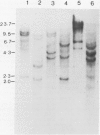
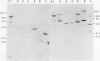
Abstract
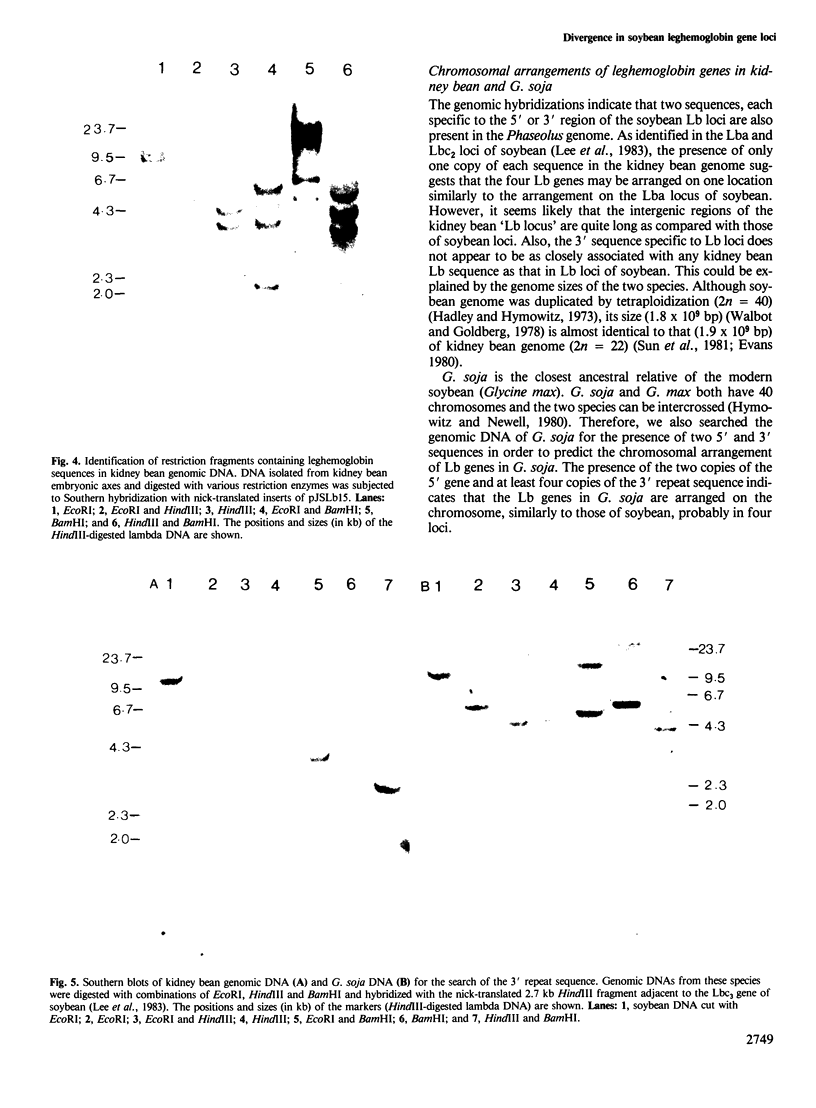
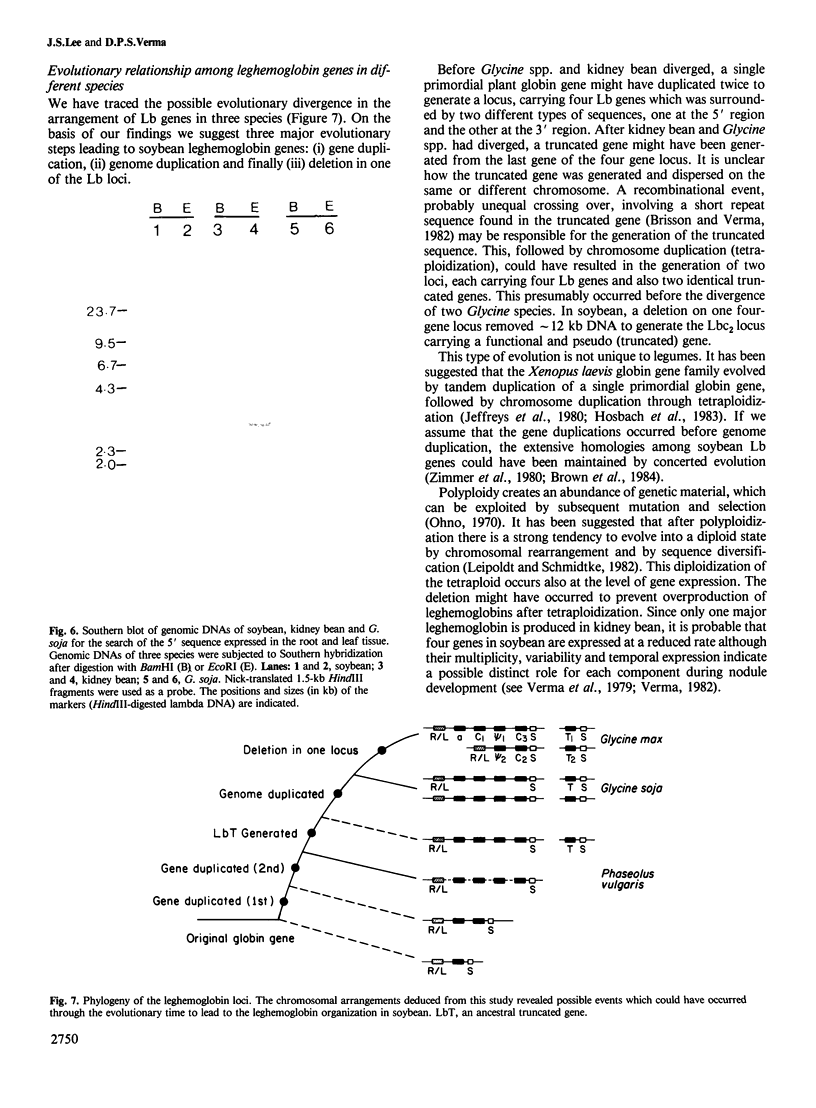
We have determined the structure of one of the leghemoglobin (Lb) genes of Phaseolus vulgaris (kidney bean) and deduced the chromosomal arrangement of leghemoglobin genes by genomic hybridizations with Lb and two other sequences, each specific to the 5' or 3' region of the soybean leghemoglobin loci. By comparing this organization with two other species of legumes, Glycine max (soybean) and G. soja (wild soybean), a phylogeny of leghemoglobin gene loci was traced. The intragenic structure of the kidney bean leghemoglobin gene shows the same intron/exon arrangement as that of soybean leghemoglobin genes and extensive sequence homologies in both coding as well as 5' and 3' non-coding regions. The presence in the kidney bean genome of four leghemoglobin genes suggests that tandem duplications of a single primordial plant globin gene had occurred to generate four leghemoglobin genes in an `Lb-locus' before Glycine and Phaseolus species diverged. Chromosome duplication by tetraploidization in Glycine generated two loci containing four genes each. A large deletion in one of the two four-gene loci in soybean resulted in the generation of the Lbc2 locus containing two leghemoglobin genes, one functional and another pseudo (LbΨ2). This pseudogene, unlike that present on the main locus, is represented by only two and a half exons and appears to be truncated. The two other truncated genes (LbT1 and LbT2) were probably generated similarly in the genome of Glycine spp. following tetraploidization before the divergence of G. max and G. soja.
Keywords: leghemoglobin, gene organization, Phaseolus vulgaris, tetraploidization
Full text
PDF
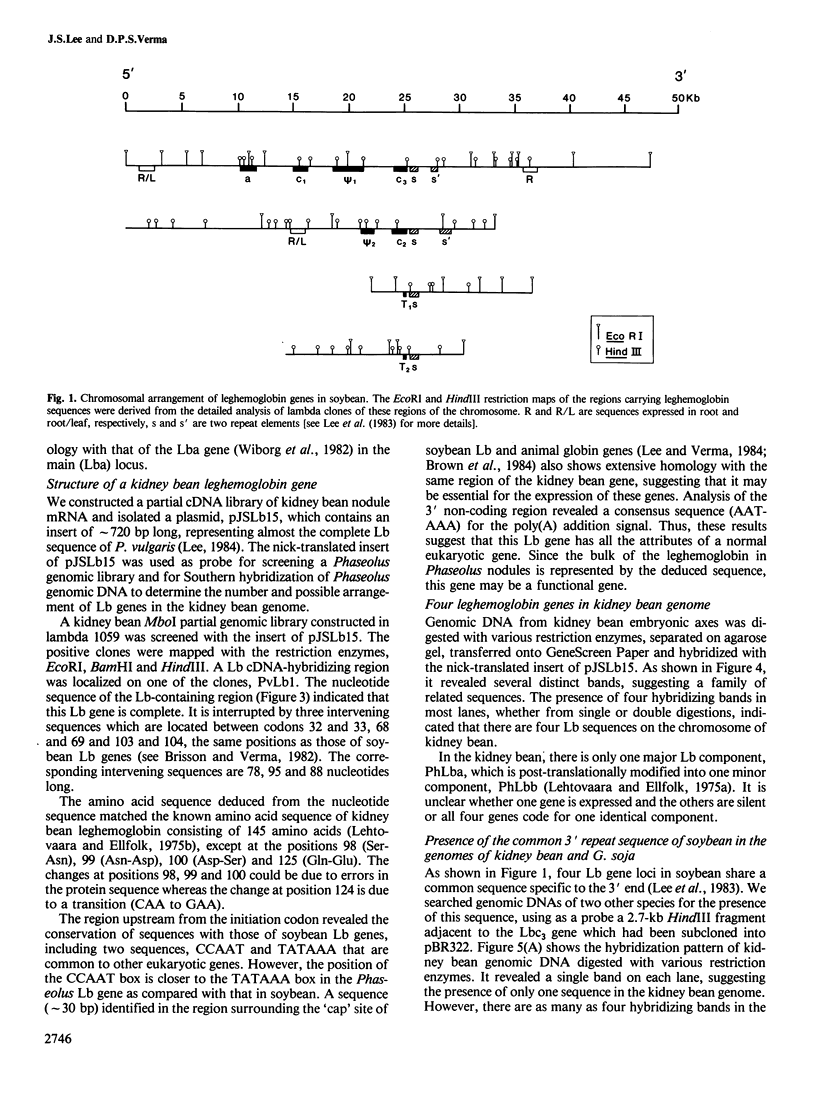
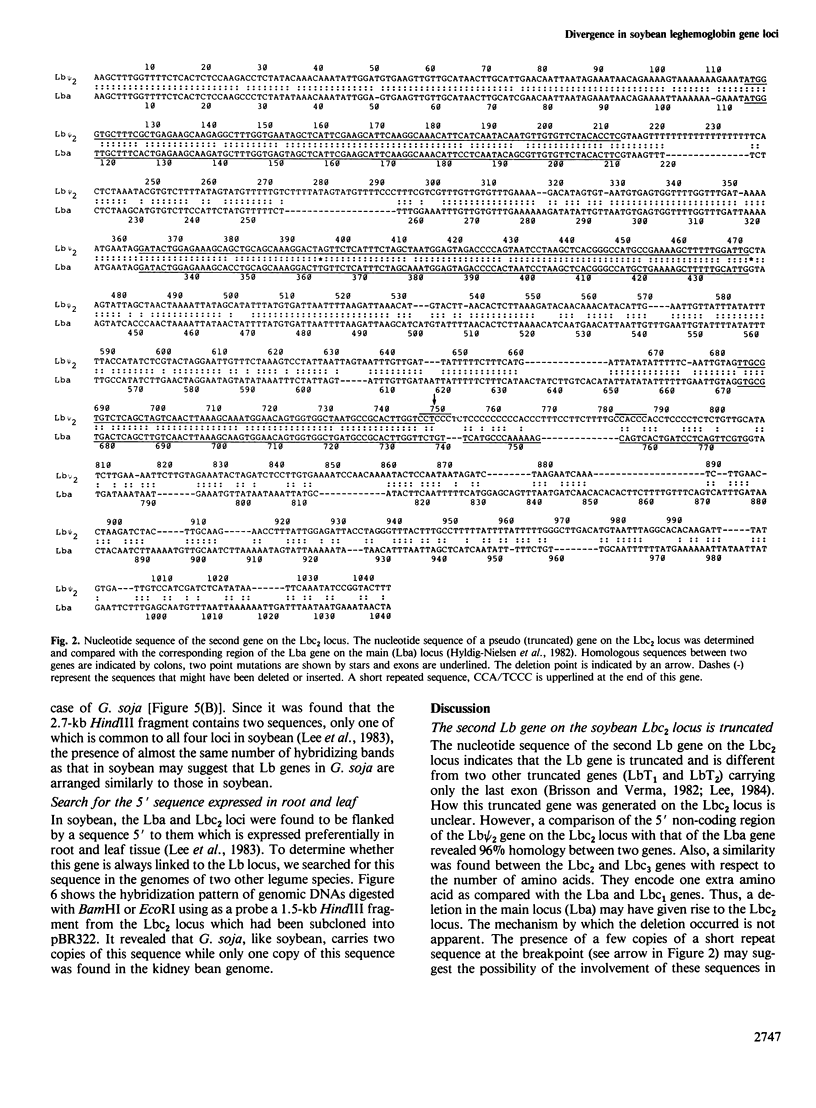



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleby C. A., Tjepkema J. D., Trinick M. J. Hemoglobin in a nonleguminous plant, parasponia: possible genetic origin and function in nitrogen fixation. Science. 1983 May 27;220(4600):951–953. doi: 10.1126/science.220.4600.951. [DOI] [PubMed] [Google Scholar]
- Auger S., Baulcombe D., Verma D. P. Sequence complexities of the poly(A)-containing mRNA in uninfected soybean root and the nodule tissue developed due to the infection by Rhizobium. Biochim Biophys Acta. 1979 Jul 26;563(2):496–507. doi: 10.1016/0005-2787(79)90068-6. [DOI] [PubMed] [Google Scholar]
- Blake C. Exons--present from the beginning? Nature. 1983 Dec 8;306(5943):535–537. doi: 10.1038/306535a0. [DOI] [PubMed] [Google Scholar]
- Blattner F. R., Williams B. G., Blechl A. E., Denniston-Thompson K., Faber H. E., Furlong L., Grunwald D. J., Kiefer D. O., Moore D. D., Schumm J. W. Charon phages: safer derivatives of bacteriophage lambda for DNA cloning. Science. 1977 Apr 8;196(4286):161–169. doi: 10.1126/science.847462. [DOI] [PubMed] [Google Scholar]
- Brisson N., Pombo-Gentile A., Verma D. P. Organization and expression of leghaemoglobin genes. Can J Biochem. 1982 Mar;60(3):272–278. doi: 10.1139/o82-032. [DOI] [PubMed] [Google Scholar]
- Brisson N., Verma D. P. Soybean leghemoglobin gene family: normal, pseudo, and truncated genes. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4055–4059. doi: 10.1073/pnas.79.13.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efstratiadis A., Posakony J. W., Maniatis T., Lawn R. M., O'Connell C., Spritz R. A., DeRiel J. K., Forget B. G., Weissman S. M., Slightom J. L. The structure and evolution of the human beta-globin gene family. Cell. 1980 Oct;21(3):653–668. doi: 10.1016/0092-8674(80)90429-8. [DOI] [PubMed] [Google Scholar]
- Fuller F., Künstner P. W., Nguyen T., Verma D. P. Soybean nodulin genes: Analysis of cDNA clones reveals several major tissue-specific sequences in nitrogen-fixing root nodules. Proc Natl Acad Sci U S A. 1983 May;80(9):2594–2598. doi: 10.1073/pnas.80.9.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S., Tatchell K., Hall B. D., Nasmyth K. A. Isolation of a gene from Drosophila by complementation in yeast. Nature. 1981 Jan 1;289(5793):33–37. doi: 10.1038/289033a0. [DOI] [PubMed] [Google Scholar]
- Hosbach H. A., Wyler T., Weber R. The Xenopus laevis globin gene family: chromosomal arrangement and gene structure. Cell. 1983 Jan;32(1):45–53. doi: 10.1016/0092-8674(83)90495-6. [DOI] [PubMed] [Google Scholar]
- Hyldig-Nielsen J. J., Jensen E. O., Paludan K., Wiborg O., Garrett R., Jørgensen P., Marcker K. A. The primary structures of two leghemoglobin genes from soybean. Nucleic Acids Res. 1982 Jan 22;10(2):689–701. doi: 10.1093/nar/10.2.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffreys A. J., Wilson V., Wood D., Simons J. P., Kay R. M., Williams J. G. Linkage of adult alpha- and beta-globin genes in X. laevis and gene duplication by tetraploidization. Cell. 1980 Sep;21(2):555–564. doi: 10.1016/0092-8674(80)90493-6. [DOI] [PubMed] [Google Scholar]
- Lee J. S., Brown G. G., Verma D. P. Chromosomal arrangement of leghemoglobin genes in soybean. Nucleic Acids Res. 1983 Aug 25;11(16):5541–5553. doi: 10.1093/nar/11.16.5541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtovaara P., Ellfolk N. The amino-acid sequence of leghemoglobin component a from Phaseolus vulgaris (kidney bean). Eur J Biochem. 1975 Jun;54(2):577–584. doi: 10.1111/j.1432-1033.1975.tb04170.x. [DOI] [PubMed] [Google Scholar]
- Lewin R. Evolutionary history written in globin genes. Science. 1981 Oct 23;214(4519):426-7, 429. doi: 10.1126/science.7291984. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Vainshtein B. K., Harutyunyan E. H., Kuranova I. P., Borisov V. V., Sosfenov N. I., Pavlovsky A. G., Grebenko A. I., Konareva N. V. Structure of leghaemoglobin from lupin root nodules at 5 angstrom resolution. Nature. 1975 Mar 13;254(5496):163–164. doi: 10.1038/254163a0. [DOI] [PubMed] [Google Scholar]
- Vanin E. F., Henthorn P. S., Kioussis D., Grosveld F., Smithies O. Unexpected relationships between four large deletions in the human beta-globin gene cluster. Cell. 1983 Dec;35(3 Pt 2):701–709. doi: 10.1016/0092-8674(83)90103-4. [DOI] [PubMed] [Google Scholar]
- Varsanyi-Breiner A., Gusella J. F., Keys C., Housman D. E., Sullivan D., Brisson N., Verma D. P. The organization of a nuclear DNA sequence from a higher plant: molecular cloning and characterization of soybean ribosomal DNA. Gene. 1979 Nov;7(3-4):317–334. doi: 10.1016/0378-1119(79)90051-9. [DOI] [PubMed] [Google Scholar]
- Verma D. P., Bal A. K. Intracellular site of synthesis and localization of leghemoglobin in root nodules. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3843–3847. doi: 10.1073/pnas.73.11.3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma D. P., Ball S., Guérin C., Wanamaker L. Leghemoglobin biosynthesis in soybean root nodules. Characterization of the nascent and released peptides and the relative rate of synthesis of the major leghemoglobins. Biochemistry. 1979 Feb 6;18(3):476–483. doi: 10.1021/bi00570a016. [DOI] [PubMed] [Google Scholar]
- Verma D. P., Nash D. T., Schulman H. M. Isolation and in vitro translation of soybean leghaemoglobin mRNA. Nature. 1974 Sep 6;251(5470):74–77. doi: 10.1038/251074a0. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller P., Jeffreys A. J., Wilson V., Blanchetot A. Organization of the human myoglobin gene. EMBO J. 1984 Feb;3(2):439–446. doi: 10.1002/j.1460-2075.1984.tb01825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiborg O., Hyldig-Nielsen J. J., Jensen E. O., Paludan K., Marcker K. A. The nucleotide sequences of two leghemoglobin genes from soybean. Nucleic Acids Res. 1982 Jun 11;10(11):3487–3494. doi: 10.1093/nar/10.11.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilbur W. J., Lipman D. J. Rapid similarity searches of nucleic acid and protein data banks. Proc Natl Acad Sci U S A. 1983 Feb;80(3):726–730. doi: 10.1073/pnas.80.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo S. L. A sensitive and rapid method for recombinant phage screening. Methods Enzymol. 1979;68:389–395. doi: 10.1016/0076-6879(79)68028-x. [DOI] [PubMed] [Google Scholar]
- Zimmer E. A., Martin S. L., Beverley S. M., Kan Y. W., Wilson A. C. Rapid duplication and loss of genes coding for the alpha chains of hemoglobin. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2158–2162. doi: 10.1073/pnas.77.4.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]