Abstract
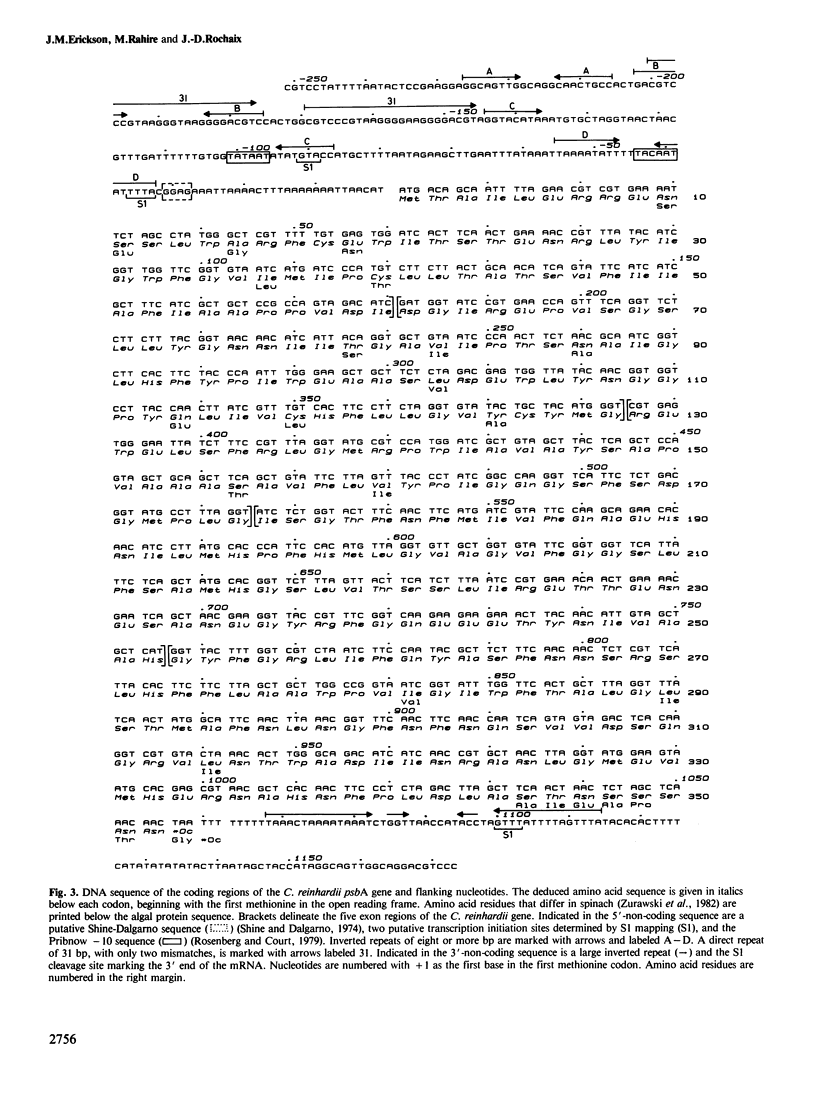
The chloroplast psbA gene from the green unicellular alga Chlamydomonas reinhardii has been localized, cloned and sequenced. This gene codes for the rapidly-labeled 32-kd protein of photosystem II, also identified as as herbicide-binding protein. Unlike psbA in higher plants which is found in the large single copy region of the chloroplast genome and is uninterrupted, psbA in C. reinhardii is located entirely within the inverted repeat, hence present in two identical copies per circular chloroplast genome, and contains four large introns. These introns range from 1.1 to 1.8 kb in size and fall into the category of Group I introns. Two of the introns contain open reading frames which are in-frame with the preceding exon sequences. We present the nucleotide sequence for the C. reinhardii psbA 5'-and 3' -flanking sequences, the coding region contained in five exons and the deduced amino acid sequence. The algal gene codes for a protein of 352 amino acid residues which is 95% homologous, excluding the last eight amino acid residues, with the higher plant protein.
Keywords: group I introns, herbicide resistance, nucleotide sequence, photosynthesis, RNA maturases
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bedbrook J. R., Link G., Coen D. M., Bogorad L. Maize plastid gene expressed during photoregulated development. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3060–3064. doi: 10.1073/pnas.75.7.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Bottomley W., Spencer D., Whitfeld P. R. Protein synthesis in isolated spinach chloroplasts: comparison of light-driven and ATP-driven synthesis. Arch Biochem Biophys. 1974 Sep;164(1):106–117. doi: 10.1016/0003-9861(74)90012-5. [DOI] [PubMed] [Google Scholar]
- Chua N. H., Gillham N. W. The sites of synthesis of the principal thylakoid membrane polypeptides in Chlamydomonas reinhardtii. J Cell Biol. 1977 Aug;74(2):441–452. doi: 10.1083/jcb.74.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covarrubias L., Cervantes L., Covarrubias A., Soberón X., Vichido I., Blanco A., Kupersztoch-Portnoy Y. M., Bolivar F. Construction and characterization of new cloning vehicles. V. Mobilization and coding properties of pBR322 and several deletion derivatives including pBR327 and pBR328. Gene. 1981 Jan-Feb;13(1):25–35. doi: 10.1016/0378-1119(81)90040-8. [DOI] [PubMed] [Google Scholar]
- Darley-Usmar V. M., Fuller S. D. Mr-values of mature subunits I and III of beef heart cytochrome c oxidase in relationship to nucleotide sequences of their genes. FEBS Lett. 1981 Nov 30;135(1):164–166. doi: 10.1016/0014-5793(81)80968-4. [DOI] [PubMed] [Google Scholar]
- Davies R. W., Waring R. B., Ray J. A., Brown T. A., Scazzocchio C. Making ends meet: a model for RNA splicing in fungal mitochondria. Nature. 1982 Dec 23;300(5894):719–724. doi: 10.1038/300719a0. [DOI] [PubMed] [Google Scholar]
- De La Salle H., Jacq C., Slonimski P. P. Critical sequences within mitochondrial introns: pleiotropic mRNA maturase and cis-dominant signals of the box intron controlling reductase and oxidase. Cell. 1982 Apr;28(4):721–732. doi: 10.1016/0092-8674(82)90051-4. [DOI] [PubMed] [Google Scholar]
- Delepelaire P. Partial characterization of the biosynthesis and integration of the Photosystem II reaction centers in the thylakoid membrane of Chlamydomonas reinhardtii. EMBO J. 1984 Apr;3(4):701–706. doi: 10.1002/j.1460-2075.1984.tb01872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dron M., Rahire M., Rochaix J. D. Sequence of the chloroplast DNA region of Chlamydomonas reinhardii containing the gene of the large subunit of ribulose bisphosphate carboxylase and parts of its flanking genes. J Mol Biol. 1982 Dec 25;162(4):775–793. doi: 10.1016/0022-2836(82)90547-2. [DOI] [PubMed] [Google Scholar]
- Erickson J. M., Rahire M., Bennoun P., Delepelaire P., Diner B., Rochaix J. D. Herbicide resistance in Chlamydomonas reinhardtii results from a mutation in the chloroplast gene for the 32-kilodalton protein of photosystem II. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3617–3621. doi: 10.1073/pnas.81.12.3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson J. M., Rushford C. L., Dorney D. J., Wilson G. N., Schmickel R. D. Structure and variation of human ribosomal DNA: molecular analysis of cloned fragments. Gene. 1981 Dec;16(1-3):1–9. doi: 10.1016/0378-1119(81)90055-x. [DOI] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Gorman D. S., Levine R. P. Cytochrome f and plastocyanin: their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardi. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1665–1669. doi: 10.1073/pnas.54.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebanier A. E., Coen D. M., Rich A., Bogorad L. Membrane proteins synthesized but not processed by isolated maize chloroplasts. J Cell Biol. 1978 Sep;78(3):734–746. doi: 10.1083/jcb.78.3.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschberg J., McIntosh L. Molecular Basis of Herbicide Resistance in Amaranthus hybridus. Science. 1983 Dec 23;222(4630):1346–1349. doi: 10.1126/science.222.4630.1346. [DOI] [PubMed] [Google Scholar]
- Hoffman-Falk H., Mattoo A. K., Marder J. B., Edelman M., Ellis R. J. General occurrence and structural similarity of the rapidly synthesized, 32,000-dalton protein of the chloroplast membrane. J Biol Chem. 1982 Apr 25;257(8):4583–4587. [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Jacq C., Banroques J., Becam A. M., Slonimski P. P., Guiso N., Danchin A. Antibodies against a fused 'lacZ-yeast mitochondrial intron' gene product allow identification of the mRNA maturase encoded by the fourth intron of the yeast cob-box gene. EMBO J. 1984 Jul;3(7):1567–1572. doi: 10.1002/j.1460-2075.1984.tb02012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karabin G. D., Farley M., Hallick R. B. Chloroplast gene for Mr 32000 polypeptide of photosystem II in Euglena gracilis is interrupted by four introns with conserved boundary sequences. Nucleic Acids Res. 1984 Jul 25;12(14):5801–5812. doi: 10.1093/nar/12.14.5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller B., Delius H. Intervening sequences in chloroplast genomes. Cell. 1984 Mar;36(3):613–622. doi: 10.1016/0092-8674(84)90341-6. [DOI] [PubMed] [Google Scholar]
- Koller B., Gingrich J. C., Stiegler G. L., Farley M. A., Delius H., Hallick R. B. Nine introns with conserved boundary sequences in the Euglena gracilis chloroplast ribulose-1,5-bisphosphate carboxylase gene. Cell. 1984 Feb;36(2):545–553. doi: 10.1016/0092-8674(84)90247-2. [DOI] [PubMed] [Google Scholar]
- Kuwabara T., Murata N. Purification and characterization of 33 kilodalton protein of spinach chloroplasts. Biochim Biophys Acta. 1979 Dec 14;581(2):228–236. doi: 10.1016/0005-2795(79)90242-3. [DOI] [PubMed] [Google Scholar]
- Malnoë P., Rochaix J. D., Chua N. H., Spahr P. F. Characterization of the gene and messenger RNA of the large subunit of ribulose 1,5-diphosphate carboxylase in Chlamydomonas reinhardii. J Mol Biol. 1979 Sep 25;133(3):417–434. doi: 10.1016/0022-2836(79)90401-7. [DOI] [PubMed] [Google Scholar]
- Marder J. B., Goloubinoff P., Edelman M. Molecular architecture of the rapidly metabolized 32-kilodalton protein of photosystem II. Indications for COOH-terminal processing of a chloroplast membrane polypeptide. J Biol Chem. 1984 Mar 25;259(6):3900–3908. [PubMed] [Google Scholar]
- Mattoo A. K., Hoffman-Falk H., Marder J. B., Edelman M. Regulation of protein metabolism: Coupling of photosynthetic electron transport to in vivo degradation of the rapidly metabolized 32-kilodalton protein of the chloroplast membranes. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1380–1384. doi: 10.1073/pnas.81.5.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattoo A. K., Pick U., Hoffman-Falk H., Edelman M. The rapidly metabolized 32,000-dalton polypeptide of the chloroplast is the "proteinaceous shield" regulating photosystem II electron transport and mediating diuron herbicide sensitivity. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1572–1576. doi: 10.1073/pnas.78.3.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
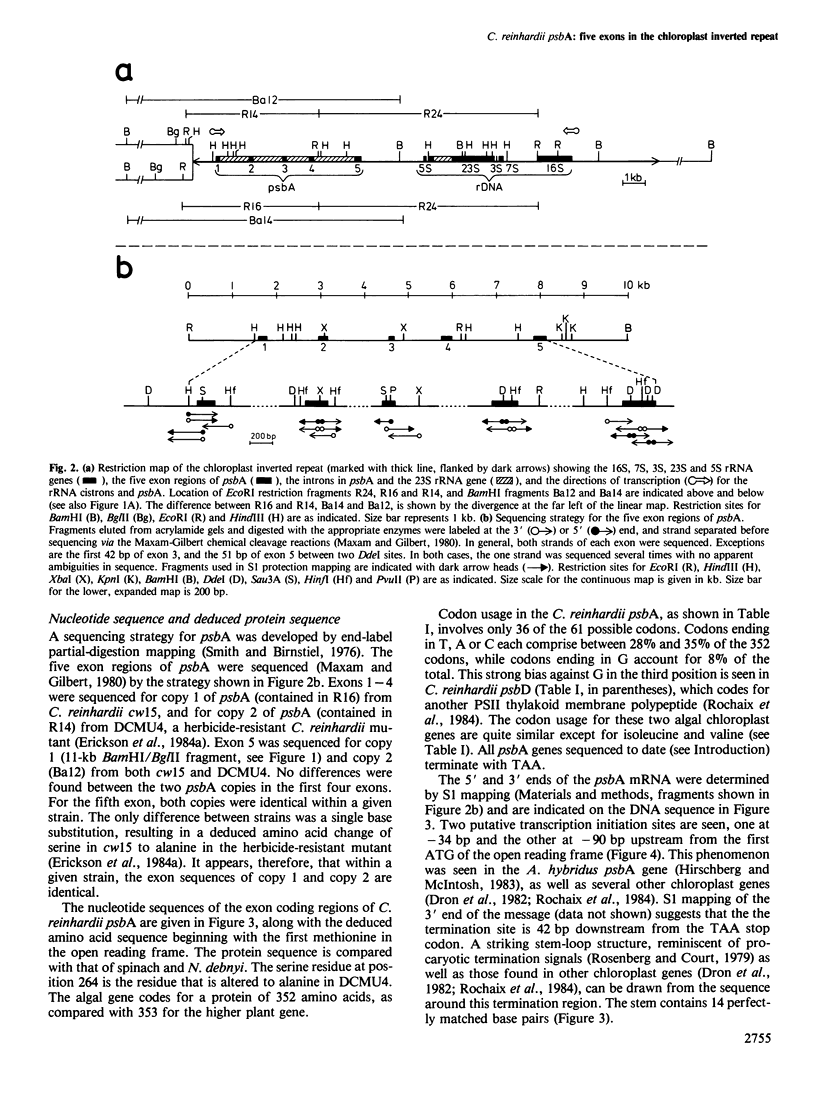
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Michel F., Dujon B. Conservation of RNA secondary structures in two intron families including mitochondrial-, chloroplast- and nuclear-encoded members. EMBO J. 1983;2(1):33–38. doi: 10.1002/j.1460-2075.1983.tb01376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel F., Jacquier A., Dujon B. Comparison of fungal mitochondrial introns reveals extensive homologies in RNA secondary structure. Biochimie. 1982 Oct;64(10):867–881. doi: 10.1016/s0300-9084(82)80349-0. [DOI] [PubMed] [Google Scholar]
- Montandon P. E., Stutz E. Nucleotide sequence of a Euglena gracilis chloroplast genome region coding for the elongation factor Tu; evidence for a spliced mRNA. Nucleic Acids Res. 1983 Sep 10;11(17):5877–5892. doi: 10.1093/nar/11.17.5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan B., Schultes N., Chen L., Bogorad L. Nucleotide sequence of a multiple-copy gene for the B protein of photosystem II of a cyanobacterium. Proc Natl Acad Sci U S A. 1984 May;81(9):2693–2697. doi: 10.1073/pnas.81.9.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohad I., Kyle D. J., Arntzen C. J. Membrane protein damage and repair: removal and replacement of inactivated 32-kilodalton polypeptides in chloroplast membranes. J Cell Biol. 1984 Aug;99(2):481–485. doi: 10.1083/jcb.99.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister K., Steinback K. E., Gardner G., Arntzen C. J. Photoaffinity labeling of an herbicide receptor protein in chloroplast membranes. Proc Natl Acad Sci U S A. 1981 Feb;78(2):981–985. doi: 10.1073/pnas.78.2.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisfeld A., Mattoo A. K., Edelman M. Processing of a chloroplast-translated membrane protein in vivo. Analysis of the rapidly synthesized 32 000-dalton shield protein and its precursor in Spirodela oligorrhiza. Eur J Biochem. 1982 May;124(1):125–129. doi: 10.1111/j.1432-1033.1982.tb05914.x. [DOI] [PubMed] [Google Scholar]
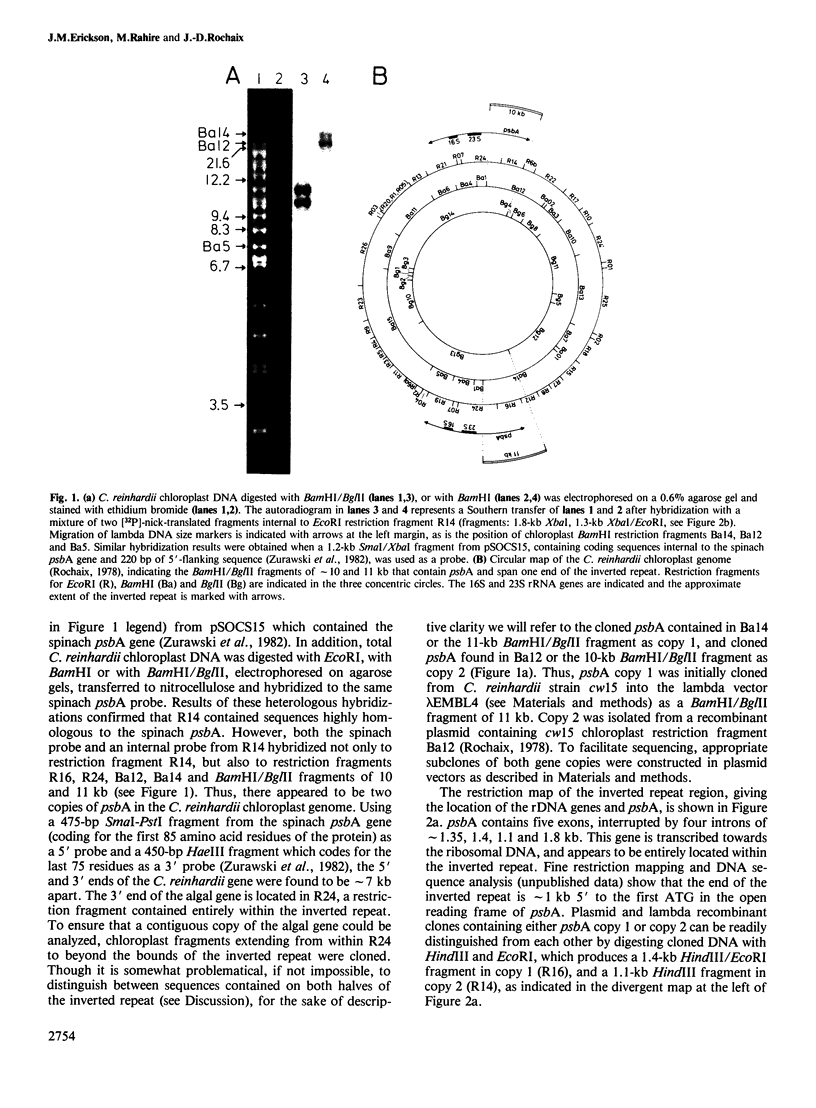
- Rochaix J. D., Malnoe P. Anatomy of the chloroplast ribosomal DNA of Chlamydomonas reinhardii. Cell. 1978 Oct;15(2):661–670. doi: 10.1016/0092-8674(78)90034-x. [DOI] [PubMed] [Google Scholar]
- Rochaix J. D. Restriction endonuclease map of the chloroplast DNA of Chlamydomonas reinhardii. J Mol Biol. 1978 Dec 25;126(4):597–617. doi: 10.1016/0022-2836(78)90011-6. [DOI] [PubMed] [Google Scholar]
- Rochaix J. D. Restriction fragments from Chlamydomonas chloroplast DNA. Methods Enzymol. 1980;65(1):785–795. doi: 10.1016/s0076-6879(80)65073-3. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. O., Birnstiel M. L. A simple method for DNA restriction site mapping. Nucleic Acids Res. 1976 Sep;3(9):2387–2398. doi: 10.1093/nar/3.9.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollner-Webb B., Reeder R. H. The nucleotide sequence of the initiation and termination sites for ribosomal RNA transcription in X. laevis. Cell. 1979 Oct;18(2):485–499. doi: 10.1016/0092-8674(79)90066-7. [DOI] [PubMed] [Google Scholar]
- Spielmann A., Stutz E. Nucleotide sequence of soybean chloroplast DNA regions which contain the psb A and trn H genes and cover the ends of the large single copy region and one end of the inverted repeats. Nucleic Acids Res. 1983 Oct 25;11(20):7157–7167. doi: 10.1093/nar/11.20.7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinback K. E., McIntosh L., Bogorad L., Arntzen C. J. Identification of the triazine receptor protein as a chloroplast gene product. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7463–7467. doi: 10.1073/pnas.78.12.7463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Vermaas W. F., Steinback K. E., Arntzen C. J. Characterization of chloroplast thylakoid polypeptides in the 32-kDa region: polypeptide extraction and protein phosphorylation affect binding of photosystem II-directed herbicides. Arch Biochem Biophys. 1984 May 15;231(1):226–232. doi: 10.1016/0003-9861(84)90382-5. [DOI] [PubMed] [Google Scholar]
- Williams J. C., Steiner L. A., Ogden R. C., Simon M. I., Feher G. Primary structure of the M subunit of the reaction center from Rhodopseudomonas sphaeroides. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6505–6509. doi: 10.1073/pnas.80.21.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youvan D. C., Bylina E. J., Alberti M., Begusch H., Hearst J. E. Nucleotide and deduced polypeptide sequences of the photosynthetic reaction-center, B870 antenna, and flanking polypeptides from R. capsulata. Cell. 1984 Jul;37(3):949–957. doi: 10.1016/0092-8674(84)90429-x. [DOI] [PubMed] [Google Scholar]
- Zurawski G., Bohnert H. J., Whitfeld P. R., Bottomley W. Nucleotide sequence of the gene for the M(r) 32,000 thylakoid membrane protein from Spinacia oleracea and Nicotiana debneyi predicts a totally conserved primary translation product of M(r) 38,950. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7699–7703. doi: 10.1073/pnas.79.24.7699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski G., Bottomley W., Whitfeld P. R. Junctions of the large single copy region and the inverted repeats in Spinacia oleracea and Nicotiana debneyi chloroplast DNA: sequence of the genes for tRNAHis and the ribosomal proteins S19 and L2. Nucleic Acids Res. 1984 Aug 24;12(16):6547–6558. doi: 10.1093/nar/12.16.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]