a more comprehensive picture of temporal trends in the quality of end-of-life cancer care in the United States but also further our knowledge of whether certain regions or regional market factors might be more conducive to improving end-of-life care.
2. Methods
2.1. Data and Study Design
We used the Surveillance, Epidemiology, and End Results (SEER)—Medicare database, a unique data source linking Medicare enrollment and claims records to tumor registries. The SEER registries currently cover approximately 28% of the U.S. population.21 We used SEER data to identify baseline patient and tumor characteristics and Medicare claims to identify indicators of interaction with the healthcare system. The study was reviewed by the Institutional Review Board of Yale University who determined that this study did not directly involve human subjects.
2.2. Patients
We identified beneficiaries who had breast, prostate, lung, colorectal, pancreas, liver, kidney, melanoma, or hematologic cancer diagnosed in 2004–2011. To make the sample of each year comparable, we limited our cohort to decedents each year who died within 3 years of diagnosis as a result of cancer. This criterion of the same range of time between cancer diagnosis and death each year, consistent with prior research,9 allowed us to avoid the potential influence of time between cancer diagnosis and death on trend results. Consequently, only the annual results from 2006 to 2011 were compared. We limited our sample to beneficiaries who were aged 66.5–94.9 years at death and enrolled in Medicare Parts A and B during the last 18 months of life. Patients were excluded if their diagnosis occurred only according to death certificate or autopsy, if they could not be assigned to a hospital referral region (HRR), or if they lived less than 3 months after cancer diagnosis. The step-wise ascertainment of our study cohort is listed in Appendix Table A1 (online only).
2.3. Measurement
2.3.1. Outcomes
We used previously developed claims-based indicators of potentially aggressive health care within the last 30 days of life,10 including (1) chemotherapy received within 14 days of death, (2) >1 emergency department (ED) visit within 30 days of death, (3) >1 hospitalization within 30 days of death, (4) ≥1 intensive care unit (ICU) admission within 30 days of death, (5) in-hospital death, and (6) hospice enrollment ≤3 days before death. We created a composite measure of aggressive end-of-life care, which was defined as the occurrence of at least one of the indicators above.
2.3.2. Covariates
We included candidate variables which are available in our database and have been used in research examining end-of-life care and/or healthcare market factors. Patient demographics included age, race, Hispanic ethnicity, gender, year of death, marital status, SEER registry, and metro status of residence.22 Socioeconomic status measures included median household income and percentage of adults with high school education or less, both derived from census data. We evaluated Elixhauser comorbidity conditions between 7 months and 18 months prior to death, adapting an approach that requires the diagnosis code to appear on an inpatient claim or two or more physician or outpatient claims greater than 30 days apart for the condition to be considered present.23 We incorporated a measure of disability status, a claim-based indicator for services commonly needed by patients with poor functional performance status.24 We also ascertained the number of outpatient clinic visits within 1 to 3 months before death. Tumor characteristics included tumor site, advanced stage, multiple-cancer diagnoses, and time between cancer diagnosis and death as reported by SEER.
We attributed each patient’s medical care to the hospital in which each patient was admitted most frequently in the last 6 months of life. If the numbers tied between hospitals, total days of hospital stay were used. We categorized hospitals according to National Cancer Institute designation. We used data from the Area Resource File to assess the following county-level market factors assigned into quartiles each year: percentage of individuals in health maintenance organizations (HMOs), and the number of physicians, radiation oncologists, hospital beds, skilled nursing facility beds, hospices, and home health agencies per 1000 people aged 65 years and older.
2.4. Statistical Analysis
We described demographic, clinical, and social factors between patients who experienced at least one indicator of aggressive care versus those who did not experience any aggressive intervention between 2006 and 2011, using t-tests for continuous variables and chi-square statistics for categorical variables. The Cochran–Armitage test was used to evaluate time trends. For each HRR, we also identified the proportion receiving aggressive care each year. We then determined the trend of aggressive end-of-life care over time for each HRR and identified the HRRs with a decrease in aggressive care. We also identified HRRs which had a lower percentage of aggressive care through the study period.
In the multivariable analyses, we used hierarchical generalized linear models (HGLMs), clustering patients by HRR, to determine the independent contribution of individual and market factors to aggressiveness of end-of-life care. To reduce variability caused by low HRR volumes, we excluded from the HGLM analysis HRRs that had less than twenty decedents during the study period. We used the variance inflation factors of independent variables using multivariate linear regression models to assess potential multicollinearity.
We estimated the adjusted proportion of decedents who experienced at least one aggressive end-of-life care intervention for each HRR, controlling for patient characteristics but not market factors. We calculated the expected proportion of aggressive end-of-life care for HRRi each year, using HGLMs with random effects as zero. Then, for HRRi, we estimated its adjusted proportion as:
where Ōi,t and Ōoverall,t are the observed proportion of aggressive end-of-life care in HRRi and in all HRRs in yeart, respectively, and Ēi,t is the expected proportion of aggressive end-of-life care in HRRi in yeart, from 2006 to 2011. This measure reflects the proportion of decedents receiving aggressive end-of-life care across HRRs after adjusting for patient factors. We created HRR quintiles according to the unadjusted proportion of decedents who received at least one aggressive end-of-life care intervention in 2006, and then followed these quintiles over time in order to investigate the trends in adjusted proportion of aggressive end-of-life care in the highest and lowest quintiles over the study period. All statistical analyses were completed using SAS, version 9.4 (SAS Institute, Cary, NC) or Stata 13 (StataCorp, College Station, TX). A two-tailed P < .05 was used to define statistical significance.
3. Results
The full study sample consisted of 132,051 beneficiaries. Overall, 44.9% of the cohort was age >80 years, about 70% had comorbidities, and 35% had stage IV disease at diagnosis (Table 1). Nearly 43% had lung cancer, followed by colorectal cancer, hematologic malignancies, and pancreas cancer. Approximately one third died within 3–6 months after diagnosis, and 20% within 6 months to 1 year.
Table 1.
Demographic and clinical characteristics of medicare beneficiaries who died of cancer, 2006–2011.
| Patients not experiencing any aggressive care (n = 66,455) |
Patients experiencing any aggressive care (n = 65,596) |
P-value | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Age-group | <.001 | ||||
| 66.5–69 | 8,200 | 12.4 | 11,560 | 17.6 | |
| 70–74 | 13,004 | 19.6 | 18,126 | 27.6 | |
| 75–79 | 14,791 | 22.3 | 18,624 | 28.4 | |
| 80–84 | 14,900 | 22.4 | 16,076 | 24.5 | |
| 85–94 | 15,540 | 23.4 | 12,819 | 19.5 | |
| Female | <.001 | ||||
| No | 33,317 | 50.1 | 29,640 | 45.2 | |
| Yes | 33,138 | 49.9 | 35,956 | 54.8 | |
| Race | <.001 | ||||
| White | 58,819 | 88.5 | 55,848 | 85.1 | |
| Black | 4,855 | 7.3 | 6,318 | 9.6 | |
| Other | 2,781 | 4.2 | 3,430 | 5.2 | |
| Hispanic | <.001 | ||||
| Non-Hispanic | 63,692 | 95.8 | 62,084 | 94.6 | |
| Hispanic | 2,763 | 4.2 | 3,512 | 5.4 | |
| Marital Status | <.001 | ||||
| Married | 31,698 | 47.7 | 34,196 | 52.1 | |
| Unmarried | 31,660 | 47.6 | 28,303 | 43.1 | |
| Unknown | 3,097 | 4.7 | 3,097 | 4.7 | |
| Metro status of residence | <.001 | ||||
| Metro | 54,083 | 81.4 | 54,251 | 81.6 | |
| Non-metro | 12,372 | 18.6 | 11,345 | 17.1 | |
| Income | <.001 | ||||
| < $33,000 | 15,302 | 23.0 | 15,953 | 24.3 | |
| $33,000–$39,999 | 10,935 | 16.5 | 10,023 | 15.3 | |
| $40,000–$49,999 | 13,662 | 20.6 | 13,203 | 20.1 | |
| $50,000–$62,999 | 12,688 | 19.1 | 12,413 | 18.9 | |
| ≥ $63,000 | 13,776 | 20.7 | 13,935 | 21.2 | |
| Unknown | 92 | 0.1 | 69 | 0.1 | |
| High School Education | <.001 | ||||
| <30% | 14,399 | 21.7 | 13,115 | 20.0 | |
| 30%–39% | 10,558 | 15.9 | 9,830 | 15.0 | |
| 40%–49% | 11,541 | 17.4 | 11,225 | 17.1 | |
| 50%–59% | 12,299 | 18.5 | 12,312 | 18.8 | |
| ≥60% | 17,566 | 26.4 | 19,045 | 29.0 | |
| Unknown | 92 | 0.1 | 69 | 0.1 | |
| Comorbidity | <.001 | ||||
| None | 19,964 | 30.0 | 16,868 | 25.7 | |
| 1 to 2 | 26,175 | 39.4 | 26,349 | 40.2 | |
| 3 or more | 20,316 | 30.6 | 22,379 | 34.1 | |
| Disability status | <.001 | ||||
| No | 56,790 | 85.5 | 56,736 | 86.5 | |
| Yes | 9,665 | 14.5 | 8,860 | 13.5 | |
| Tumor site | <.001 | ||||
| Lung | 28,780 | 43.3 | 27,844 | 42.4 | |
| Colorectal | 9,948 | 15.0 | 8,074 | 12.3 | |
| Hematologic malignancies | 6,560 | 9.9 | 10,180 | 15.5 | |
| Pancreas | 6,627 | 10.0 | 5,018 | 7.6 | |
| Breast | 4,227 | 6.4 | 4,217 | 6.4 | |
| Prostate | 3,719 | 5.6 | 4,163 | 6.3 | |
| Skin | 2,287 | 3.4 | 2,172 | 3.3 | |
| Kidney | 2,189 | 3.3 | 2,049 | 3.1 | |
| Liver | 2,118 | 3.2 | 1,879 | 2.9 | |
| Stage IV at diagnosis | <.001 | ||||
| Not stage IV | 42,135 | 63.4 | 43,126 | 65.7 | |
| Stage IV | 24,320 | 36.6 | 22,470 | 34.3 | |
| Multiple cancers | <.001 | ||||
| No | 60,572 | 91.1 | 58,167 | 88.7 | |
| Yes | 5,883 | 8.9 | 7,429 | 11.3 | |
| Year of death | <.001 | ||||
| 2006 | 11,378 | 17.1 | 10,738 | 16.4 | |
| 2007 | 11,673 | 17.6 | 11,362 | 17.3 | |
| 2008 | 11,306 | 17.0 | 11,172 | 17.0 | |
| 2009 | 10,939 | 16.5 | 10,827 | 16.5 | |
| 2010 | 10,829 | 16.3 | 10,958 | 16.7 | |
| 2011 | 10,330 | 15.5 | 10,539 | 16.1 | |
| Time between cancer diagnosis and death | <.001 | ||||
| 3–6 months | 22,344 | 33.6 | 23,187 | 35.3 | |
| 6 months–1 year | 13,664 | 20.6 | 13,282 | 20.2 | |
| 1–2 years | 19,588 | 29.5 | 18,533 | 28.3 | |
| 2–3 years | 10,859 | 16.3 | 10,594 | 16.2 | |
| Outpatient visits within 1 to 3 months before death | <.001 | ||||
| None | 11,545 | 17.4 | 2,398 | 3.7 | |
| 1 to 4 | 15,355 | 23.1 | 8,680 | 13.2 | |
The proportion of beneficiaries receiving at least one potentially aggressive end-of-life intervention increased from 48.6% to 50.5% over time (trend test P < .001; Fig. 1). There was a slight but significant increase repeated hospitalizations (from 14.1% to 14.8%; P = .014), repeated ED visits (from 34.3% to 36.6%; P < .001), and very short hospice enrollment (from 11.2% to 12.9%; P < .001), while increase in ICU admission was more substantial (from 16.2% to 21.3%; P < .001). In contrast, in-hospital death declined in the study period (from 23.5% to 20.9%; P < .001). End-of-life chemotherapy use (approximately 4.5% from 2006 to 2011) did not change significantly over time (trend test P = .12).
Fig. 1.
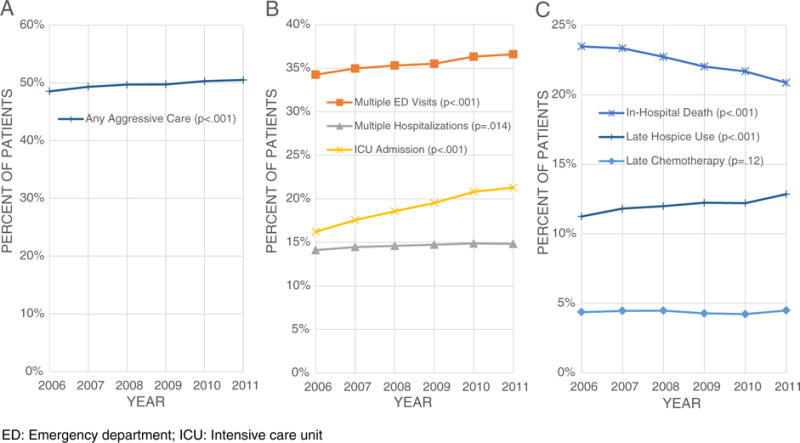
Trends of aggressive end-of-life care: (A) proportion of patients with cancer receiving any aggressive end-of-life care; (B) proportion of patients with cancer receiving hospital-based service in last month of life; (C) proportion end-of-life medical care of patients with cancer. ED: emergency department; ICU: intensive care unit.
In multivariable analyses, we found decedents who were younger, male, non-white, Hispanic, married, and resided in non-metropolitan areas or areas with higher proportions with more than high school education were more likely to have aggressive care, compared with those who were older, female, white, non-Hispanic, unmarried, and resided in metropolitan areas or areas with areas with lower proportions with more than high school education (Table 2; and Appendix Table A2 for detailed results). Compared with patients without comorbidity, having three or more comorbidities was associated with increased odds of receiving aggressive care (odds ratio [OR], 1.08; 95% confidence interval [CI], 1.04 to 1.12). In contrast, having disability was associated with decreased odds of receiving aggressive care (OR, 0.92; 95% CI, 0.89 to 0.96). Cancer type, cancer stage at diagnosis, and time between cancer diagnosis and death also played a significant role in predicting aggressive care. For example, compared with patients with breast cancer, patients with hematologic malignancies were more likely to receive aggressive end-of-life care (OR, 1.21; 95% CI, 1.14 to 1.29), whereas patients with pancreatic cancer were less likely to receive aggressive end-of-life care (OR, 0.61; 95% CI, 0.57 to 0.66).
Table 2.
Multi-level logistic regression of factors associated with aggressive care.
| Odds ratio | 95% CI
|
||
|---|---|---|---|
| Lower | Upper | ||
| Demographic factors | |||
| Age-group | |||
| 66.5–69 | REF | ||
| 70–74 | 0.96 | 0.92 | 1.01 |
| 75–79 | 0.86 | 0.82 | 0.89 |
| 80–84 | 0.76 | 0.73 | 0.80 |
| 85–94 | 0.65 | 0.62 | 0.68 |
| Sex | |||
| Male | REF | ||
| Female | 0.82 | 0.80 | 0.85 |
| Race | |||
| White | REF | ||
| Black | 1.29 | 1.23 | 1.35 |
| Other | 1.34 | 1.26 | 1.43 |
| Hispanic | |||
| Non-Hispanic | REF | ||
| Hispanic | 1.20 | 1.13 | 1.28 |
| Marital status | |||
| Married | REF | ||
| Unmarried | 0.94 | 0.91 | 0.97 |
| Metro status of residence | |||
| Metro | REF | ||
| Non-metro | 1.09 | 1.03 | 1.15 |
| Income | |||
| <$33,000 | REF | ||
| $33,000–$39,999 | 0.95 | 0.91 | 0.99 |
| $40,000–$49,999 | 0.97 | 0.93 | 1.01 |
| $50,000–$62,999 | 0.96 | 0.91 | 1.01 |
| ≥ $63,000 | 0.94 | 0.89 | 1.00 |
| High school education | |||
| <30% | REF | ||
| 30%–39% | 1.05 | 1.00 | 1.10 |
| 40%–49% | 1.06 | 1.01 | 1.12 |
| 50%–59% | 1.12 | 1.06 | 1.18 |
| ≥60% | 1.16 | 1.09 | 1.23 |
| Clinical factors | |||
| Comorbidity | |||
| None | REF | ||
| 1 to 2 | 1.06 | 1.03 | 1.10 |
| 3 or more | 1.08 | 1.04 | 1.12 |
| Disability | |||
| No | REF | ||
| Yes | 0.92 | 0.89 | 0.96 |
| Tumor site | |||
| Lung | 0.82 | 0.77 | 0.86 |
| Colorectal | 0.73 | 0.69 | 0.78 |
| Hematologic malignancies | 1.21 | 1.14 | 1.29 |
| Pancreas | 0.61 | 0.57 | 0.66 |
| Breast | REF | ||
| Prostate | 0.92 | 0.86 | 1.00 |
| Skin | 0.81 | 0.75 | 0.89 |
| Kidney | 0.78 | 0.72 | 0.85 |
| Liver | 0.69 | 0.63 | 0.76 |
| Stage IV at diagnosis | |||
| Not Stage IV | REF | ||
| Stage IV | 0.85 | 0.83 | 0.87 |
| Multiple cancers | |||
| No | REF | ||
| Yes | 1.18 | 1.13 | 1.23 |
| Time between cancer diagnosis and death | |||
| 3–6 months | REF | ||
| 6 months–1 year | 1.12 | 1.07 | 1.16 |
| 1–2 years | 1.13 | 1.09 | 1.17 |
| 2–3 years | 1.14 | 1.09 | 1.20 |
| Year of death | |||
| 2006 | REF | ||
| 2007 | 1.04 | 1.00 | 1.09 |
| 2008 | 1.06 | 1.01 | 1.10 |
| 2009 | 1.06 | 1.01 | 1.10 |
| 2010 | 1.09 | 1.04 | 1.14 |
| 2011 | 1.10 | 1.05 | 1.15 |
| Outpatient visits within 1 to 3 months before death | |||
| None | REF | ||
| 1 to 4 | 2.92 | 2.76 | 3.08 |
| 5 to 10 | 5.40 | 5.12 | 5.70 |
| 11 to 19 | 6.46 | 6.12 | 6.82 |
| 20 or more | 6.81 | 6.44 | 7.19 |
| Market factors | |||
| County-level HMO penetration rate | |||
| Q1 (lowest quartile) | REF | ||
| Q2 | 0.95 | 0.91 | 1.00 |
| Q3 | 0.88 | 0.83 | 0.94 |
| Q4 | 0.90 | 0.84 | 0.97 |
| County-level radiation oncologist number per 1000 people 65 years and older | |||
| Q1 (lowest quartile) | REF | ||
| Q2 | 0.94 | 0.89 | 1.00 |
| Q3 | 0.96 | 0.90 | 1.02 |
| Q4 | 0.93 | 0.87 | 0.99 |
CI: confidence interval; NCI: National Cancer Institute; HMO: health maintenance organization.
Model included all variables in the Table as well as hospital factor (NCI Designation) and market factors (hospice number, physician number, hospital bed number, skilled nursing bed number, and home health agency number per 1000 people 65 years and older at county level).
Detailed results can be found in the Appendix Table A2.
Only two market factors that we examined were associated with aggressive end-of-life intervention in the multivariable analysis. Decedents who were in the areas with higher HMO penetration rate were less likely to receive aggressive end-of-life care (OR, 0.90; 95% CI, 0.84 to 0.97), compared with decedents who were in the areas with lower HMO penetration rate. Decedents who were in the areas with a higher number of radiation oncologists per 1000 people aged 65 years and older were less likely to receive aggressive end-of-life care (OR, 0.93; 95% CI, 0.87 to 0.99), compared with decedents in the lowest quintile area. The supply of hospice, physicians, hospitals, home health agency, or skill nursing facilities within HRRs was not associated with receipt of aggressive care (Appendix Table A2).
The use of aggressive end-of-life care varied substantially across HRRs, ranging from 40.3% to 58.3% (unadjusted). In the analysis of temporal trends, few HRRs had a decrease in aggressive end-of-life care during the study period (Fig. 2). Although 5 HRRs had an absolute decrease in end-of-life care of >2%, only one HRR (Ogden, Utah) had a statistically significantly decreased trend of 6.8% (P = .023). Seven HRRs (including Santa Cruz, CA; Cedar Rapids, Des Moines, Iowa City, and Mason City, IA; Fort Oglethorpe, GA; and Tacoma, WA) had low levels of end-of-life care intensity across the stud period. Additionally, the magnitude of regional variation in end-of-life care did not change substantially: the difference in the proportion of patients receiving potentially aggressive end-of-life care between HRRs in the lowest and the highest quintiles was 20.5% (37.4% vs. 58.0%) in 2006 and 19.8% (40.2% vs. 60.1%) in 2011. After adjusting for patient characteristics, the difference between the highest and lowest quintile areas was 15.8% (44.4% vs 60.2%) in 2011 (Fig. 3).
Fig. 2.

Trends of aggressive end-of-life care in 92 hospital referral regions among SEER-Medicare areas. Each line represents a hospital referral region (HRR), showing the regression for trend in aggressive end-of-life care. Five HRRs (highlighted in orange) had a trend of >2% decrease. SEER: Surveillance, Epidemiology, and End Results.
Fig. 3.
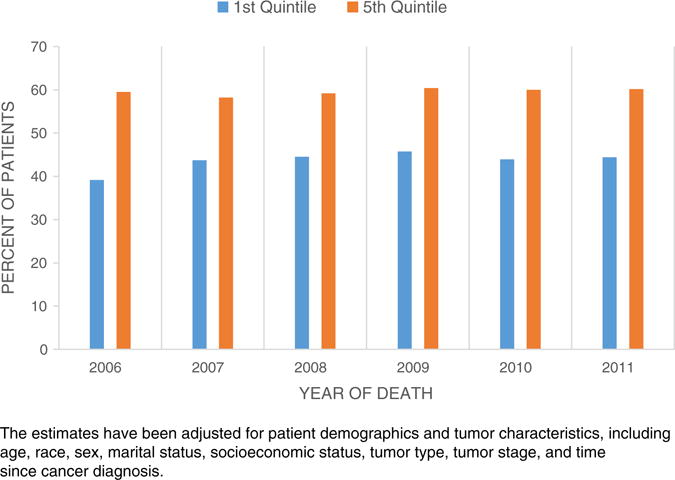
Proportions of decedents receiving aggressive end-of-life care between the highest and lowest quintile areas by year. The estimates have been adjusted for patient demographics and tumor characteristics, including age, race, sex, marital status, socioeconomic status, tumor type, tumor stage, and time since cancer diagnosis.
4. Discussion
Our analysis found that nearly 50% of Medicare beneficiaries who died as a result of cancer between 2006 and 2011 experienced some form of potentially aggressive end-of-life care. Not only has there been no improvement in the overall use of aggressive end-of-life care, there was actually a slight increase in aggressive care over time. Despite a growing focus on providing appropriate end-of-life care, our finding that aggressive end-of-life cancer care is increasing over time in the Medicare program underscores the difficulties in improving end-of-life cancer care.
Our findings build upon prior work in important ways. First, consistent with prior literature,18,25 there was substantial regional variation of end-of-life care. Unlike prior work, we further described the trends in variation, to identify regions that had marked improvement. Of the 92 regions in our sample, only a single region (Ogden, Utah) had a statistically significant reduction in use of aggressive end-of-life care (manifested by a decrease of 6.8% from 2006 to 2011). It has been reported that patients in Ogden, Utah, spent an average of 39.5 days per patient in hospice in the last 6 months of life in 2007, the longest time in hospice care in the United States.26 It is unclear whether the improvement over time in Ogden is associated with trends in early referral for hospice care; future research to identify factors associated with the decrease in these HRRs may help to highlight mechanisms and interventions to improve end-of-life care in other regions.
Second, we found several patient characteristics were associated with aggressive end-of-life care. Consistent with prior literature,9,16 older patients were less likely to receive aggressive care. As treatment decisions for older terminally ill patients with cancer are complex, it is important for patients and providers to discuss benefits and harms attributable to treatments. Recent research suggested that the effectiveness of chemotherapy in reducing cancer-specific mortality may decrease with age.27 Another study, using caregiver’s rating of patient quality of life near death as a proxy, indicated that palliative chemotherapy could not improve patients’ quality of life near death.28 These two studies highlight the importance of patient-physician communication about end-of-life treatment decisions among the older population.
Third, patients who were male, non-white, or Hispanic were more likely to receive aggressive care.15 Additionally, decedents who were unmarried were less likely to receive aggressive end-of-life care, compared with those who were married, indicating the necessity of studying the importance of cancer caregivers when it comes to patients’ end-of-life care. For instance, prior research has suggested that social support can have great impact of cancer detection, treatment, and survival.29 Interestingly, patients with comorbidities were more likely to receive aggressive care, whereas patients with disability were less likely to receive aggressive care. It is possible that comorbidities were associated with increased healthcare utilization, receiving medical intervention for comorbidities or for the increased side effects attributed to cancer care; thus, comorbidities led to an increase in aggressive end-of-life care. In contrast, patients with poor performance status (as the disability measure intended to capture) might be more likely to receive palliative care, which would result in a decrease in aggressive end-of-life care. Future research examining which factors lead patients with poor performance status to receive less aggressive care is needed. Nevertheless, the results suggest that comorbidities and disability measures capture different components of patient health, and should be included in related models.
Fourth, tumor characteristics were also associated with care aggressiveness. Compared with patients with breast cancer, patients with cancer of lung, colorectal, pancreas, liver, kidney, or skin were less likely to receive aggressive care. In contrast, patients with hematologic malignancy were more likely to receive aggressive care, which is consistent with prior literature.16 Also, patients with advanced cancer at diagnosis or with a shorter time between cancer diagnosis and death were associated with less aggressive care, potentially reflecting disease progression and patient perception about his/her disease. For example, patients who respond well to an initial treatment may be encouraged to receive aggressive care for a recurrence. Our study design including patients who died within 3 years after cancer diagnosis allowed us to analyze the relation between time since diagnosis and care aggressiveness. In contrast, prior literature generally examined end-of-life care patterns for decedents who died within a year after cancer diagnosis9; thus, proportions of receiving aggressive care among all cancer decedents may be even higher than the reported estimates.
Fifth, prior literature has indicated that higher HMO penetration was related with less aggressive EOL care in the fee-for-service markets, so-called spillover effects. It is well known that, compared with fee-for-service providers, managed care providers performed better end-of-life care, using measures of higher rates of hospice use and longer hospice stays.30 We found that patients who resided in the areas with higher HMO penetration rates were less likely to receive aggressive care, suggesting that some spillover effects may exist. Literature regarding managed care’s spillover effects on cancer screening is inconclusive,31,32 and launching a new wave of studies examining spillover effects on cancer care quality has been suggested.33 Using a rigorous approach and validated quality measures, we demonstrated an association between HMO penetration and performance of end-of-life care. In contrast, other market factors such as numbers of hospital, hospice, or physician at county level were not associated with aggressive care. Our results indicate that healthcare supply factors may not play an important role regarding end-of-life cancer care quality among Medicare fee-for-service beneficiaries died due to cancer. Future research is needed to examine this relationship in other settings and patient populations.
Concurrent with an increase in most of the indicators of aggressive care we examined, we did find that in-hospital deaths decreased over time. The finding regarding in-hospital deaths was not surprising in that the proportion of decedents receiving hospice care continued increasing34 and hospice use could decrease the likelihood of in-hospital death.9 However, an increase in hospice service and/or a decrease in in-hospital death did not necessarily imply a decrease in care aggressiveness. Researchers have raised concerns that hospice may be an “add-on” to a growing pattern of more utilization of intensive end-of-life care.17,20 Thus, efforts to improve end-of-life care may require consideration of both quantity and quality of hospice use.35,36
We acknowledge several limitations. Our population is limited to elderly patients in fee-for-service Medicare with nine types of cancer. The results may not be generalizable to younger patients or those in HMO plans. Second, we lack information regarding patient preferences, although prior literature has demonstrated that patient preferences explain little of regional variation in end-of-life healthcare utilization.3,37 We also lack information regarding palliative consultation, which could decrease aggressive end-of-life care. Third, while our claim-based indicators of aggressive end-of-life care have been validated, the composite measure may not capture all aggressive care. For instance, late hospice enrollment (within 7 days of death) has been proposed as a measure of poor quality of end-of-life care.36,38 Additionally, our composite measure may miss decedents who have never enrolled in hospice. Thus, our observations may actually underestimate the rates of aggressive end-of-life care. Finally, our cohort was created backward from decedents. Such a retrospective design may lead to biased conclusions for the quality of care provided to dying patients because care received by decedents is not equivalent to care received by individuals who are dying.39 Prospective designs, however, may also be limited by the difficulty of accurately assessing prognosis. Using both retrospective and prospective approaches, Setoguchi et al. found similar physician and hospital patterns of end-of-life care regarding the use of opiate analgesia.40 Future prospective studies confirming our findings are needed.
In conclusion, approximately 50% of cancer decedents in the fee-for-service Medicare Program received aggressive end-of-life care. Despite emphasis on improving end-of-life care in the United States, end-of-life care for patients with cancer appeared more aggressive over time. Regional variation of end-of-life cancer care was substantial, with no evidence of decreased variation over time. There may be important opportunities to learn from areas with a recent decrease in aggressive care. Identifying the underlying mechanisms that led to improved end-of-life care in these areas could influence the testing and adoption of new models in other regions.
Supplementary Material
Acknowledgments
The collection of the California cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract N01-PC-35136 awarded to the Northern California Cancer Center, contract N01-PC-35139 awarded to the University of Southern California, and contract N02-PC-15105 awarded to the Public Health Institute; and the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under agreement #U55/CCR921930-02 awarded to the Public Health Institute. The authors of this report are responsible for its content. The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California, Department of Public Health the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should be inferred. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database. The interpretation and reporting of the SEER-Medicare data are the sole responsibility of the authors.
Funding
This investigation was supported by a Pilot Grant and a P30 Cancer Center Support Grant (CCSG), both from Yale Comprehensive Cancer Center. Dr. Pollack is supported by a career development award from the National Cancer Institute and the Office of Behavioral and Social Science Research (1K07CA151910). Dr. Wang is supported by a career development award from the Agency for Healthcare Research and Quality (1K01HS023900).
Role of the Sponsors
None of the funders had any role in the conduct of the study; in the collection, management, analysis, or interpretation of the data; or in the preparation, review, or approval of the manuscript.
Dr. Gross receives support from Medtronic, Inc. and Johnson & Johnson. Drs. Gross and Hall receive support from the 21st Century Oncology. These supports were not used for any portion of the current manuscript.
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.jgo.2015.11.007.
Footnotes
Disclosures and Conflict of Interest Statements
None of the other coauthors have conflicts to report.
Author Contributions
Dr. Hall had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Wang, Pollack, Bradley, and Gross.
Acquisition of data: Gross.
Analysis and interpretation of data: Wang, Hall, Pollack, Adelson, Bradley, Long, and Gross.
Drafting of the manuscript: Wang, Hall, Long, and Gross.
Critical revision of the manuscript for important intellectual content: Wang, Hall, Pollack, Adelson, Bradley, Long, and Gross.
Statistical analysis: Wang, Hall, and Gross.
Obtained funding: Wang, Gross.
Administrative, technical, or material support: Wang and Gross.
Study supervision: Wang and Gross.
References
- 1.Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300(14):1665–1673. doi: 10.1001/jama.300.14.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steinhauser KE, Clipp EC, McNeilly M, Christakis NA, McIntyre LM, Tulsky JA. In search of a good death: observations of patients, families, and providers. Ann Intern Med. 2000;132(10):825–832. doi: 10.7326/0003-4819-132-10-200005160-00011. [DOI] [PubMed] [Google Scholar]
- 3.Barnato AE, Herndon MB, Anthony DL, et al. Are regional variations in end-of-life care intensity explained by patient preferences? A study of the US Medicare population. Med Care. 2007;45(5):386–393. doi: 10.1097/01.mlr.0000255248.79308.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fisher ES, Wennberg DE, Stukel TA, Gottlieb DJ, Lucas FL, Pinder EL. The implications of regional variations in Medicare spending. Part 1: the content, quality, and accessibility of care. Ann Intern Med. 2003;138(4):273–287. doi: 10.7326/0003-4819-138-4-200302180-00006. [DOI] [PubMed] [Google Scholar]
- 5.Fisher ES, Wennberg DE, Stukel TA, Gottlieb DJ, Lucas FL, Pinder EL. The implications of regional variations in Medicare spending. Part 2: health outcomes and satisfaction with care. Ann Intern Med. 2003;138(4):288–298. doi: 10.7326/0003-4819-138-4-200302180-00007. [DOI] [PubMed] [Google Scholar]
- 6.Yong C, Onukwugha E, Mullins CD, Seal B, Hussain A. The use of health services among elderly patients with stage IV prostate cancer in the initial period following diagnosis. J Geriatr Oncol. 2014;5(3):290–298. doi: 10.1016/j.jgo.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Hewitt M, Simone JV, editors. Institute of Medicine. Ensuring quality cancer care. Washington, DC: National Academy Press; 1999. [PubMed] [Google Scholar]
- 8.Foley KM, Gelband H, editors. Institute of Medicine. Improving palliative care for cancer. Washington, DC: National Academy Press; 2001. (Summary: 1–42) [Google Scholar]
- 9.Earle CC, Neville BA, Landrum MB, Ayanian JZ, Block SD, Weeks JC. Trends in the aggressiveness of cancer care near the end of life. JCO. 2004;22(2):315–321. doi: 10.1200/JCO.2004.08.136. [DOI] [PubMed] [Google Scholar]
- 10.Earle CC, Park ER, Lai B, Weeks JC, Ayanian JZ, Block S. Identifying potential indicators of the quality of end-of-life cancer care from administrative data. JCO. 2003;21(6):1133–1138. doi: 10.1200/JCO.2003.03.059. [DOI] [PubMed] [Google Scholar]
- 11.Grunfeld E, Lethbridge L, Dewar R, et al. Towards using administrative databases to measure population-based indicators of quality of end-of-life care: testing the methodology. Palliat Med. 2006;20(8):769–777. doi: 10.1177/0269216306072553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Institute of Medicine. Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life, 2014. Available at, http://www.nap.edu/openbook.php?record_id=18748 (Accessed March 3, 2015)
- 13.American Society of Clinical Oncology. IOM Report on End-of-Life Care: A Closer Look. Available at, http://www.asco.org/advocacy/iom-report-end-life-care-closer-look (Accessed June 4, 2015)
- 14.HPNA, NBCHPN, and HPNF Response to the IOM Report: Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life. Available at, http://origin.library.constantcontact.com/download/get/file/1109774303066-233/Joint+Response+to+the+IOM+Report.pdf (Accessed June 4, 2015)
- 15.Earle CC, Landrum MB, Souza JM, Neville BA, Weeks JC, Ayanian JZ. Aggressiveness of cancer care near the end of life: is it a quality-of-care issue? JCO. 2008;26(23):3860–3866. doi: 10.1200/JCO.2007.15.8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ho TH, Barbera L, Saskin R, Lu H, Neville BA, Earle CC. Trends in the aggressiveness of end-of-life cancer care in the universal health care system of Ontario, Canada. JCO. 2011;29(12):1587–1591. doi: 10.1200/JCO.2010.31.9897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wright AA, Hatfield LA, Earle CC, Keating NL. End-of-life care for older patients with ovarian cancer is intensive despite high rates of hospice use. JCO. 2014;32(31):3534–3539. doi: 10.1200/JCO.2014.55.5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morden NE, Chang CH, Jacobson JO, et al. End-of-life care for Medicare beneficiaries with cancer is highly intensive overall and varies widely. Health Aff. 2012;31(4):786–796. doi: 10.1377/hlthaff.2011.0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goodman DC, Morden NE, Chang C-H, Fisher ES, Wennberg JE. Trends in Cancer Care Near the End of Life: A Dartmouth Atlas of Health Care Brief. Available at, http://www.dartmouthatlas.org/downloads/reports/Cancer_brief_090413.pdf Accessed March 20, 2015. [PubMed]
- 20.Teno JM, Gozalo PL, Bynum JP, et al. Change in end-of-life care for Medicare beneficiaries: site of death, place of care, and health care transitions in 2000, 2005, and 2009. JAMA. 2013;309(5):470–477. doi: 10.1001/jama.2012.207624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Cancer Institute. SEER-Medicare: How the SEER and Medicare Data Are Linked. Available at, www.appliedresearch.cancer.gov/seermedicare/overview/linked.html (Assessed September 20, 2010)
- 22.Bach PB, Guadagnoli E, Schrag D, Schussler N, Warren JL. Patient demographic and socioeconomic characteristics in the SEER-Medicare database applications and limitations. Med Care. 2002;40(8 Suppl) doi: 10.1097/00005650-200208001-00003. (IV-19-25) [DOI] [PubMed] [Google Scholar]
- 23.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Davidoff AJ, Zuckerman IH, Pandya N, et al. A novel approach to improve health status measurement in observational claims-based studies of cancer treatment and outcomes. J Geriatr Oncol. 2013;4(2):157–165. doi: 10.1016/j.jgo.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Earle CC, Neville BA, Landrum MB, et al. Evaluating claims-based indicators of the intensity of end-of-life cancer care. Int J Qual Health Care. 2005;17(6):505–509. doi: 10.1093/intqhc/mzi061. [DOI] [PubMed] [Google Scholar]
- 26.Goodman DC, Esty AR, Fisher ES, Chang C-H. A Report of the Dartmouth Atlas Project: Trends and Variation in End-of-Life Care for Medicare Beneficiaries with Severe Chronic Illness. Avilable at, http://www.dartmouthatlas.org/downloads/reports/EOL_Trend_Report_0411.pdf (Assessed June 3, 2015) [PubMed]
- 27.Du XL, Zhang Y, Parikh RC, Lairson DR, Cai Y. Comparative effectiveness of chemotherapy regimens in prolonging survival for two large population-based cohorts of elderly adults with breast and colon cancer in 1992–2009. J Am Geriatr Soc. 2015;63(8):1570–1582. doi: 10.1111/jgs.13523. [DOI] [PubMed] [Google Scholar]
- 28.Prigerson HG, Bao Y, Shah MA, et al. Chemotherapy use, performance status, and quality of life at the end of life. JAMA Oncol. 2015;1(6):778–784. doi: 10.1001/jamaoncol.2015.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aizer AA, Chen MH, McCarthy EP, et al. Marital status and survival in patients with cancer. JCO. 2013;31(31):3869–3876. doi: 10.1200/JCO.2013.49.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCarthy EP, Burns RB, Ngo-Metzger Q, Davis RB, Phillips RS. Hospice use among Medicare managed care and fee-for-service patients dying with cancer. JAMA. 2003;289(17):2238–2245. doi: 10.1001/jama.289.17.2238. [DOI] [PubMed] [Google Scholar]
- 31.Keating NL, Landrum MB, Meara E, Ganz PA, Guadagnoli E. Do increases in the market share of managed care influence quality of cancer care in the fee-for-service sector? JNCI. 2005;97(4):257–264. doi: 10.1093/jnci/dji044. [DOI] [PubMed] [Google Scholar]
- 32.Baker LC, Phillips KA, Haas JS, Liang SY, Sonneborn D. The effect of area HMO market share on cancer screening. Health Serv Res. 2004;39(6 Pt 1):1751–1772. doi: 10.1111/j.1475-6773.2004.00316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lipscomb J. Managed care market penetration, spillover effects, and the quality of cancer care. JNCI. 2005;97(4):242–244. doi: 10.1093/jnci/dji068. [DOI] [PubMed] [Google Scholar]
- 34.While number of patients receiving hospice care increases, larger percentage have short length of service [news release] Alexandria, VA: National Hospice and Palliative Care Organization; Nov 27, 2012. ( http://www.nhpco.org/press-room/press-releases/hospice-facts-figures. Accessed October 27, 2014) [Google Scholar]
- 35.Teno JM, Gozalo PL. Quality and costs of end-of-life care: the need for transparency and accountability. JAMA. 2014;312(18):1868–1869. doi: 10.1001/jama.2014.14949. [DOI] [PubMed] [Google Scholar]
- 36.Wang SY, Aldridge MD, Gross CP, et al. Geographic variation of hospice use patterns at the end of life. J Palliat Med. 2015;18(9):771–780. doi: 10.1089/jpm.2014.0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Teno JM, Mitchell SL, Kuo SK, et al. Decision-making and outcomes of feeding tube insertion: a five-state study. J Am Geriatr Soc. 2011;59(5):881–886. doi: 10.1111/j.1532-5415.2011.03385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.American Society of Clinical Oncology. Summary of Current QOPI Measures. Available at, http://www.asco.org/institute-quality/summary-current-qopi-measures (Assessed on August 25, 2015)
- 39.Bach PB, Schrag D, Begg CB. Resurrecting treatment histories of dead patients: a study design that should be laid to rest. JAMA. 2004;292(22):2765–2770. doi: 10.1001/jama.292.22.2765. [DOI] [PubMed] [Google Scholar]
- 40.Setoguchi S, Earle CC, Glynn R, et al. Comparison of prospective and retrospective indicators of the quality of end-of-life cancer care. JCO. 2008;26(35):5671–5678. doi: 10.1200/JCO.2008.16.3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


