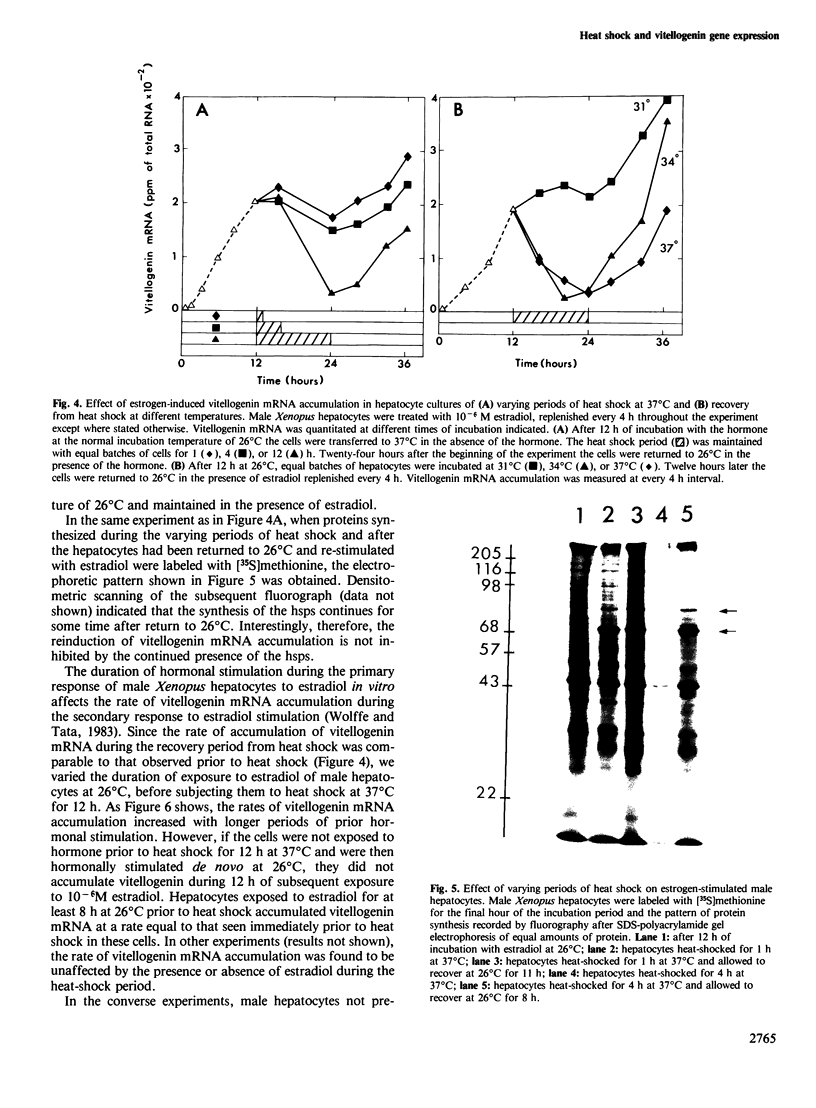
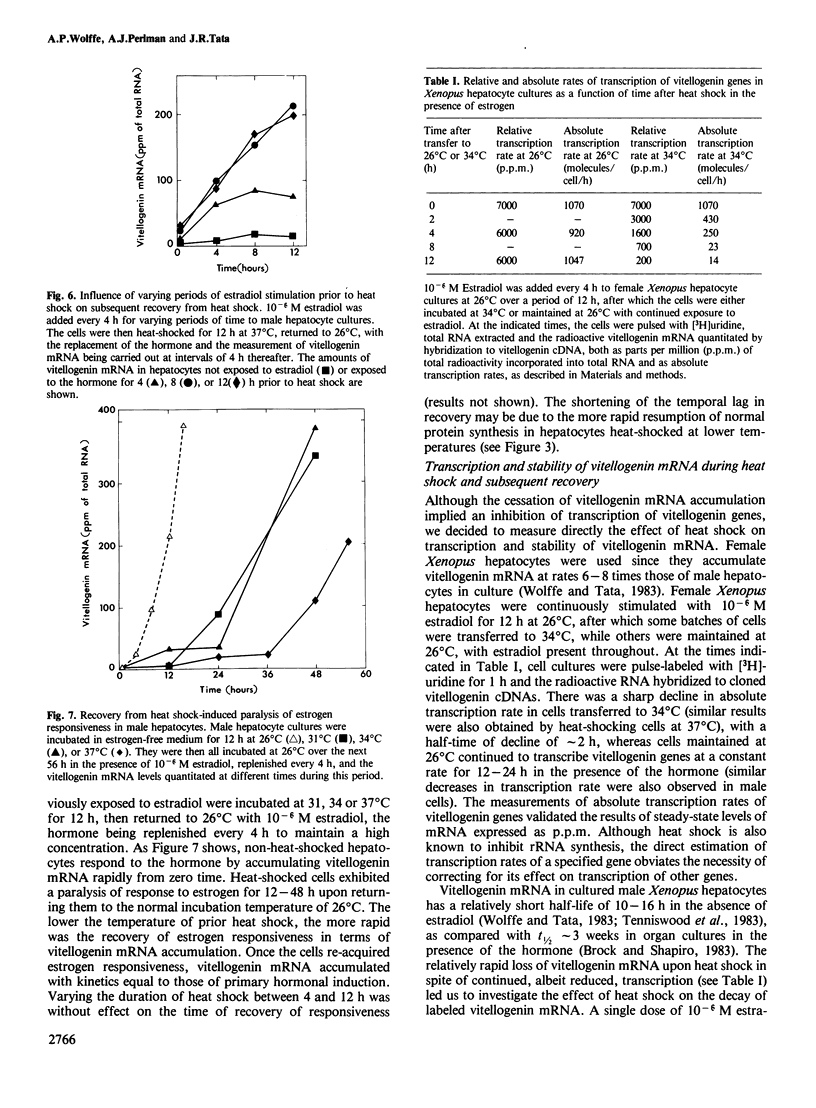
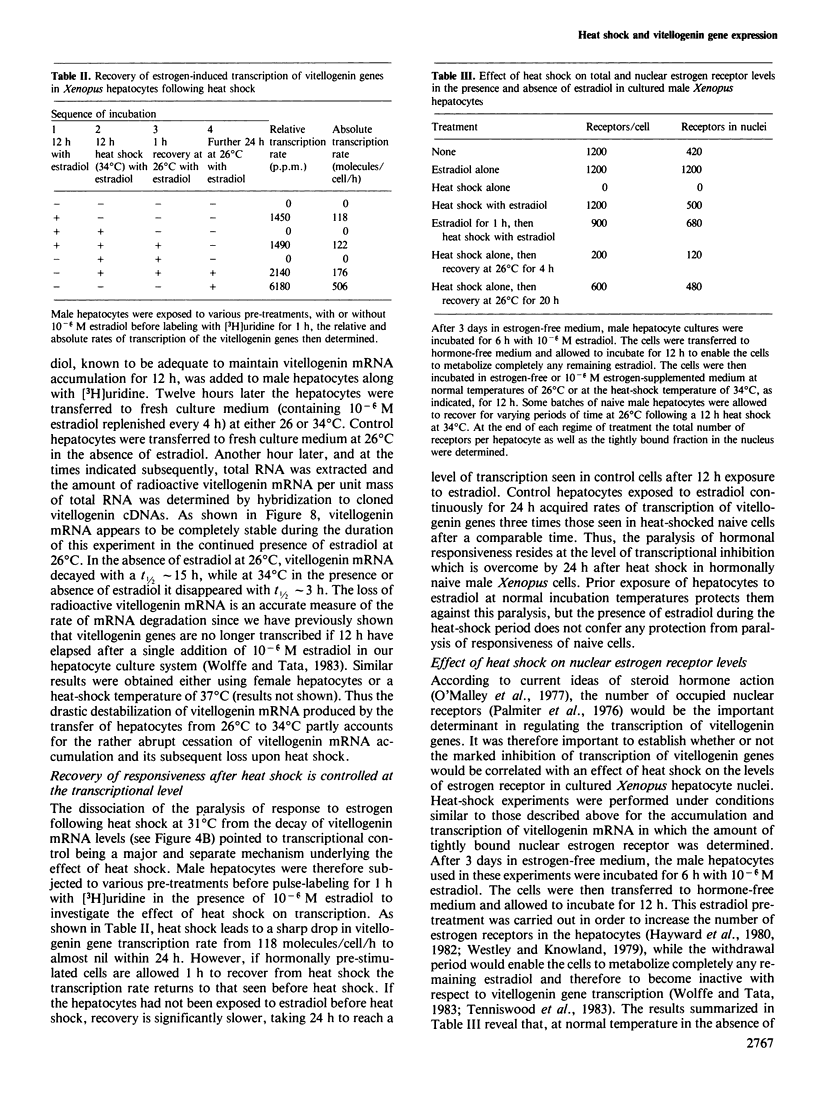
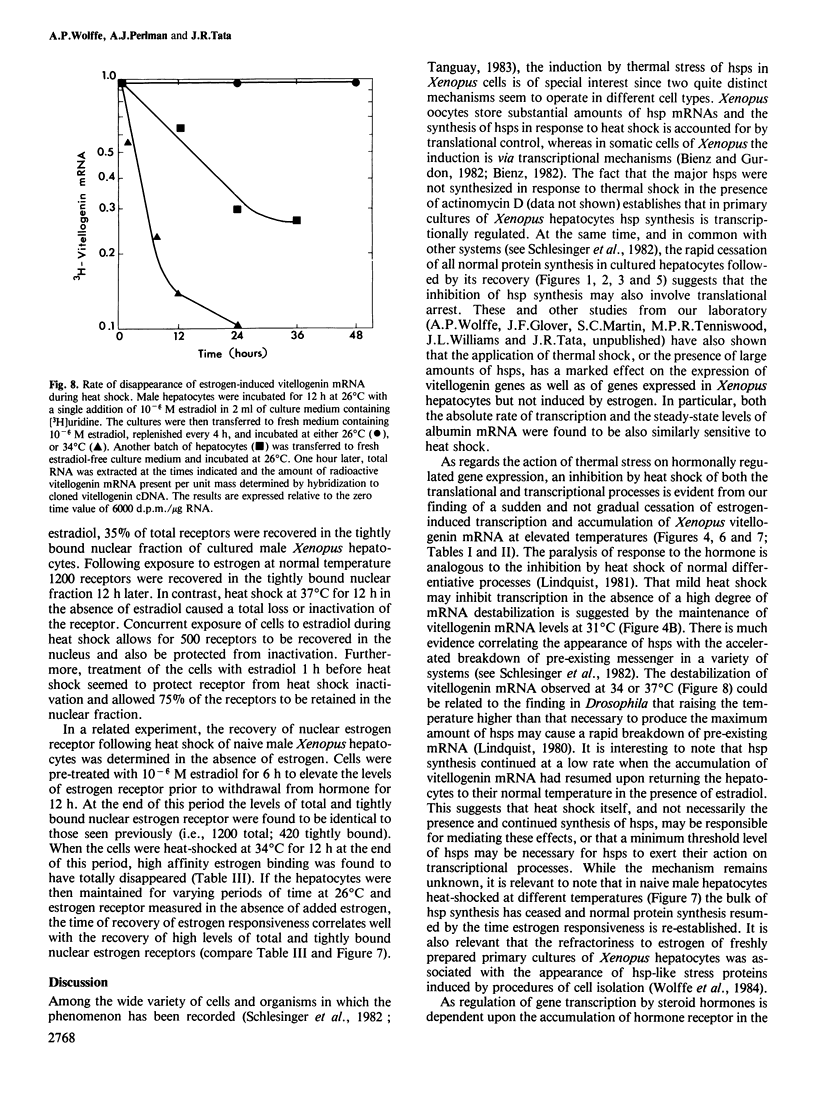
Abstract
We have investigated the effect of heat shock on primary cultures of male and female Xenopus laevis hepatocytes as a function of estrogen-induced vitellogenin gene expression. Coincident with the induction of heat-shock protein (hsp) synthesis, thermal stress abolishes the estrogen activated transcription and accumulation of vitellogenin mRNA, at the same time causing the destabilization of vitellogenin mRNA accumulated by prior treatment with the hormone. Exposure of the cells to estrogen before heat shock allows an immediate resumption of vitellogenin gene transcription on return to 26 degrees C. Heat shock applied to cells from hormonally naive male Xenopus extends the lag period preceding vitellogenin gene transcription upon return to normal temperatures. This transient and reversible paralysis of estrogen responsiveness is paralleled by reversible changes in the amount of nuclear estrogen receptor in the hepatocytes. Heat shock therefore offers a novel approach in the manipulation and analysis of the early stages of steroid hormonal regulation of gene expression.
Full text
PDF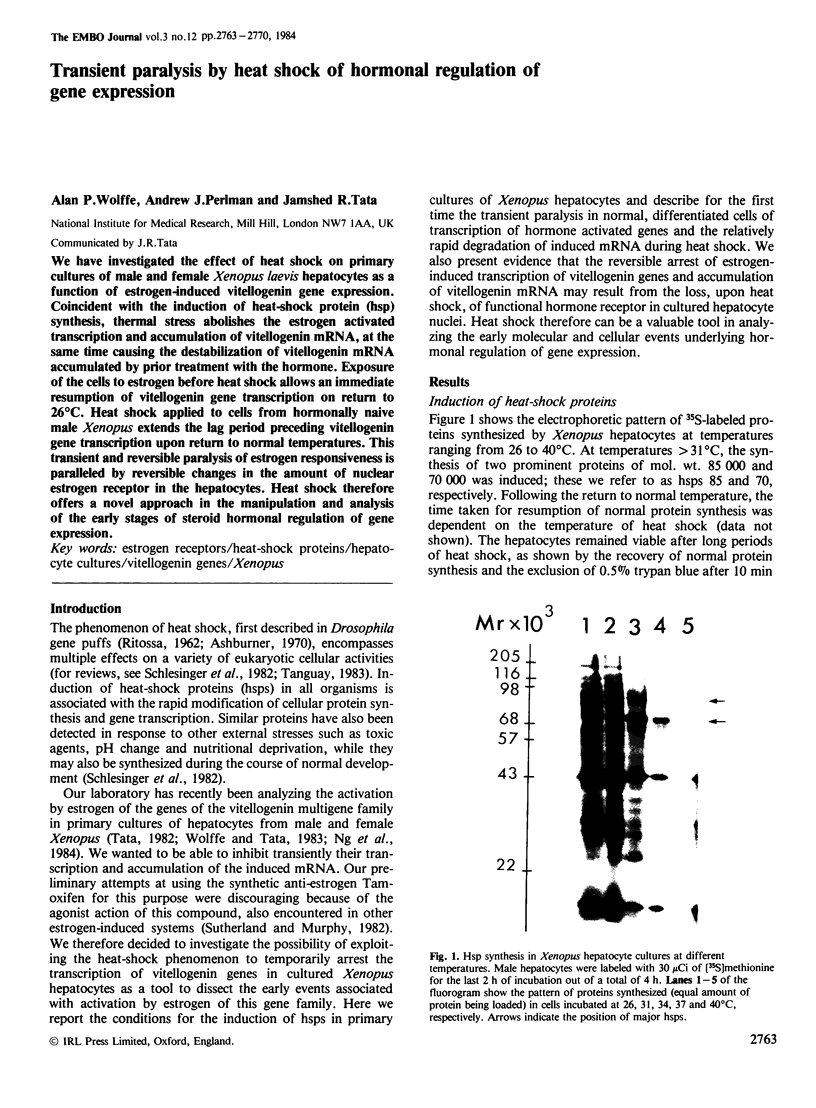


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashburner M. Patterns of puffing activity in the salivary gland chromosomes of Drosophila. V. Responses to environmental treatments. Chromosoma. 1970;31(3):356–376. doi: 10.1007/BF00321231. [DOI] [PubMed] [Google Scholar]
- Bienz M., Gurdon J. B. The heat-shock response in Xenopus oocytes is controlled at the translational level. Cell. 1982 Jul;29(3):811–819. doi: 10.1016/0092-8674(82)90443-3. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brock M. L., Shapiro D. J. Estrogen stabilizes vitellogenin mRNA against cytoplasmic degradation. Cell. 1983 Aug;34(1):207–214. doi: 10.1016/0092-8674(83)90151-4. [DOI] [PubMed] [Google Scholar]
- Clemens M. J., Lofthouse R., Tata J. R. Sequential changes in the protein synthetic activity of male Xenopus laevis liver following induction of egg-yolk proteins by Estradiol-17 beta. J Biol Chem. 1975 Mar 25;250(6):2213–2218. [PubMed] [Google Scholar]
- Hayward M. A., Brock M. L., Shapiro D. J. The role of estrogen receptor in Xenopus laevis vitellogenin gene expression. Am J Physiol. 1982 Jul;243(1):C1–C6. doi: 10.1152/ajpendo.1982.243.1.E1. [DOI] [PubMed] [Google Scholar]
- Hayward M. A., Mitchell T. A., Shapiro D. J. Induction of estrogen receptor and reversal of the nuclear/cytoplasmic receptor ratio during vitellogenin synthesis and withdrawal in Xenopus laevis. J Biol Chem. 1980 Dec 10;255(23):11308–11312. [PubMed] [Google Scholar]
- Ireland R. C., Berger E., Sirotkin K., Yund M. A., Osterbur D., Fristrom J. Ecdysterone induces the transcription of four heat-shock genes in Drosophila S3 cells and imaginal discs. Dev Biol. 1982 Oct;93(2):498–507. doi: 10.1016/0012-1606(82)90137-3. [DOI] [PubMed] [Google Scholar]
- Lindquist S. Regulation of protein synthesis during heat shock. Nature. 1981 Sep 24;293(5830):311–314. doi: 10.1038/293311a0. [DOI] [PubMed] [Google Scholar]
- Lindquist S. Varying patterns of protein synthesis in Drosophila during heat shock: implications for regulation. Dev Biol. 1980 Jun 15;77(2):463–479. doi: 10.1016/0012-1606(80)90488-1. [DOI] [PubMed] [Google Scholar]
- Ng W. C., Wolffe A. P., Tata J. R. Unequal activation by estrogen of individual Xenopus vitellogenin genes during development. Dev Biol. 1984 Mar;102(1):238–247. doi: 10.1016/0012-1606(84)90188-x. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Moore P. B., Mulvihill E. R. A significant lag in the induction of ovalbumin messenger RNA by steroid hormones: a receptor translocation hypothesis. Cell. 1976 Aug;8(4):557–572. doi: 10.1016/0092-8674(76)90224-5. [DOI] [PubMed] [Google Scholar]
- Schaltmann K., Pongs O. Identification and characterization of the ecdysterone receptor in Drosophila melanogaster by photoaffinity labeling. Proc Natl Acad Sci U S A. 1982 Jan;79(1):6–10. doi: 10.1073/pnas.79.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searle P. F., Tata J. R. Vitellogenin gene expression in male Xenopus hepatocytes during primary and secondary stimulation with estrogen in cell cultures. Cell. 1981 Mar;23(3):741–746. doi: 10.1016/0092-8674(81)90437-2. [DOI] [PubMed] [Google Scholar]
- Sutherland R. L., Murphy L. C. Mechanisms of oestrogen antagonism by nonsteroidal antioestrogens. Mol Cell Endocrinol. 1982 Jan;25(1):5–23. doi: 10.1016/0303-7207(82)90165-4. [DOI] [PubMed] [Google Scholar]
- Tanguay R. M. Genetic regulation during heat shock and function of heat-shock proteins: a review. Can J Biochem Cell Biol. 1983 Jun;61(6):387–394. doi: 10.1139/o83-053. [DOI] [PubMed] [Google Scholar]
- Tenniswood M. P., Searle P. F., Wolffe A. P., Tata J. R. Rapid estrogen metabolism and vitellogenin gene expression in Xenopus hepatocyte cultures. Mol Cell Endocrinol. 1983 Jun;30(3):329–345. doi: 10.1016/0303-7207(83)90068-0. [DOI] [PubMed] [Google Scholar]
- Wangh L. J., Osborne J. A., Hentschel C. C., Tilly R. Parenchymal cells purified from Xenopus liver and maintained in primary culture synthesize vitellogenin in response to estradiol-17 beta and serum albumin in response to dexamethasone. Dev Biol. 1979 Jun;70(2):479–499. doi: 10.1016/0012-1606(79)90040-x. [DOI] [PubMed] [Google Scholar]
- Westley B., Knowland J. An estrogen receptor from Xenopus laevis liver possibly connected with vitellogenin synthesis. Cell. 1978 Oct;15(2):367–374. doi: 10.1016/0092-8674(78)90005-3. [DOI] [PubMed] [Google Scholar]
- Westley B., Knowland J. Estrogen causes a rapid, large and prolonged rise in the level of nuclear estrogen receptor in Xenopus laevis liver. Biochem Biophys Res Commun. 1979 Jun 13;88(3):1167–1172. doi: 10.1016/0006-291x(79)91531-6. [DOI] [PubMed] [Google Scholar]
- Wolffe A. P., Tata J. R. Coordinate and non-coordinate estrogen-induced expression of A and B groups of vitellogenin genes in male and female Xenopus Hepatocytes in culture. Eur J Biochem. 1983 Feb 1;130(2):365–372. doi: 10.1111/j.1432-1033.1983.tb07162.x. [DOI] [PubMed] [Google Scholar]