Abstract
Two new sesterterpenoids, 1 and 2, were isolated from the sponge Luffariella variabilis. Their planar structures were characterized with spectroscopic analyses. The sole chiral center of compound 1 was elucidated as 12R by comparing observed and calculated optical rotation values. The configurations of compound 2 were determined by NMR and electronic circular dichroism (ECD) studies. Furthermore, compound 2 showed cytotoxicity at IC50 1.0 µM against NBT-T2 cells.
Keywords: sesterterpenoid, sponge, cytotoxicity
1. Introduction
Among sessile marine macroorganisms, sponges have remained the richest sources of new bioactive molecules [1]. Those belonging to the family Thorectidae are known as sources of sesterterpenoids and other molecules [2,3,4,5,6,7,8,9,10,11,12]. The title sponge Luffariella variabilis, a representative species of this family, has been studied by several research groups and found to be prolific in secondary metabolites [2,3,4,7,11,12]. Of the metabolites, manoalide was originally reported as an antibiotic by Scheuer [2] from a Palauan specimen. It was found to have additional biological activity such as anti-inflammatory [3], inhibitory activity against phospholipase A2 [4] and NS3 helicase of hepatitis C virus [5], and has been distributed as a reagent for life science studies. Additional members of sesterterpenoids with a similar molecular scaffold have been reported from different collections of sponges of the same genus [6,7,8,9,10,11,12]. Here, we report structures of two new sesterterpenoids isolated from a specimen of L. variabilis collected at a deeper coral reef.
2. Results and Discussion
2.1. Structure Elucidation
As a part of collaborative efforts to search for antiviral molecules [13], we constructed a small library of Okinawan marine invertebrates. Although the lipophilic extract of the title sponge did not inhibit the growth of target viruses, it showed moderate cytotoxicity with a unique 1H-NMR spectrum. It was then sequentially separated with silica gel columns to give compounds 1 and 2 as glassy or oily substances.
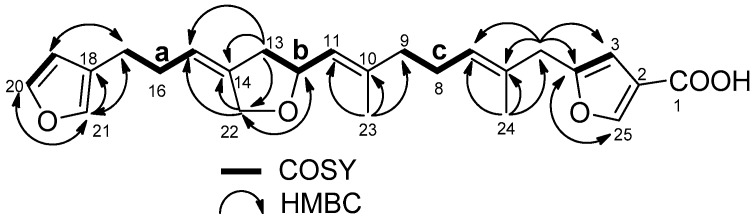
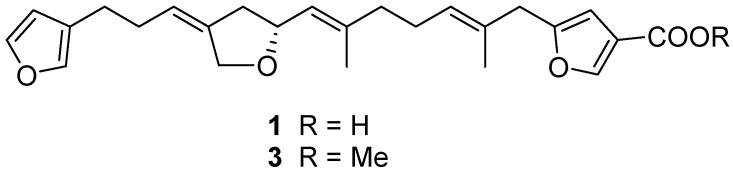
The molecular formula of compound 1 was determined as C25H30O5 by observing its sodiated ion at m/z 433.19909 (∆ + 1.2 ppm). As eight out of eleven degrees of unsaturation can be accounted for by the presence of one carbonyl (1715 cm−1, δC 167.0 s) and seven double bonds (14 signals in the range from δC 156.4 to 106.0), the molecule was determined to contain three rings. Of the double bonds, two were incorporated into a β-substituted furan (δH 7.34 brs, 7.21 brs, 6.26 brs; δC 142.7 d, 138.9 d, 124.5 s, 110.9 d), while another two were assigned to a disubstituted furan moiety (δH 7.96 brs, 6.38 brs; δC 156.4 s, 147.7 d, 119.0 s, 106.0 d) after NMR inspection (Figures S1 and S2). Using 2D NMR (Figures S3–S5) analyses, three other double bonds were involved in the following three substructures: (a) –CH2–CH2–CH=C< (δH 5.33 m; δC 139.8 s, 119.2 d), (b) –CH2–CH(O–)–CH=C< (δH 5.24 m; δC 139.7 s, 124.9 d), and (c) –CH2–CH2–CH=C< (δH 5.24 m; δC 130.9 s, 127.2 d) (Figure 1). The remaining pieces of the molecule were an oxymethylene (δH 4.39 d, 4.20 d; δC 68.3), an sp3 hybridized methylene (δH 3.28 s; δC 38.1), and two vinyl methyls (δH 1.70 brs, 1.60 brs; δcC 16.7, 15.9).
Figure 1.
Substructures a–c and selected HMBC correlations of compound 1.
The oxymethylene group was incorporated into a five-membered ether ring connecting the substructures a and b, as shown by the HMBC cross peaks: H-22/C-12,14,15, H-12/C-22, and H-13/C-14,15,22. The sp3 hybridized methylene at δH 3.28 was flanked by the disubstituted furan and an olefin connected to substructure c by observing H-5/C-3,4,6,7,24 and H-24/C-5,6,7. Finally, additional correlations of H-23/C-9,10,11, H-17/C-19,21, and H-19,21/C-17 (Figure 1) allowed us to connect all of the above structural units except for the carbonyl group. The carbonyl group was at first judged as an ester from the chemical shift, however, it gave a methyl ester 3 (m/z 447.21457; δH 3.81 s; 1731 cm−1, Figure S6) on treatment with TMSCHN2, confirming it as a carboxylic acid. Both the 1H and 13C-NMR signals (δH 7.96; δC 147.7) for C-25 shifted more downfield than other furan α-positions such as C-21 (δH 7.21; δC 138.9), likely caused by conjugation with the carbonyl group. These chemical shifts are also comparable to those of β-carboxylic furans [14]. The double bond geometry at C-6 and C-10 was assigned as E by the chemical shifts of the vinyl methyls (δC 15.9 (C-24) and 16.7 (C-23)) and nuclear Overhauser effects (NOEs) (H-5/H-7, H-9/H-11, H-23/H-12), while the configuration at C-14 was determined as Z with an NOE (H-13/H-15). With all the NMR signals assigned, the planar structure was determined, as depicted in Figure 2 and Table 1.
Figure 2.
Structures of compounds 1 and 3.
Table 1.
13C and 1H-NMR data of compounds 1 and 2 in CDCl3.
| No. | 1 | 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| 13C | 1H (J in Hz) | 13C | 1H (J in Hz) | |||||
| 1 | 167.0 | s | - | - | 166.5 | s | - | - |
| 2 | 119.0 | s | - | - | 128.2 | s | - | - |
| 3 | 106.0 | d | - | 6.38 brs | 140.0 | d | - | 6.63 brd (6.7) |
| 4 | 156.4 | s | - | - | 23.8 | t | a | 2.34 m |
| - | - | - | - | - | - | - | b | 2.21 ddd (3.1, 6.7, 17.7) |
| 5 | 38.1 | t | - | 3.28 s (2H) | 81.9 | d | - | 4.30 dd (3.1, 12.9) |
| 6 | 130.9 | s | - | - | 38.9 | s | - | - |
| 7 | 127.2 | d | - | 5.24 m | 31.8 | t | α | 1.56 m |
| - | - | - | - | - | - | - | β | 1.30 m |
| 8 | 26.3 | t | - | 2.16 m (2H) | 17.4 | t | - | 1.51 m (2H) |
| 9 | 39.2 | t | - | 2.06 m (2H) | 37.8 | t | α | 0.97 dt (3.1, 12.6) |
| - | - | - | - | - | - | - | β | 1.67 m |
| 10 | 139.7 | s | - | - | 38.9 | s | - | - |
| 11 | 124.9 | d | - | 5.24 m | 46.6 | d | - | 1.73 dd (2.6, 11.3) |
| 12 | 75.9 | d | - | 4.56 dt (8.4, 5.9) | 17.5 | t | α | 1.55 m |
| - | - | - | - | - | - | - | β | 1.47 m |
| 13 | 39.4 | t | a | 2.58 dd (5.9, 15.0) | 41.6 | t | α | 1.65 m |
| - | - | - | b | 2.23 m | - | - | β | 1.70 m |
| 14 | 139.8 | s | - | - | 73.0 | s | - | - |
| 15 | 119.2 | d | - | 5.33 m | 58.8 | d | - | 1.05 brs |
| 16 | 29.9 | t | - | 2.16 m (2H) | 26.0 | t | a | 1.68 m |
| - | - | - | - | - | - | - | b | 1.54 m |
| 17 | 24.6 | t | - | 2.48 t (7.5) (2H) | 28.4 | t | a | 2.44 m |
| - | - | - | - | - | - | - | b | 2.39 m |
| 18 | 124.5 | s | - | - | 125.4 | s | - | - |
| 19 | 110.9 | d | - | 6.26 brs | 110.9 | d | - | 6.29 brs |
| 20 | 142.7 | d | - | 7.34 brs | 142.6 | d | - | 7.35 brt (1.5) |
| 21 | 138.9 | d | - | 7.21 brs | 138.6 | d | - | 7.25 brs |
| 22 | 68.3 | t | a | 4.39 d (13.0) | 30.5 | q | - | 1.18 s |
| - | - | - | b | 4.20 d (13.0) | - | - | - | - |
| 23 | 16.7 | q | - | 1.70 brs | 15.9 | q | - | 1.03 s |
| 24 | 15.9 | q | - | 1.60 brs | 17.4 | q | - | 0.87 s |
| 25 | 147.7 | d | - | 7.96 brs | 16.9 | q | - | 1.92 s |
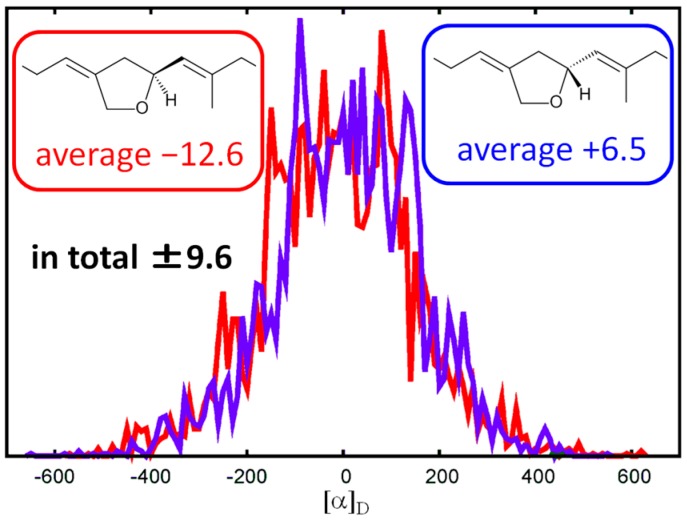
To elucidate the absolute configuration, we calculated the specific rotation values of (12S)-1 and (12R)-1 with molecular dynamics (MD) simulations and time-dependent density functional theory (TDDFT). After generating 5000 structures from a 500-ns MD trajectory for each chiral molecule in CH2Cl2 solutions, we calculated the specific rotation values with the TDDFT method. The resulting averaged optical rotation values of (12S)-1 and (12R)-1 were calculated as −9.6 and +9.6 (Figure 3). Since the natural 1 presented a specific rotation value of [α]D +11, it is likely to have 12R configuration.
Figure 3.
Distribution of calculated specific rotation values.
Compound 2 showed a sodiated ion at m/z 423.25046 (∆ − 1.6 ppm) in high resolution electrospray ionization mass spectrometry (HRESIMS), establishing the molecular formula C25H36O4 with eight degrees of unsaturation. As in compound 1, a β-substituted furan moiety was deduced from the signals for α-positions at δH 7.35, 7.25; δC 142.6, 138.6 and for β-positions at δH 6.29; δC 125.4, 110.9 (Figures S7 and S8). The presence of an α,β-unsaturated δ-lactone moiety was inferred by observing an ester carbonyl (1705 cm−1; δH 166.5 s, C-1), conjugated olefinic signals (δH 6.63 brd; δC 128.2, 140.0, C-2,3), allylic methylene protons at δH 2.34 m and 2.21 ddd (H-4a,4b), and an oxymethine signal at δH 4.30; δC 81.9 (C-5). The lactone moiety was also confirmed by HMBC correlations (Figure S10) from a vinyl methyl at δH 1.92 s (H-25) to C-1,2,3 (δC 166.5, 128.2, 140.0) and from the vinyl proton H-3 to C-1,4,5,25 and COSY cross peaks (H-5/H-4a,4b and H-3/H-4a,25, Figure S11) and by a comparison of NMR data with those of schisanlactone B and colossolactone G [15,16]. The remaining two degrees of unsaturation are assignable to two additional rings.
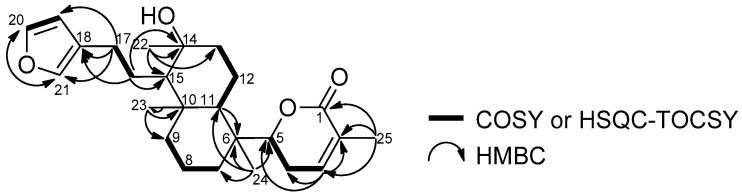
By analyzing the HMBC correlations from three singlet methyls at δH 1.18 (H-22), 1.03 (H-23), and 0.87 (H-24), it was straightforward to construct a partial structure starting from C-24 via C-6,7,11,10,9,15,14,13 to C-22. The two remaining methylenes at C-8 (δC 17.4) and C-12 (δC 17.5) were involved in the bicyclic portion by Heteronuclear Single Quantum Correlation-Total Correlation Spectroscopy (HSQC-TOCSY, Figure S12) correlations (H-7α C-8,9, H-11/C-12,13). Therefore, the decaline ring was confirmed. Two allylic methylene protons at δH 2.44 and 2.39 (H-17a,17b) were assigned next to the furan by observing HMBC correlations to furan carbons (H-17a,17b/C-18,19,21) and another methylene at C-16 (H-16/C-14,15,17,18). Since the δ-lactone moiety was confirmed to be attached to C-6 by observing an HMBC cross peak (H-24/C-5), the whole planar structure of compound 2 was determined, as depicted in Figure 4.
Figure 4.
Selected 2D NMR correlations of compound 2.
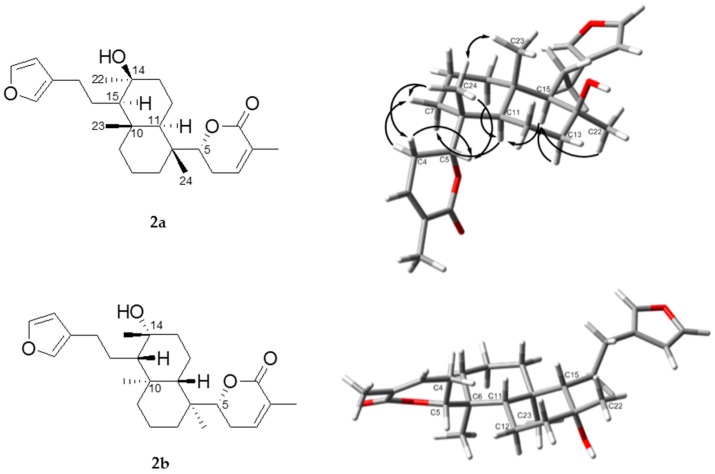
The absolute configuration of the δ-lactone was determined as R on the basis of its positive Cotton effect at 253 nm (∆ε + 27.6, Figure S13), which sign is the same as that of (+)-parasorbic acid with the same δ-lactone moiety [17]. The bicyclic portion takes the trans-decaline ring system with chair conformation by observing NOEs for H-15/H-11,22 and H-23/H-24 (Figure S14). Therefore, two candidate structures, 2a and 2b, are possible for the absolute configuration of compound 2. In both cases, there is steric hindrance between the methylene protons at C-4 and at C-12. In fact, we could not observe any NOE between the methylene protons, confirming the restricted rotation between C-5/C-6. The DFT calculation also showed that one conformer is dominant with respect to the rotation between C-5/C-6 (Figure 5). The observed NOE between H-4b/H-24 and the absence of NOEs between H-4a,4b/H-11 support the structure 2a, but not 2b. In conclusion, the configurations of compound 2 were elucidated as 5R, 6R, 10S, 11R, 14S, and 15R.
Figure 5.
Structures of 2a and 2b and selected NOEs in arrows. The structures were optimized with the density functional theory (DFT) method at the IEFPCM (CHCl3)/B3LYP/6-31G(d) level. Red color means oxygen atom.
2.2. Biological Activity
Compounds 1–3 were tested against cultured NBT-T2 cells by MTT assay. Compounds 1 and 2 showed cytotoxicity at IC50 47.5 and 1.0 µM, while compound 3 did not.
3. Conclusions
Two new furanosesterterpenoids 1 and 2 were isolated from the Okinawan sponge L. variabillis, and their absolute stereochemistry was elucidated with spectroscopic analyses and calculation. Of the two molecules, compound 2 may be the first member with this skeleton. Specimens of L. variabilis collected at shallower coral reefs in Okinawa usually contain manaoalide as the major constituent, while the current specimen collected from a deeper reef at 60 m contained new sesterterpenoids without manoalide. We have previously encountered metabolite variation with depth [18], and the current work may be another example of this.
4. Materials and Methods
4.1. General Experimental Procedure
NMR spectra were recorded on a Bruker AVANCE III 500 spectrometer (Billerica, MA, USA). ESI mass spectra were taken on a Jeol JMS-T 100LP mass spectrometer (Tokyo, Japan). HPLC separation was done on a unit equipped with a Shimadzu LC-10AD pump (Kyoto, Japan), a Shimadzu SPD-10A UV detector, a Shodex RI-101 refractive index detector (Tokyo, Japan), and a Nacalai Cosmosil 5SL-II column (4.6 × 250 mm) (Kyoto, Japan). Specific rotation was observed on a Jasco P-1010 polarimeter (Tokyo, Japan). FTIR spectrum was measured on a Jasco FT/IR-300 spectrophotometer. ECD spectrum was obtained on a Jasco J-820 spectropolarimeter.
4.2. Specimen
The specimen was collected by hand using a trimix rebreather at a depth of 60 m from a reef near Iriomote Island, Okinawa, in July 2013, and kept frozen until extraction. The specimen was identified as Luffariella variabilis by one of the authors (NJdV) and deposited at the Naturalis Biodiversity Center with the code RMNH POR 8677.
4.3. Extraction and Isolation
After thawing, the sponge (57 g, wet) was cut and macerated ca. 12 h in acetone (150 mL), three times. The acetone solution was filtered and concentrated under vacuum, and the residue was partitioned between EtOAc and H2O. The organic layer gave an extract (770 mg) after concentration. A portion (260 mg) of the extract was separated on a Merck silica gel 60 (63–200 µm) column using solvents stepwise, namely, n-hexane, n-hexane-EtOAc (9-1, 7-3, 1-1), EtOAc, EtOAc-MeOH (1-1), and MeOH, to give eight fractions. A portion (18.2 mg) of the seventh fraction (54.4 mg) was passed through a silica Sep-Pak® 3 cc Vac cartridge (200 mg) with EtOAc to remove polar impurities, and its first fraction (14.6 mg) was subjected to HPLC (Cosmosil 5SL-II, n-hexane-EtOAc, 2-3) to give compound 1 (2.0 mg, 3.3% from the extract). A further amount of compound 1 was obtained with similar treatment. A portion (12.7 mg) of the fourth fraction was purified via HPLC (Cosmosil 5SL-II, n-hexane-EtOAc, 4-1) to give compound 2 (7.0 mg, 6.5%).
Compound 1: Glass. 1H and 13C-NMR (CDCl3) see Table 1. HRESIMS m/z 433.19909, calcd. for C25H30O5Na 433.19854 (∆ + 1.2 ppm). FTIR (neat) 2923, 1715, 1695, 1549, 1295, 1198, 767 cm−1. [α] + 11 (c 0.14, CH2Cl2).
Compound 2: Colorless oil. 1H and 13C-NMR (CDCl3) see Table 1. HRESIMS m/z 423.25046, calcd. for C25H36O4Na 423.25113 (∆ − 1.6 ppm). FTIR (neat) 3498, 2928, 1705, 1243, 1136, 781 cm−1. ECD (MeOH) nm (∆ε) 253 nm (+27.6). [α] + 68 (c 0.18, MeOH). UV (MeOH) 212 nm (logε 4.1).
4.4. Methylation of Compound 1 to 3
Eight drops of a solution of TMSCHN2 in hexane were added to compound 1 (0.2 mg) in MeOH (200 µL). After allowing the mixture to stand for 5 min at room temperature, it was concentrated with N2 flow to give 0.2 mg of the ester 3.
Compound 3: HRESIMS m/z 447.21457, calcd. for C26H32O5Na 447.21419 (∆ +0.8 ppm). FTIR (neat) 1731 cm−1. 1H-NMR (CDCl3) δ 7.88 (1H, s, H-25), 7.34 (1H, bs, H-20), 7.21 (1H, bs, H-21), 6.35 (1H, bs, H-3), 6.27 (1H, bs, H-19), 5.33 (1H, m, H-15), 5.23 (1H, m, H-7), 5.23 (1H, m, H-11), 4.55 (1H, dt, J = 5.8, 8.7 Hz, H-12), 4.38 (1H, d, J = 13.3 Hz, H-22a), 4.20 (1h, d, J = 13.3 Hz, H-22b), 3.81 (3H, s, OMe), 3.28 (2H, s, H-5), 2.58 (1H, dd, J = 5.5, 15.3 Hz, H-13a), 2.48 (2H, t, J = 7.4 Hz, H-17), 2.22 (1H, m, H-13b), 2.16 (2H, m, H-8), 2.16 (2H, m, H-16), 2.05 (2H, m, H-9), 1.70 (3H, s, H-23), 1.60 (3H, s, H-24).
4.5. Cytotoxicity Assay
NBT-T2 cells were incubated at 37 °C under 5% CO2 for 24 h in a 96-well plate containing 100 µL of Dulbecco’s Modified Eagle’s Medium (DMEM). An aliquot (1 µL) of DMSO solution of compounds 1, 2, and 3 was dispensed into each well in triplicate for each concentration and the cells were incubated for 48 h. Doxorubicin was used as a positive control, showing cytotoxicity at IC50 0.5 µM. A 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) solution was prepared by dissolving 5 mg of MTT in 1 mL of phosphate-buffered saline (PBS), and the solution was added to 10 mL of DMEM. After removing the culture media and dispensing 100 µL of the MTT solution, the wells were incubated for additional 3 h. The solution was then removed and 100 µL of DMSO was added to each well. The absorbance of each well was measured at 570 nm using a microplate reader. IC50 values were obtained by analyzing the data on Kaleida Graph software (version 4.1, Synergy Software, Reading, PA, USA).
4.6. Calculation of Specific Rotation Value
We first performed the MD simulation to sample various conformations of compound 1 in CH2Cl2 solution. The system consisted of one compound 1 and 3375 CH2Cl2 molecules. A cubic unit cell with the periodic boundary condition was employed, using a box length of 72.5 Å. The general Amber force field (GAFF) [19] was used for compound 1 and CH2Cl2. Long-range electrostatic interactions were treated with the particle mesh Ewald method. Bonds involving hydrogen were constrained by using the SHAKE method. The equations of motion were integrated using the leapfrog algorithm with a time step of 1 fs at a temperature of 300 K. The MD simulation was performed for 500 ns. Next, 5000 geometries of compound 1 taken from the MD trajectory were optimized with the DFT method at the IEFPCM(CH2Cl2)/B3LYP/6-31G(d) level. Finally, specific rotation values were calculated with the TDDFT method at the IEFPCM(CH2Cl2)/B3LYP/aug-cc-pVDZ level [20]. We performed this procedure for both 12S and 12R configurations. The distributions of specific rotation values for (12S)-1 and (12R)-1 were almost antisymmetric (Figure 1), indicating that the number of samples was sufficient. The specific rotation values averaged over 5000 samples were −12.6 and +6.5 for (12S)-1 and (12R)-1, respectively. Further averaging the values over the two configurations, the values of (12S)-1 and (12R)-1 were −9.6 and +9.6, respectively. The MD simulations and TDDFT calculations were performed using the AMBER 12 [21] and Gaussian 09 [22] program packages at the Research Center for Computational Science, Okazaki, Japan.
Acknowledgments
This research was financially supported by a grant of Okinawa Intellectual Cluster Programs from Okinawa Science and Technology Center (OSTC), an affiliated organization of Okinawa Prefecture. At the initial stage of this study, we were indebted to researchers of Meiji Seika Pharma Co., Ltd. (Yokohama, Japan). This research was also funded by MEXT/JSPS (No. 16H00778, 16KT0165 and 17K05757). The authors thank Tohru Naruse, University of the Ryukyus, for the assistance on the collection of the sponge.
Supplementary Materials
The following are available online at www.mdpi.com/1660-3397/15/8/249/s1. Figure S1: 1H-NMR spectrum of compound 1; Figure S2: 13C-NMR spectrum of compound 1; Figure S3: HSQC spectrum of compound 1; Figure S4: HMBC spectrum of compound 1; Figure S5: COSY spectrum of comound 1; Figure S6: 1H-NMR spectrum of compound 3; Figure S7: 1H-NMR spectrum of compound 2; Figure S8: 13C-NMR spectrum of compound 2; Figure S9: HSQC spectrum of compound 2; Figure S10: HMBC spectrum of compound 2; Figure S11: COSY spectrum of compound 2; Figure S12: HSQC-TOCSY spectrum of compound 2; Figure S13: ECD spectrum of compound 2; Figure S14: NOESY spectrum of compound 2.
Author Contributions
Peni Ahmadi did all the chemical and biological analyses and prepared a draft. Masahiro Higashi calculated the specific rotation values to estimate the absolute configuration of compound 1 and analyzed conformation of structures 2a and 2b. Nicole J. de Voogd identified the sponge. Junichi Tanaka conceived this study, collected the sponge, and revised the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Blunt J.W., Copp B.R., Keyzers R.A., Munro M.H.G., Prinsep M.R. Marine Natural Products. Nat. Prod. Rep. 2015;32:116–211. doi: 10.1039/C4NP00144C. [DOI] [PubMed] [Google Scholar]
- 2.De Silva E.D., Scheuer P.J. Manoalide, an antibiotic sesterterpenoid from the marine sponge Luffariella variabilis (polejaff) Tetrahedron Lett. 1980;21:1611–1614. doi: 10.1016/S0040-4039(00)77766-5. [DOI] [Google Scholar]
- 3.De Silva E.D., Scheuer P.J. Three new sesterterpenoids antibiotic from the marine sponge Luffariella variabilis (polejaff) Tetrahedron Lett. 1981;22:3147–3150. doi: 10.1016/S0040-4039(01)81849-9. [DOI] [Google Scholar]
- 4.Kernan M.R., Faulkner D.J., Jacobs R.S. The luffariellins, novel anti-inflammatory sesterterpenes of chemotaxonomic importance from the marine sponge Luffariella variabilis. J. Org. Chem. 1987;52:3081–3083. doi: 10.1021/jo00390a021. [DOI] [Google Scholar]
- 5.Salam K.A., Furuta A., Noda N., Tsuneda S., Sekiguchi Y., Yamashita A., Moriishi K., Nakanoshi M., Tsubuki M., Tani H., et al. Inhibition of hepatitis C virus NS3 helicase by manoalide. J. Nat. Prod. 2012;75:650–654. doi: 10.1021/np200883s. [DOI] [PubMed] [Google Scholar]
- 6.König G.M., Wright A.D., Sticher O. Four new antibacterial sesterterpenes from a marine sponge of the genus Luffariella. J. Nat. Prod. 1992;55:174–178. doi: 10.1021/np50080a004. [DOI] [PubMed] [Google Scholar]
- 7.Potts B.C.M., Capon R.J., Faulkner D.J. Luffalactone and (4E,6E)-dehydromanoalide from the sponge Luffariella variabilis. J. Org. Chem. 1992;57:2965–2967. doi: 10.1021/jo00036a043. [DOI] [Google Scholar]
- 8.Tsuda M., Shigemori H., Ishibashi M., Sasaki T., Kobayashi J. Luffariolides A-E, new cytotoxic sesterterpenes from the Okinawan marine sponge Luffariella sp. J. Org. Chem. 1992;57:3503–3507. doi: 10.1021/jo00038a051. [DOI] [Google Scholar]
- 9.Kobayashi J., Zeng C.-M., Ishibashi M. Luffariolides F and G, new manoalide derivatives from the Okinawan marine sponge Luffariella sp. J. Nat. Prod. 1993;56:435–439. doi: 10.1021/np50093a020. [DOI] [PubMed] [Google Scholar]
- 10.Tsuda M., Endo T., Mikami Y., Fromont J., Kobayashi J. Luffariolides H and J, new sesterterpenes from a marine sponge Luffariella species. J. Nat. Prod. 2002;65:1507–1508. doi: 10.1021/np0202071. [DOI] [PubMed] [Google Scholar]
- 11.Ettinger-Epstein P., Motti C.A., de Nys R., Wright A.D., Battershill C.N., Tapiolas D.M. Acetylated sesterterpenes from the Great Barrier reef sponge Luffariella variabilis. J. Nat. Prod. 2007;70:648–651. doi: 10.1021/np060240d. [DOI] [PubMed] [Google Scholar]
- 12.Gauvin-Bialecki A., Aknin M., Smadja J. 24-O-ethylmanoalide, a manoalide-related sesterterpene from the marine sponge Luffariella cf. variabilis. Molecules. 2008;13:3148–3191. doi: 10.3390/molecules13123184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmadi P., Haruyama T., Kobayashi N., de Voogd N.J., Tanaka J. Spongian diterpenes from the sponge Hyatella aff. intestinalis. Chem. Pharm. Bull. 2017 doi: 10.1248/cpb.c17-00297. [DOI] [PubMed] [Google Scholar]
- 14.Park S.K., Scheuer P.J. Isolation and structure determination of two furanosesquiterpenes from the soft coral Sinularia iochmodes. J. Korean Chem. Soc. 1994;38:749–752. [Google Scholar]
- 15.Liu J.-S., Huang M.-F., Ayer W.A., Bigam G. Schisanlactone B, a new triterpenoid from a Schisandra sp. Tetrahedron Lett. 1983;24:2355–2358. doi: 10.1016/S0040-4039(00)81923-1. [DOI] [Google Scholar]
- 16.Lakornwong W., Kanokmedhakul K., Kanokmedhakul S., Kongsaeree P., Prabpai S., Sibounavong P., Soytong K. Triterpene lactones from cultures of Ganoderma sp. KM01. J. Nat. Prod. 2014;77:1545–1553. doi: 10.1021/np400846k. [DOI] [PubMed] [Google Scholar]
- 17.Beecham A.F. The CD of αβ-unsaturated lactones. Tetrahedron. 1972;28:5543–5554. doi: 10.1016/S0040-4020(01)93618-X. [DOI] [Google Scholar]
- 18.Faricha A., Ahmadi P., de Voogd N.J., Tanaka J. Two isospongian diterpenes from the sponge Luffariella sp. Nat. Prod. Commun. 2017;12:1011–1012. [Google Scholar]
- 19.Wang J., Wolf R.M., Caldwell J.W., Kollman P.A., Case D.A. Development and testing of a general Amber force field. J. Comput. Chem. 2004;25:1157–1174. doi: 10.1002/jcc.20035. [DOI] [PubMed] [Google Scholar]
- 20.Stephens P.J., Devlin F.J., Cheeseman J.R., Frisch M.J. Calculation of optical rotation using density functional theory. J. Phys. Chem. A. 2001;105:5356–5371. doi: 10.1021/jp0105138. [DOI] [Google Scholar]
- 21.Case D.A., Darden T.A., Cheatham T.E., III, Simmerling C.L., Wang J., Duke R.E., Luo R., Walker R.C., Zhang W., Merz K.M., et al. AMBER 12. University of California; San Francisco, CA, USA: 2012. [Google Scholar]
- 22.Frisch M.J., Trucks G.W., Schlegel H.B., Scuseria G.E., Robb M.A., Cheeseman J.R., Scalmani G., Barone V., Mennucci B., Petersson G.A., et al. Gaussian 09. Gaussian, Inc.; Wallingford, CT, USA: 2009. Revision D.01. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.