Abstract
Although vitamin D deficiency is prevalent among obese individuals, its cause is poorly understood. Few studies have measured vitamin D concentrations in adipose of obese (OB) subjects, and none have included normal weight controls (C). The goal of this study was to investigate whether the relationship between body composition, serum 25-hydroxyvitamin D (25OHD), vitamin D in subcutaneous (SQ) and omental (OM) adipose, and total adipose stores of vitamin D differ among OB and C. Obese women undergoing bariatric surgery and normal-weight women undergoing abdominal surgery for benign gynecologic conditions were enrolled. Subjects had measurements of serum 25OHD by high-performance liquid chromatography (HPLC) and body composition by dual-energy X-ray absorptiometry (DXA). Vitamin D concentrations in SQ and OM adipose were measured by mass spectroscopy. Thirty-six women were enrolled. Serum 25OHD was similar between groups (OB 27 ± 2 versus C 26 ± 2 ng/mL; p = 0.71). Adipose vitamin D concentrations were not significantly different in either SQ (OB 34 ± 9 versus C 26 ± 12 ng/g; p = 0.63) or OM compartments (OB 51 ± 13 versus C 30 ± 18 ng/g; p = 0.37). The distribution of vitamin D between SQ and OM compartments was similar between groups. Serum 25OHD was directly related to adipose vitamin D in both groups. Total body vitamin D stores were significantly greater in OB than in C (2.3 ± 0.6 versus 0.4 ± 0.8 mg, respectively; p < 0.01). In summary, although OB had significantly greater total vitamin D stores than C, the relationship between serum 25OHD and fat vitamin D and the overall pattern of distribution of vitamin D between the OM and SQ fat compartments was similar. Our data demonstrate that obese subjects have greater adipose stores of vitamin D. They support the hypotheses that the enlarged adipose mass in obese individuals serves as a reservoir for vitamin D and that the increased amount of vitamin D required to saturate this depot may predispose obese individuals to inadequate serum 25OHD.
Keywords: DISORDERS OF CALCIUM/PHOSPHATE METABOLISM, NUTRITION, BONE-FAT INTERACTIONS
Introduction
Vitamin D deficiency is prevalent among obese individuals.(1–4) The reason for this association is not clear, although several causative factors have been proposed, including low dietary intake of vitamin D and less sunlight exposure among obese compared with normal-weight individuals.(5,6) It has also been hypothesized that low levels of fat-soluble vitamin D are directly related to the large amounts of adipose tissue in obese individuals. Some authors have postulated that vitamin D is sequestered in the excess adipose tissue, leading to less bioavailability,(7) whereas others suggest that low serum 25-hydroxy vitamin D (25OHD) may be a result of volumetric dilution of vitamin D in the large adipose stores.(8) Few studies have directly measured vitamin D concentrations in the adipose tissue of obese or normal-weight subjects. The goal of this study was to further investigate the relationship between vitamin D deficiency and obesity by measuring vitamin D storage in different adipose compartments in obese patients and normal weight controls. We hypothesized that although serum 25OHD would be lower in obese individuals compared with normal-weight controls, concentrations of vitamin D would not. Based upon greater fat mass, obese subjects would have greater total vitamin D stores compared with normal-weight individuals.
Materials and Methods
This was a single-center study conducted at the Metabolic Bone Diseases Unit and Departments of Surgery and Obstetrics and Gynecology at Columbia University Medical Center (CUMC). Eligible patients were women aged 18 to 70 years old. Cases were severely obese women referred for bariatric surgery (body mass index [BMI] >35 kg/m2). Control patients were normal-weight patients (BMI <25 kg/m2) undergoing elective laparoscopic abdominal surgery for benign gynecologic indications. Exclusion criteria included known metabolic bone disease, current bisphosphonate treatment, transplant recipients, renal insufficiency (defined as Cr >1. 5 mg/dL), malignancy (except if cured for >5 years), endocrinopathy (including hyperthyroidism, Cushing’s), liver disease, intestinal malabsorptive disorders, and past or current use of glucocorticoids. Subjects were excluded from the study if they were using vitamin D supplements at doses >1200 IU daily.
Serum concentrations of 25OHD and body composition measurements were obtained preoperatively. Body composition measurements included assessment of total fat mass, lean mass, and percent fat mass via dual-energy X-ray absorptiometry (DXA) on QDR 4500A and Delphi W model densitometers (Hologic Inc, Waltham, Massachusetts, USA). Short-term in vivo precision for the body composition measurements at CUMC is 1%. Serum 25OHD2 and 25OHD3 were measured by ultra-performance liquid chromatography combined with tandem mass spectrometry (UPLC-MS/MS) using a 1290 UPLC and a 6410 Tandem Mass Spectrometer (Agilent, Santa Clara, CA, USA). Interassay coefficient of variation (CV) is 2.9% for 25OHD2 and 5.4% for 25OHD3.
Measurement of vitamin D2 and D3 in adipose tissue
Biopsies of subcutaneous fat and omental fat were obtained intra-operatively. Vitamin D2 and D3 were measured in adipose tissue by LC-MS/MS performed in the Heartland Assays Laboratory. The assay was originally described for high-performance liquid chromatography (HPLC) analysis(9) and modified for LC-MS/MS quantitation. Briefly, each specimen of 1 to 2 g of fat was added to 5mL of 50% methanolic KOH. Samples and standards were spiked with [6, 19, 19-d3]-vitamin D3 and [6, 19, 19-d3]-vitamin D2 for recovery estimates. After 1 to 2 hours at 60°C, 5 mL of water was added, samples were vortexed, and lipids were extracted 2× with hexane/ethyl acetate (85/15) by vortexing vigorously for 5 minutes. Samples were centrifuged to separate organic (hexane/ethyl acetate) and aqueous (methanol/water) layer. The hexane/ethyl acetate was removed, dried under vacuum, and the lipids applied to a Varian 0.5 g silica SPE column in 1 mL of 90/10 hexane/methylene chloride followed by a wash with the same solvent. The vitamin D was eluted from the column using a solution of 99.8/0.2 methylene chloride/isopropanol. The solvent was removed under vacuum and the sample purified using a quantitative Agilent Zorbax Silica column (0. 45 × 25 cm) developed with a mixture of 90% hexane/methylene chloride (85/15) and isopropanol/methanol (2/1, solvent B). Samples were collected, dried, and further purified using an Agilent Zorbax NH2 column developed in 93/7 hexane/solvent B. The vitamin D was collected and final quantitation of the vitamin D2 and vitamin D3 was achieved by LC-MS/MS using an Agilent 1200 Series Rapid Resolution LC (RRLC) system coupled with an Agilent 6460 Triple Quadrupole LC/MS with multimode ionization (MMI). Calibration curves were constructed for vitamin D2 and Vitamin D3. The inter- and intra-assay CVs for quantification were ≤12%. The lower limit of detection was 1.2 ng/g of tissue. Standard addition studies were performed to verify the accuracy of vitamin D quantitation using 10 samples spiked with 60 ng of vitamin D3 or vitamin D2. These studies revealed an accuracy of 98% to 108%. Extraction efficiencies of the [6, 19, 19-d3]-vitamin D3 and [6, 19, 19-d3]-vitamin D2 were similar and averaged 58 ± 5%. These estimates for recovery and accuracy were comparable to other published results.(10–14)
Statistical analysis
All analyses were conducted using SAS (version 9. 4; SAS Institute, Cary, NC, USA).
Two-sided p values < 0. 05 were considered to indicate statistical significance. Descriptive data are presented as mean ± standard deviation (SD). Normality testing was performed and positively skewed parameters were log-transformed before analysis. Differences in characteristics between groups were assessed using Student’s t test or chi-square. Three repeated-measures one-way ANOVAs (D2, D3, and total) were used to test the between-group differences in serum versus omental versus subcutaneous compartments using a Scheffé adjustment for post hoc comparisons. No adjustment was taken for the multiplicity of the three ANOVAs. The relationship between serum and adipose vitamin D was examined using Spearman correlations. Total body vitamin D stored in adipose tissue was estimated using DXA body composition measurement of fat mass, in grams, and the following formula: total vitamin D stores in adipose tissue = [(truncal fat mass) × (concentration total vitamin D in omental fat] + [(total limb fat mass) × (concentration of total vitamin D in subcutaneous fat].
Sample size estimates based on previously published data of concentrations of 103 ± 42 nmol/kg in obese subjects by Blum and colleagues(12) suggested that for 80% power with 5% two-tailed alpha, 15 subjects per group were required. A preliminary analysis to verify assumptions of the power analysis revealed higher serum 25OHD levels than anticipated in the obese group. We, therefore, increased enrollment to the obese group to 21 to improve our confidence that the outcomes were accurately estimated in this group.
Results
This study enrolled 21 obese and 15 control women. The baseline characteristics of the study groups are shown in Table 1. Groups were of similar age and race/ethnicity. Mean BMI in the obese group was 44 ± 4 kg/m2 compared with 23 ± 2 kg/m2 in the control group (p < 0.0001). Contrary to our expectations, serum 25OHD did not differ between groups (27 ± 2 ng/mL in obese versus 26 ± 2 ng/mL in control; p = 0. 71), likely reflecting the fact that most (n = 15; 71%) obese subjects were on supplemental vitamin D compared with only two controls. All obese subjects reported using cholecalciferol-containing supplements, with a mean daily dose of 747 ± 550 IU. One control reported using a daily multivitamin containing 100 IU of ergocalciferol and the other a vitamin with 400 IU of cholecalciferol.
Table 1.
Baseline Characteristics of Groups (Mean ± SD)
| Obese (n = 21) | Controls (n = 15) | p Value | |
|---|---|---|---|
| Age (years) | 42 ± 12 | 40 ± 8 | 0.56 |
| Ethnicity | 0.20 | ||
| Black | 2 (10%) | 0 | |
| White | 14 (67%) | 13 (93%) | |
| Hispanic | 5 (23%) | 1 (7%) | |
| Height (cm) | 177 ± 113 | 176 ± 48 | 0.99 |
| Weight (kg) | 107 ± 33 | 59 ± 11 | <0.0001 |
| BMI (kg/m2) | 44 ± 4 | 23 ± 2 | <0.0001 |
| % fat mass | 49.6 ± 4.7 | 29.5 ± 5.8 | <0.0001 |
| % lean mass | 50.4 ± 4.7 | 70.5 ± 5.8 | <0.0001 |
| % truncal mass | 50.9 ± 5.3 | 25.3 ± 7.1 | <0.0001 |
| % subtotal body fat | 51.0 ± 4.8 | 30.4 ± 6.2 | <0.0001 |
Vitamin D2 and D3 were assayed in both omental and subcutaneous fat compartments (Table 2). In 3 control subjects, there was insufficient subcutaneous fat and only omental samples were obtained. All subjects had measurable concentrations of vitamin D3 in both compartments. In contrast, vitamin D2 concentrations were undetectable in subcutaneous fat in 10% (2/21) of obese and 42% (5/12) of control subjects. Omental vitamin D2 concentrations were undetectable in 14% (3/21) of obese and 27% (4/15) of control subjects. The presence of vitamin D2 in fat was not significantly associated with current reported intake of supplements containing ergocalciferol.
Table 2.
Vitamin D in Fat and Serum (Mean ± SD)
| Obese | Control | p Value | |
|---|---|---|---|
| Fat compartment vitamin D (ng/g) | |||
| SUBQ vitamin D2 | 14.9 ± 5.2 | 2.2 ± 7.1 | 0.16 |
| SUBQ vitamin D3 | 19.6 ± 6.3 | 23.5 ± 8.5 | 0.88 |
| SUBQ total vitamin D | 34.2 ± 9.1 | 25.7 ± 12.3 | 0.63 |
| Omental vitamin D2 | 22.8 ± 8.6 | 3.7 ± 11.6 | 0.44 |
| Omental vitamin D3 | 28.0 ± 8.4 | 26.3 ± 11.4 | 0.72 |
| Omental total vitamin D | 50.6 ± 13.1 | 29.7 ± 17.6 | 0.37 |
| Serum 25(OH)D (ng/mL) | |||
| 25OHD2 | 5.1 ± 1.1 | 2.6 ± 0.8 | 0.08 |
| 25OHD3 | 21.8 ± 1.8 | 23.4 ± 2.4 | 0.54 |
| 25OHD total | 26.9 ± 1.6 | 25.9 ± 2.2 | 0.71 |
| Total body vitamin D stores (mg) | |||
| Vitamin D2 | 0.99 ± 0.4 | 0.04 ± 0.50 | 0.001 |
| Vitamin D3 | 1.31 ± 0.31 | 0.40 ± 0.42 | 0.01 |
| Vitamin D total | 2.29 ± 0.55 | 0.44 ± 0.76 | 0.002 |
The results of these analyses were unchanged after controlling for the season in which vitamin D measurements were performed. Neither concentration of vitamin D in omental or subcutaneous fat, nor serum 25OHD2 or 25OHD3 varied by season.
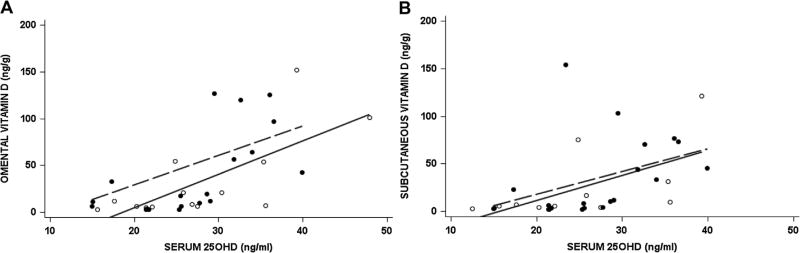
Serum and adipose concentrations of vitamin D were closely related in both groups (Fig. 1). The concentration of vitamin D in subcutaneous fat was significantly correlated with serum 25OHD in obese (r = 0. 66, p < 0. 01) and control subjects (r = 0. 64, p < 0. 03). Similarly, there was a significant correlation between omental vitamin D concentration and serum 25OHD in both obese (r = 0. 61, p < 0. 01) and control subjects (r = 0. 68, p < 0. 01).
Fig. 1.
Relationship between total serum 25OHD with total vitamin D in omental (A) and subcutaneous fat (B). Obese subjects are denoted by the dashed line and controls by the solid line.
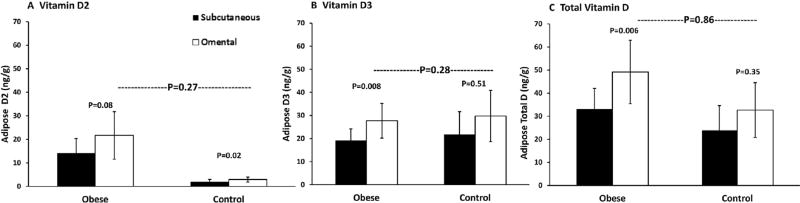
As expected, total body vitamin D stores, calculated using body composition measurements and fat biopsy data, were significantly greater in the obese compared with control subjects (Table 2). In contrast, the distribution of vitamin D between fat compartments did not differ according to degree of adiposity (Fig. 2). The distribution of vitamin D between the omental and subcutaneous fat compartments was similar in the obese and control groups (p between groups = 0.9). Total vitamin D concentrations were numerically higher in omental fat compared with subcutaneous fat in both obese and control subjects. However, the difference in total vitamin D and vitamin D3 between compartments was statistically significant only in the obese group (Fig. 2). Significantly higher vitamin D2 in omental fat was noted in both obese and control groups.
Fig. 2.
Distribution of vitamin D in subcutaneous (black bar) and omental (white bar) compartments among obese and control subjects. Vitamin D2 (A), D3 (B), and total D (C).
Discussion
To our knowledge, this is the first study that has measured vitamin D in both subcutaneous and omental fat compartments in obese and normal-weight control subjects. The data extend the limited prior information on vitamin D in the adipose tissue of the obese, confirming with increased accuracy their substantially greater total body stores of vitamin D and demonstrating that concentrations of vitamin D varied according to compartment. Specifically, vitamin D was higher in omental compared with subcutaneous fat. The findings suggest that vitamin D metabolism has more similarities than differences across the weight spectrum. Obese and non-obese individuals had comparable positive associations between serum 25OHD and concentration of vitamin D in their adipose tissue, and the overall distribution of vitamin D in different fat compartments similarly did not vary according to weight. These findings support the hypothesis of volumetric dilution as a cause for vitamin D deficiency in the obese. They suggest that a greater amount of vitamin D must be deposited to saturate the large mass of adipose tissue, which in the setting of inadequate intake may lead to lower serum levels.
We found a significant direct correlation between serum 25OHD and total vitamin D in both subcutaneous and omental fat and found a similar relationship in both the obese and non-obese subjects. These data amplify similar findings from most(12,14) but not all(13,15,16) prior studies, which assessed primarily subcutaneous adipose tissue and reported associations with both 25OHD(14) and serum vitamin D3.(12) Similarly, in a recent animal study, serum and adipose vitamin D were closely related.(17)
Prior studies on vitamin D storage in adipose tissue in non-obese(8) and obese(12–15) subjects have also focused primarily on subcutaneous fat.(12–15) Heaney and colleagues investigated the concentration of vitamin D2 and D3 in the subcutaneous tissue of non-obese but overweight individuals and also found that subjects had greater levels of D3 compared with D2 in their adipose tissue.(9) Despite similar 25OHD levels to our cohort, the vitamin D3 concentration in subcutaneous fat from this study was higher than that found in our control group, which may relate to differences in our study populations. They did not have data on omental adipose tissue. Blum and colleagues found a concentration of vitamin D3 in subcutaneous fat similar to our levels in an obese cohort(12) but lacked a normal BMI control group and data on omental fat. Only two prior studies of obese patients undergoing bariatric study measured vitamin D in omental fat. Unfortunately, in one study, data were only available on 4 subjects, making it impossible to draw conclusions about vitamin D distribution between compartments.(15) In the second study, higher total vitamin D and vitamin D3 concentrations were reported in omental versus subcutaneous fat, similar to our findings.(16) Neither of these studies included a control group.
We found that the overall distribution of vitamin D3 between the omental and subcutaneous fat compartments was similar in the obese and control subjects; both groups had higher concentrations of total vitamin D and vitamin D3 in omental fat. That this difference was only significant in the obese group may have been because of the smaller sample size and greater variability in values found in the control group. Few studies have measured vitamin D concentrations in both omental and subcutaneous adipose and, therefore, our data provide important evidence of the variability of vitamin D stores in different fat depots. This supports the findings of prior work in humans and animals.(16,18) Whether vitamin D metabolism varies in these different compartments is an important question that merits further exploration.
Vitamin D2, previously measured in only one study,(15,16) was undetectable in many subjects, particularly in the control group. Surprisingly, we did not find a significant relationship between vitamin D2 supplementation and concentration of vitamin D2 in adipose tissue. It is conceivable that vitamin D2 stores reflected past use in several subjects, as we did not have information regarding prior dosing or duration of supplementation. In addition, vitamin D2 may have been included in other supplements of which patients may have been unaware.
As expected, total vitamin D stores were higher in the obese group than in the control group. Prior studies have typically calculated total body vitamin D assuming similar fat concentrations throughout all adipose tissue.(9,13) Our data suggest that this assumption is not accurate. Our study calculated total vitamin D stores using the actual (and different) vitamin D3 concentrations measured in the various fat compartments, allowing a more accurate estimation. However, it is important to note that our method has not yet been validated. It also does not take into account intrahepatic or intramuscular fat stores, which may be substantial in obese individuals. If these compartments had been taken into account, it is likely that we would have observed an even greater difference in total adipose vitamin D stores between obese and control subjects. Alternative methods such as MRI would be necessary to directly quantify these depots.
The finding of increased adipose vitamin D stores in the obese supports the idea that the greater fat mass is a reservoir for vitamin D, which when filled results in larger total stores. However, because of this increased requirement, for a similar vitamin D intake, the serum 25OHD concentration may be lower in an obese compared with a non-obese subject. Several studies demonstrating that serum 25OHD increases with diet-induced weight loss further support this theory.(19,20) The available data after bariatric surgery is more difficult to interpret. Although increased 25OHD levels have been reported, findings are confounded by postoperative vitamin D supplementation.(16,21)
Although these data offer some insight into vitamin D metabolism in obesity, additional work is necessary to fully explain the etiology of low levels of 25OHD in the obese. The finding of a similar association in obese and non-obese subjects between serum 25OHD and total vitamin D in both subcutaneous and omental fat suggests that movement of vitamin D between fat and serum compartments is similar in normal-weight and obese individuals. These results support the hypothesis that lower 25OHD levels commonly found in the obese are at least in part related to volumetric dilution.(8) We cannot, however, conclude that no other hypothesis has merit. Although the finding of higher vitamin D in omental versus subcutaneous fat in the obese but not normal-weight subjects could reflect the smaller sample size of the control group, it could also suggest that some component of sequestration of vitamin D exists as well.
Our study has several limitations. First, the sample size was relatively small. Second, we cannot draw conclusions about vitamin D distribution in a more vitamin D–deficient obese cohort. Ideally, this study would have been conducted in a group of vitamin D–deficient obese patients. The subjects used represent a convenience sample of sequential subjects who agreed to enroll at our center. The fact that our obese subjects had better vitamin D status than expected likely reflects increased awareness of vitamin D deficiency by the medical community and the public and subsequently increased use of vitamin D supplements. This finding has been demonstrated in other recent studies examining bariatric surgery candidates.(22) Although it would have been preferable to only include subjects who were not using any supplementation, based on current clinical practice and the prevalence of supplement use in the general population, it was not possible to find many subjects who were not using any supplements. We also did not have information on sun exposure. However, we did show that our analyses were unchanged after controlling for the season in which vitamin D measurements were performed and that the concentration of vitamin D in omental and subcutaneous fat, and serum 25-hydroxyvitamin D2 and D3, did not vary by season. We assessed vitamin D intake using a questionnaire for supplement use but did not use diet diaries to determine intakes. This may have led to an underestimation of total vitamin D intake, though it would have affected both groups equally. Finally, we do not have data on 25OHD levels in tissue, nor of vitamin D2 or D3 in serum, although we did find a relationship between the serum 25OHD and vitamin D2 and D3 in adipose tissue. This information would have enabled us to more precisely investigate the relationships between serum and adipose vitamin D. Despite these limitations, our study has important strengths, including its novel design that assessed several vitamin D metabolites in two disparate fat compartments in both obese and normal-weight individuals.
In summary, this is the first controlled study investigating vitamin D concentrations in subcutaneous and omental fat in severely obese individuals. We demonstrated that vitamin D2 and D3 are measurable in subcutaneous and omental fat of individuals across the weight spectrum. We found that although obese subjects had significantly greater total vitamin D stores, the relationship between serum 25OHD and fat vitamin D and the overall pattern of distribution of vitamin D between the omental and subcutaneous fat compartments was similar in obese and normal-weight individuals. Our data support the hypothesis that the large amount of adipose tissue in obese individuals serves as a reservoir for vitamin D. The increased amount of vitamin D required to saturate this large reservoir may therefore predispose obese individuals to inadequate serum 25OHD. Future, larger studies are necessary to confirm these findings and further elucidate the nature of vitamin D storage and metabolism in the obese population.
Supplementary Material
Acknowledgments
This work was supported by K23 DK084337 (EMS) and K24 DK074457 (SJS).
Footnotes
Disclosures
All authors state that they have no conflicts of interest.
Authors’ roles: Study design: EMS, SC, and SJS. Study conduct: EMS, AC, and SJS. Data collection: EMS, AC, M Bucovsky, M Bessler, BS, JE, and JB. Data analysis: EMS, AC, SC, RH, and CZ. Data interpretation: EMS, AC, and SJS. Drafting manuscript: EMS, AC, and SJS. Revising manuscript content and approving final version of manuscript: all authors. EMS takes responsibility for the integrity of the data analysis.
References
- 1.Stein EM, Strain G, Sinha N, et al. Vitamin D insufficiency prior to bariatric surgery: risk factors and a pilot treatment study. Clin Endocrinol (Oxf) 2009;71(2):176–83. doi: 10.1111/j.1365-2265.2008.03470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Censani M, Stein EM, Shane E, et al. Vitamin D deficiency is prevalent in morbidly obese adolescents prior to bariatric surgery. ISRN Obes. 2013;2013 doi: 10.1155/2013/284516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Samuel L, Borrell LN. The effect of body mass index on adequacy of serum 25-hydroxyvitamin D levels in US adults: the National Health and Nutrition Examination Survey 2001 to 2006. Ann Epidemiol. 2014;24(10):781–4. doi: 10.1016/j.annepidem.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 4.Bell NH, Epstein S, Greene A, Shary J, Oexmann MJ, Shaw S. Evidence for alteration of the vitamin D-endocrine system in obese subjects. J Clin Invest. 1985;76(1):370–3. doi: 10.1172/JCI111971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hypponen E, Power C. Hypovitaminosis D in British adults at age 45 y: nationwide cohort study of dietary and lifestyle predictors. Am J Clin Nutr. 2007;85(3):860–8. doi: 10.1093/ajcn/85.3.860. [DOI] [PubMed] [Google Scholar]
- 6.Compston JE, Vedi S, Ledger JE, Webb A, Gazet JC, Pilkington TR. Vitamin D status and bone histomorphometry in gross obesity. Am J Clin Nutr. 1981;34(11):2359–63. doi: 10.1093/ajcn/34.11.2359. [DOI] [PubMed] [Google Scholar]
- 7.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72(3):690–3. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 8.Drincic AT, Armas LA, Van Diest EE, Heaney RP. Volumetric dilution, rather than sequestration best explains the low vitamin D status of obesity. Obesity. 2012;20(7):1444–8. doi: 10.1038/oby.2011.404. [DOI] [PubMed] [Google Scholar]
- 9.Heaney RP, Recker RR, Grote J, Horst RL, Armas LA. Vitamin D(3) is more potent than vitamin D(2) in humans. J Clin Endocrinol Metab. 2011;96(3):E447–52. doi: 10.1210/jc.2010-2230. [DOI] [PubMed] [Google Scholar]
- 10.Hollis BW. Individual quantitation of vitamin D2, vitamin D3, 25-hydroxyvitamin D2, and 25-hydroxyvitamin D3 in human milk. Anal Biochem. 1983;131(1):211–9. doi: 10.1016/0003-2697(83)90157-4. [DOI] [PubMed] [Google Scholar]
- 11.Hollis BW, Roos BA, Draper HH, Lambert PW. Vitamin D and its metabolites in human and bovine milk. J Nutr. 1981;111(7):1240–8. doi: 10.1093/jn/111.7.1240. [DOI] [PubMed] [Google Scholar]
- 12.Blum M, Dolnikowski G, Seyoum E, et al. Vitamin D(3) in fat tissue. Endocrine. 2008;33(1):90–4. doi: 10.1007/s12020-008-9051-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Didriksen A, Burild A, Jakobsen J, Fuskevag OM, Jorde R. Vitamin D3 increases in abdominal subcutaneous fat tissue after supplementation with vitamin D3. Eur J Endocrinol. 2015;172(3):235–41. doi: 10.1530/EJE-14-0870. [DOI] [PubMed] [Google Scholar]
- 14.Piccolo BD, Dolnikowski G, Seyoum E, et al. Association between subcutaneous white adipose tissue and serum 25-hydroxyvitamin D in overweight and obese adults. Nutrients. 2013;5(9):3352–66. doi: 10.3390/nu5093352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pramyothin P, Biancuzzo RM, Lu Z, Hess DT, Apovian CM, Holick MF. Vitamin D in adipose tissue and serum 25-hydroxyvitamin D after roux-en-Y gastric bypass. Obesity. 2011;19(11):2228–34. doi: 10.1038/oby.2011.170. [DOI] [PubMed] [Google Scholar]
- 16.Beckman LM, Earthman CP, Thomas W, et al. Serum 25(OH) vitamin D concentration changes after Roux-en-Y gastric bypass surgery. Obesity. 2013;21(12):E599–606. doi: 10.1002/oby.20464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burild A, Lauridsen C, Faqir N, Sommer HM, Jakobsen J. Vitamin D3 and 25-hydroxyvitamin D3 in pork and their relationship to vitamin D status in pigs. J Nutr Sci. 2016;5:e3. doi: 10.1017/jns.2015.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lawson DE, Douglas J, Lean M, Sedrani S. Estimation of vitamin D3 and 25-hydroxyvitamin D3 in muscle and adipose tissue of rats and man. Clin Chim Acta. 1986;157(2):175–81. doi: 10.1016/0009-8981(86)90223-8. [DOI] [PubMed] [Google Scholar]
- 19.Gangloff A, Bergeron J, Pelletier-Beaumont E, et al. Effect of adipose tissue volume loss on circulating 25-hydroxyvitamin D levels: results from a 1-year lifestyle intervention in viscerally obese men. Int J Obes. 2015;39(11):1638–43. doi: 10.1038/ijo.2015.118. [DOI] [PubMed] [Google Scholar]
- 20.Christensen P, Bartels EM, Riecke BF, et al. Improved nutritional status and bone health after diet-induced weight loss in sedentary osteoarthritis patients: a prospective cohort study. Eur J Clin Nutr. 2012;66(4):504–9. doi: 10.1038/ejcn.2011.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DiGiorgi M, Daud A, Inabnet WB, et al. Markers of bone and calcium metabolism following gastric bypass and laparoscopic adjustable gastric banding. Obes Surg. 2008;18(9):1144–8. doi: 10.1007/s11695-007-9408-4. [DOI] [PubMed] [Google Scholar]
- 22.Stein EM, Carrelli A, Young P, et al. Bariatric surgery results in cortical bone loss. J Clin Endocrinol Metab. 2013;98(2):541–9. doi: 10.1210/jc.2012-2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.