Abstract
Objective
To investigate gender-specific trajectories in well-being among older people with coronary heart disease (CHD) and to compare them to those of healthy people.
Method
4,496 participants from the first three waves of the English Longitudinal Study of Ageing (2002/03-2006/07). We measured well-being using quality of life (CASP-19) and depressive caseness (3 or more symptoms on the CESD-8).
Results
After adjustment, at 2 and 4 years follow-up women had 3 points higher quality of life than men (p<0.001). When looking at each quality of life’s domain we found that women reported higher scores of Autonomy compared to men. The gender difference in the probability of having depressive caseness reduced to 7 percentage points at 4-year follow-up from 13 percentage points in the previous occasions. Men’s quality of life declined progressively over-time by 3 points (p<0.001) (equivalent to the effect of having diabetes) but no changes in prevalence of depressive caseness were found. Women’s quality of life only declined after 4-year follow-up by less than 2 points (p<0.001), while in the same period their probability of reporting depressive caseness reduced by 6 percentage points (p<0.001).
Conclusions
Women had better quality of life than men in the two and four years following a CHD event, and were not more likely than men to report depressive caseness in the long term. Men’s quality of life deteriorated progressively over time, among women it did not deteriorate in the first two years following a CHD event; women had a long-term improvement in depressive caseness.
Keywords: depressive caseness, quality of life, CHD, trajectories
INTRODUCTION
Recent trends indicate that the death rate from coronary heart disease (CHD) reduced by at least two fifths between 2000 and 2010 for men and women aged 55 to 64 in the UK (British Heart Foundation, 2010). Concurrent with the substantial reduction in deaths from CHD, there has been a growing recognition of the importance of outcomes other than survival following CHD, such as well-being. Reduced quality of life and increased risk of depression are common following CHD (Kristofferzon et al., 2005; Norris et al., 2007) and are associated with increased mortality (Lesperance et al., 1996; 2000; Ziegelstein et al., 2000; Bogg et al., 2000; Ferketich et al., 2000; Bush et al., 2001; Carney et al., 2003; Lane et al.; 2005; Parashar et al., 1996), with poor adherence to recommended behaviours and lifestyle changes after the cardiac event (Ziegelstein et al., 2000; 2001)and with an increased risk of readmission because of cardiac complications (Lauzon et al., 2003).
Results from studies exploring longitudinal changes in well-being among men and women hospitalized after CHD reported that women had significantly lower health-related quality of life than men (Norris et al., 2007; Bogg et al., 2000; Westin et al., 1999; Brink et al., 2005) and were more likely to be depressed (Norris et al., 2007) one year post-CHD. However, other studies reported contrasting findings, with no difference between men and women in their prevalence of depression post-CHD (Brink et al., 2005; Wiklund et al., 1993). Results from a community study with a five year follow-up showed that women had a high initial risk for depression with a significant decrease after two years, while the risk for depression for men was only increased in the two to five years after myocardial infarction (Bjerkeset et al., 2005).
Despite the increasing interest in the well-being of people with CHD (Norris et al., 2007, Norris et al., 2007; Bogg et al., 2000; Westin et al., 1999; Brink et al., 2005; Wiklund et al., 1993; Bjerkeset et al., 2005), results from the majority of previous studies have mainly been based on small samples with short follow-up periods (Norris et al., 2007,7,Bogg et al., 2000; Westin et al., 1999; Brink et al., 2005; Wiklund et al., 1993). Research has typically focused exclusively on people with CHD, so direct comparisons with a population free from CHD and other major diseases on well-being have not been possible. Therefore, questions concerning long-term outcome, gender differences, and the role of explaining factors still remain unanswered. Moreover, most of the studies have measured quality of life using a health-related measure such as the short-form-36, which may not be optimal as these measures are based on proxies (such as health) “which draw on a set of normative assumptions about what a particular condition implies for a person’s quality of life without necessarily taking close account of a person’s current life experience” (Wiggins et al., 2008).A recent avenue of research looks at positive, rather than negative functioning and this is particularly interesting given that health life expectancy has been improving. Given this interest, an alternative measure of quality of life has been specifically developed for old age, called CASP (Hyde et al., 2003), comprising four domains (‘control’, ‘autonomy’, ‘pleasure’ and ‘self-realization’). CASP has been based on theories of need satisfaction (Doyal and Gough, 1991), which assume that quality of life at older ages is conceptualized as the degree to which human needs are satisfied in the above mentioned four domains (Hyde et al., 2003). This measure differs from health-related measures by focusing on positive aspects of quality of life and by being independent of health and other factors that might influence it (Hyde et al., 2003; Wiggins et al., 2008).
Using a large population-based sample of older adults living in England, the aims of our study are to explore gender-specific changes in well-being over a six year period among people with CHD and to compared them with a group of individuals free from CHD and other major conditions. In line with current recommendations to distinguish between different aspects of well-being (Dolan et al., 2011; Kahneman et al., 2006) we use quality of life, and depressive caseness.
METHODS
Study population
We analysed data from the first three waves (2002-2003 to 2006-2007) of the English Longitudinal Study of Ageing (ELSA) (Steptoe et al., 2012), a panel study where the same individuals are re-interviewed every two years. The ELSA sample was designed to represent people aged 50 and over, living in private households in England. The original sample size was 11,391 at wave 1 (2002-03) (individual response rate of 67%). The analytical sample of this study consists of 4,996 participants with a CHD event occurring (n=895 18%) in the two years preceding the baseline interview (2002-03) and of healthy participants without known longstanding conditions (n=3,601) at baseline. A detailed explanation of the selection of the CHD and healthy participants is given in the next section. Data were collected through face-to-face interviews and self-completion questionnaires. Ethical approval for the ELSA study was provided by the local Ethics Committee and patients or their representatives gave informed consent.
Measures
Well-being
We measured quality of life using the CASP-19 (Hyde et al., 2003) self-completion questionnaire. CASP-19 contains 19 items covering four conceptual domains of individual needs that are particularly relevant in later life: Control, Autonomy, Self-realization and Pleasure. The instrument has four items for the control domain and five for each of the others. Each item is assessed on a four-point Likert scale. The resulting scale scores of Control (range 0 to 12, mean 8.6 s.d 2.5), Autonomy (range 1 to 15, mean 10.8 s.d. 2.6), Self-realization (range 0 to 15, mean 10.6 s.d. 3.1) and Pleasure (range 0 to 15, mean 13.5 s.d. 2.1) are summed to form an index which ranges from 0 to 57 with higher scores indicating better quality of life (sample specific Cronbach’s alpha=0.78).The psychometric properties of CASP-19 are fully described elsewhere (Hyde et al., 2003; Wiggins et al., 2008). In terms of size of effects, a reduction of around 7 quality of life points is associated with having a limiting long-standing illness compared to those without (Netuveli et al., 2006).
We used the eight-item version of the Centre for Epidemiologic Study Depression scale (CESD-8) administered in the face-to-face interview to measure depressive symptoms (Radloff, 1977). The questions asked the degree to which the respondent had experienced (or not) depressive symptoms such as restless sleep and being unhappy, over the past month. The total score, which ranges from 0 to 8,was dichotomized with score ≥3 to indicate depression caseness, in line with previous studies that have used this abridged version of the scale (Steffick, 2000; Chou, 2007; Blane et al., 2008; Rice et al., 2009). Caseness does not here signify diagnosed depression. The dichotomy was used in preference to a continuous scale because of the skewed distribution of number of symptoms.
Coronary Heart Disease
Our main exposure was experience of angina and/or a myocardial infarction (henceforth referred to as coronary heart diseases CHD) during the two years prior to baseline. Exposure was identified from the face-to-face interview in which participants were asked whether a doctor had ever told them that they suffered from angina or heart attack and, if so, whether they had angina symptoms or myocardial infarction in the past two years. We compared people with CHD with a reference population of individuals that at baseline had never been diagnosed with CHD, stroke, diabetes, hypertension, pulmonary disease, Alzheimer, Parkinson’s, cancer and did not report any limiting longstanding illness (referred to Healthy group). The CHD and Healthy groups include people who might experience CHD and other chronic diseases after baseline.
Covariates
Covariates considered in this study include: gender, age, cohabiting status (0“cohabiting with a partner (married or not)” 1“not cohabiting with a partner”), employment status (three categories, paid employment, completely retired and other), educational attainment (0“yes” 1“no”), quintile of non-pension wealth, smoking status (0“never smoked and ex-smoker” 1“current smoker”), frequency of alcohol consumption (0“less than three times a week” 1“three times a week and more”), physical activity (0“physically active” 1“inactive”) whether or not often troubled with pain, one or more limitation with activities of daily living (ADLs), score of positive support (Stafford et al., 2011) received from spouse, children, other relatives and friends(continuous variable with higher scores indicating greater positive support), and number of close friends (continuous variable). These time-varying factors were selected as relevant to the well-being outcomes included in the analyses.
Statistical Analysis
We used random intercepts models (Goldstein, 2003) to examine changes in well-being over time. First, we estimated a linear random intercepts model for quality of life using the index score obtained from the sum of the scores of each domains, Control, Autonomy, Self-realisation and Pleasure. We then estimated linear random intercepts models for each of the four domains in order to explore whether different trajectories for the four distinct domains emerge. Lastly a logit random intercepts model was estimated for depressive caseness. The models included an interaction term between gender and time in order to assess gender-specific changes in the outcomes over time. The models were run separately for the CHD and Healthy groups. From the logit random intercepts model, average predicted probabilities were estimated and presented graphically, in the text they were referred to as percentages (probabilities multiplied by 100).
To handle missing data due to loss to follow-up and item non-response we used a multiple imputation technique suitable for longitudinal data: the recently proposed two-fold fully conditional specification (Nevalainen et al., 2009). We created five imputed data sets and we combined the results estimates according to Rubin’s rule (1996).When using multiple imputation methods it is recommended to include auxiliary variables predictive of missingness in the imputation model (Sterne et al., 2009)even if they are not of interest in the substantive model. We therefore added five variables (measured at baseline) which were found to be predictive of non-response (Scholes et al., 2008) to the imputation model, these variables are: year of HSE interview, Government Office Region, housing tenure, number of people in the household and ethnicity. Statistical significance was assessed with a p-value ≤0.01, whereas a p-value up to 0.05 was considered as borderline significant. All analyses were run in Stata version 11.1.
RESULTS
Table 1 shows baseline characteristics of participants by disease status and gender. Men and women with CHD were on average older and had lower quality of life than men and women from the Healthy group. The prevalence of those reporting depressive caseness was higher in men and women with CHD compared to people in the Healthy group. The prevalence of being retired, in low education, with poor wealth, physically inactive, drinking alcohol on three or more days a week, often troubled with pain and with one or more limitations with ADLs was higher in men and women with CHD compared with men and women from the Healthy group. Women with CHD were also more likely than women from the Healthy group not to be cohabiting and less likely to be in the richest quintiles of wealth (5th and 4th). Men with CHD received on average lower positive social support than men from the Healthy group.
Table 1.
Baseline characteristics by gender and disease status. England 2002-03
| Men CHD group (n=518) | Men Healthy groupa (n=1,702) | Women CHD group (n=377) | Women Healthy groupa (1,899) | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Mean (SD) | Mean (SD) | P-value | Mean (SD) | Mean (SD) | P-value | |
| Age | 68.1 (10.1) | 64.5 (9.6) | <.001 | 69.9 (10.2) | 63.1 (10.3) | <.001 |
| Quality of life | 40.1 (9.5) | 44.8 (7.5) | <.001 | 39.3 (9.2) | 44.8 (7.6) | <.001 |
| Positive support | 26.6 (5.4) | 25.9 (5.5) | .010 | 27.9 (5.2) | 28.2 (5.2) | 0.279 |
| Close friends | 8.1 (6.0) | 7.8 (5.3) | 0.242 | 7.8 (5.0) | 8.1 (6.0) | 0.236 |
| %(95%CI) | %(95%CI) | %(95%CI) | %(95%CI) | |||
| Depressive caseness | 22.7 (20.6; 24.7) | 14.2 (13.2; 15.1) | <.001 | 33.2 (30.4; 35.9) | 22.5 (21.4; 23.6) | <.001 |
| Not cohabiting | 26.8 (24.6; 29.0) | 23.1 (21.9; 24.2) | 0.084 | 49.3 (46.4; 52.3) | 36.7 (35.5; 38.0) | <.001 |
| Completely retired | 69.6 (67.3; 71.9) | 45.4 (44.0; 46.8) | <.001 | 68.1 (65.4; 70.8) | 42.9 (41.6; 44.2) | <.001 |
| Otherb | 8.7 (7.3; 10.1) | 5.5 (4.9; 6.1) | .001 | 22.3 (19.9; 24.7) | 17.9 (16.9; 18.9) | <0.05 |
| Low education | 47.2 (44.7; 49.7) | 39.0 (37.7; 40.4) | <.001 | 48.8 (45.9; 51.7) | 39.1 (37.8; 40.4) | <.001 |
| Wealth 5th (richest) | 17.0 (13.7;22.0) | 26.9 (24.7;29.0) | <.001 | 12.7 (9.4;16.1) | 25.1 (23.2;27.0) | <.001 |
| Wealth 4th | 18.5 (16.6; 20.5) | 22.5 (21.3; 23.6) | 0.053 | 15.6 (13.5; 17.8) | 20.0 (18.9; 21.0) | .048 |
| Wealth 3rd | 19.8 (17.8; 21.7) | 19.9 (18.8; 21.0) | 0.960 | 20.9 (18.5; 23.2) | 20.5 (19.5; 21.6) | 0.861 |
| Wealth 2nd | 20.7 (18.6; 22.7) | 18.6 (17.5; 19.6) | 0.287 | 19.7 (17.4; 22.0) | 19.6 (18.5; 20.6) | 0.964 |
| Wealth 1st (poorest) | 23.4 (21.3; 25.5) | 14.3 (13.4; 15.3) | <.001 | 31.5 (28.8; 34.2) | 17.2 (16.3; 18.2) | <.001 |
| Current smoker | 13.8 (12.1; 15.6) | 16.3 (15.3; 17.3) | 0.171 | 14.0 (11.9; 16.0) | 17.8 (16.8; 18.8) | 0.074 |
| Physically inactive | 69.9 (67.6; 72.2) | 52.6 (51.2; 54.0) | <.001 | 80.9 (78.6; 83.2) | 63.1 (61.8; 64.3) | <.001 |
| Drinks alcohol ≥3 days a week | 36.3 (33.9; 38.7) | 43.0 (41.6; 44.4) | .007 | 36.3 (19.3; 24.2) | 28.8 (27.6; 30.0) | .004 |
| Often troubled with pain | 26.3 (24.1; 28.5) | 14.2 (13.3; 15.2) | <.001 | 26.3 (33.8; 39.4) | 16.3 (15.4; 17.3) | <.001 |
| 1 or more ADLs | 27.8 (25.6; 30.0) | 10.5 (9.7; 11.3) | <.001 | 33.5 (30.8; 36.3) | 11.6 (10.8; 12.5) | <.001 |
Notes:
people that at baseline had never been diagnosed with CHD, stroke, diabetes, hypertension, pulmonary disease, Alzheimer, Parkinson’s, cancer or any limiting longstanding illness.
Permanently unable to work, not currently in paid employment, looking after home or family. Results based on five imputed data sets.
Changes in quality of life
Table 2 reports the changes over time in quality of life by gender among people with CHD and the Healthy group obtained using random intercepts models (imputed data), results are presented fully adjusted. Figure 1 shows graphically the results reported in Table 2. Among men with CHD the adjusted mean quality of life score at baseline was 47.4, which decreased to 46.5 at two year follow-up (borderline significant p=0.034) and to 44.6 at four year follow-up (p<0.001). The adjusted mean quality of life score at baseline for women with CHD was 48.2, which remained stable at two year follow-up (48.4), and then decreased to 46.5 at four year follow-up (p<0.001). At two year follow-up and at four year follow-up women had significantly higher quality of life than men (2004-05:coeff 1.82 95%CI 0.83-2.81, p<0.001; 2006-07: coeff 1.82 95%CI 0.83-2.80 p<0.001) (Table 2 and Figure 1).
Table 2.
Changes over time in quality of life among people with CHD and the Healthy group. England 2002-03 to 2006-07
| CHD group (N=895) | Healthy groupa (N=3,601) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Coef.c | Std. Err. | P-value | Coef.c | Std. Err. | P-value | |
|
|
||||||
| Fully adjustedb | ||||||
| Women vs Men (2002-03) | 0.83 | 0.50 | 0.094 | 0.24 | 0.23 | 0.289 |
| Women vs Men (2004-05) | 1.82 | 0.50 | <0.001 | 0.49 | 0.23 | 0.034 |
| Women vs Men (2006-07) | 1.82 | 0.50 | <0.001 | 0.42 | 0.23 | 0.066 |
| Estimated average Men (2002-03) | 47.38 | 0.64 | <0.001 | 49.03 | 0.25 | <0.001 |
| Estimated average Men (2004-05) | 46.54 | 0.62 | <0.001 | 47.92 | 0.25 | <0.001 |
| Estimated average Men (2006-07) | 44.65 | 0.63 | <0.001 | 46.15 | 0.25 | <0.001 |
| Between variance | 20.65 | 0.04 | 19.17 | 0.02 | ||
| Within variance | 30.62 | 0.02 | 24.95 | 0.01 | ||
Notes:
People that at baseline had never been diagnosed with CHD, stroke, diabetes, hypertension, pulmonary disease, Alzheimer, Parkinson’s, cancer or any limiting longstanding illness.
Adjusted for age, cohabiting status, employment status, education, wealth, smoking status, physical activity, frequency of alcohol consumption, pain, number of limitations with ADLs, depressive caseness.
Estimated using random intercept models with interaction terms between time and sex. Results based on five imputed data sets
Figure 1.
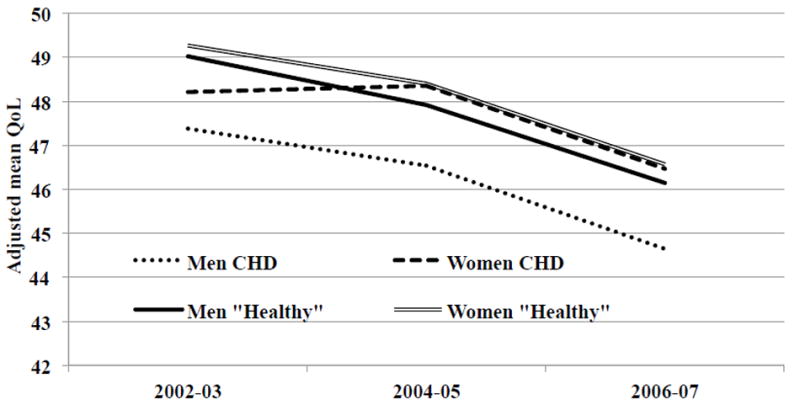
Trajectories over time of quality of life among people with CHD and the Healthy group, by gender. England 2002-03 to 2006-07
Results adjusted for gender, age, quadratic effect of age, cohabiting status, employment status, educational attainment, wealth, smoking status, alcohol consumption, physical activity, pain, ADLs, depressive caseness, positive support and number of close friends and family. Results based on five imputed data sets.
Among men and women in the Healthy group, quality of life decreased substantially over time (Table 2 and Figure 1). Men and women in the Healthy group had similar changes in quality of life over time and only at two year follow-up was the quality of life of women slightly higher than that of men (2004-05: coeff 0.49 95%CI 0.36-0.93 p=0.016);the difference was borderline significant and not clinically important.
The adjusted quality of life of men with CHD was over 1.4 points lower than the quality of life of men from the Healthy group, at each measurement occasion. The adjusted quality of life of women with CHD was 1.1 points lower than the quality of life of women from the Healthy group at baseline, while at two and four year follow-ups it was almost the same.
Table 3 shows changes over time in each domain of quality of life by gender among people with CHD and the Healthy group. To simplify interpretation results are presented as fully adjusted mean estimated using random intercepts models (imputed data). For each domain of quality of life, the score decreases over time in men and women from CHD group and from the Healthy group. The Autonomy domain is the aspect of quality of life for which men and women in the CHD group reported gender differences, while for the other domains the results were not statistically significant. Among people in the healthy group, only at wave 2 (2004-05) women reported higher scores of Autonomy compared to men, while on all other occasions and for all other domains of quality of life they reported similar results.
Table 3.
Changes over time in each domain of quality of life among people with CHD and the Healthy group. England 2002-03 to 2006-07
| CHD group (N=895) | Healthy groupa (N=3,601) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Men | Women | P-value | Men | Women | P-value | |
|
|
||||||
| Fully adjustedb | ||||||
| Control | ||||||
| Estimated average 2002-03 | 9.77 | 10.10 | 0.055 | 9.78 | 9.65 | 0.113 |
| Estimated average 2004-05 | 9.53 | 9.48 | 0.817 | 9.62 | 9.60 | 0.795 |
| Estimated average 2006-07 | 8.75 | 9.04 | 0.215 | 8.93 | 8.93 | 0.908 |
| Autonomy | ||||||
| Estimated average 2002-03 | 11.58 | 11.98 | 0.027 | 11.74 | 11.92 | 0.026 |
| Estimated average 2004-05 | 11.29 | 11.83 | <0.010 | 11.71 | 12.06 | <0.010 |
| Estimated average 2006-07 | 10.84 | 11.24 | <0.001 | 11.46 | 11.58 | 0.326 |
| Self-realisation | ||||||
| Estimated average 2002-03 | 11.67 | 11.86 | 0.347 | 12.00 | 12.07 | 0.416 |
| Estimated average 2004-05 | 11.42 | 11.53 | 0.598 | 11.84 | 11.76 | 0.472 |
| Estimated average 2006-07 | 11.07 | 11.19 | 0.607 | 11.49 | 11.47 | 0.790 |
| Pleasure | ||||||
| Estimated average 2002-03 | 13.87 | 14.18 | 0.104 | 14.08 | 14.25 | 0.013 |
| Estimated average 2004-05 | 13.74 | 13.96 | 0.240 | 13.94 | 14.08 | 0.148 |
| Estimated average 2006-07 | 13.38 | 13.60 | 0.398 | 13.69 | 13.86 | 0.057 |
Notes:
People that at baseline had never been diagnosed with CHD, stroke, diabetes, hypertension, pulmonary disease, Alzheimer, Parkinson’s, cancer or any limiting longstanding illness.
Adjusted for age, cohabiting status, employment status, education, wealth, smoking status, physical activity, frequency of alcohol consumption, pain, number of limitations with ADLs, depressive symptoms. Results based on five imputed data sets.
Results obtained from random intercept models with interaction terms between time and sex.
Changes in depressive caseness
Changes in prevalence of depressive caseness estimated using adjusted random intercepts models are presented in Table 4.Women with CHD had higher odds of depressive caseness than men at baseline and two-year follow-up, independent of covariates adjustment (2002-03: OR 1.99 95%CI 1.24-3.19 p<0.01; 2004-05: OR 1.76 95%CI 1.08-2.86 p=0.023). The odds of depressive caseness were constant over time among men with CHD, while women with CHD were less likely to report depressive caseness at four year follow-up compared to baseline, however, the difference was borderline significant (OR: 0.63 95%CI 0.40-0.97 p=0.024).
Table 4.
Changes over time in depressive caseness among people with CHD and the Healthy group. England 2002-03 to 2006-07
| CHD group (N=895) | Healthy groupa (N=3,601) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| ORc | Std. Err. | P-value | ORc | Std. Err. | P-value | |
|
|
||||||
| Fully adjustedb | ||||||
| Women vs Men (2002-03) | 1.99 | 0.48 | <0.010 | 2.31 | 0.30 | <0.001 |
| Women vs Men (2004-05) | 1.76 | 0.44 | 0.023 | 1.78 | 0.23 | <0.001 |
| Women vs Men (2006-07) | 1.18 | 0.30 | 0.517 | 2.56 | 0.35 | <0.001 |
| Men’s depression (2002-03) | 1 | 1 | ||||
| Men’s depression (2004-05) | 1.20 | 0.24 | 0.359 | 1.17 | 0.14 | 0.212 |
| Men’s depression (2006-07) | 1.06 | 0.22 | 0.779 | 0.85 | 0.11 | 0.203 |
| Women’s depression (2002-03) | 1 | 1 | ||||
| Women’s depression (2004-05) | 1.06 | 0.23 | 0.774 | 0.90 | 0.09 | 0.274 |
| Women’s depression (2006-07) | 0.63 | 0.14 | 0.024 | 0.94 | 0.10 | 0.551 |
| Between variance | 20.65 | 0.04 | 19.17 | 0.02 | ||
| Within variance | 30.62 | 0.02 | 24.95 | 0.01 | ||
Notes:
People that at baseline had never been diagnosed with CHD, stroke, diabetes, hypertension, pulmonary disease, Alzheimer, Parkinson’s, cancer or any limiting longstanding illness.
Adjusted for age, cohabiting status, employment status, education, wealth, smoking status, physical activity, frequency of alcohol consumption, pain, number of limitations with ADLs.
Estimated using random intercept models with interaction terms between time and sex. Results based on five imputed data sets.
From the models presented in Table 4 we estimated the adjusted predicted probabilities of depressive caseness and presented these results graphically in Figure 2. The probabilities are presented in the text as percentages. The probabilities of depressive caseness were fairly constant over time for men with CHD and men and women from the Healthy group. For women with CHD the prevalence of depressive caseness was constant between baseline and two-year follow-up but was 8 percentage points lower at four year follow-up (2006-07) compared to baseline (p<0.001).At baseline and at two year follow-up women had 13 percentage points higher probability of reporting depressive caseness compared to men (p=0.023), this difference reduced to 7 percentage points at four year follow-up (p=0.517).
Figure 2.
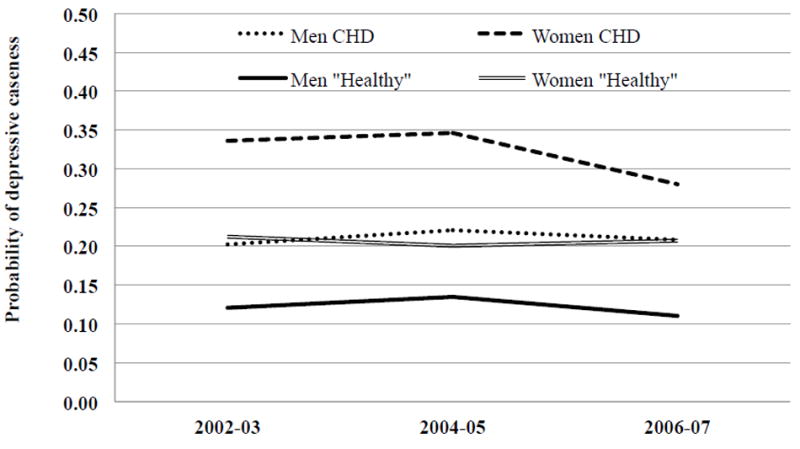
Predicted probabilities of depressive caseness among people with CHD and the Healthy group, by gender. England 2002-03 to 2006-07
Results adjusted for gender age, cohabiting status, employment status, educational attainment, wealth, smoking status, alcohol consumption, physical activity, pain, ADLs, positive support and number of close friends and family. Results based on five imputed data sets.
After adjusting for covariates, men with CHD had 10 percentage points higher probability of depressive caseness compared with in the Healthy group only at four year follow-up (p=0.024). Baseline differences in depressive caseness between people with CHD and those in the Healthy group, shown in Table 1, were no longer statistically significant when the model was adjusted for covariates. At each assessment women with CHD had the same probability of reporting 3 or more depressive symptoms as women from the Healthy group after adjustment. Gender differences in depressive caseness were found at each assessment in the Healthy group.
DISCUSSION
In this large population-based study of older people living in England we found gender-specific changes over time for quality of life and depressive caseness among people with CHD. For men, quality of life decreased progressively over time by approximately three points (which in the whole ELSA sample is equivalent to the effect of having diabetes), while for women it remained stable in the first two years, only declining at four year follow-up by less than two points. We showed that at two year follow-up and four year follow-up women had on average three points higher quality of life than men. It was found that the domain of quality of life for which men and women report differences is Autonomy.
Changes in prevalence of 3 or more depressive symptoms also differed according to gender. The probability of this depressive caseness did not change significantly over time for men. For women the prevalence at two year follow-up was the same as at baseline, while at four year follow-up prevalence had reduced below the baseline level. We found gender differences in depressive caseness independent of covariates at baseline and at two year follow-up but not at four year follow-up.
In the Healthy group men and women reported similar trajectories of quality of life and depressive caseness. Women were more likely than men to have depressive caseness at each assessment; whereas quality of life only differed by gender at two year follow-up and was higher for women than men..
After adjustment we found that men with CHD had lower quality of life than men from the Healthy group at each follow-up, while their probability of depressive caseness was higher at four year follow-up only. Women with CHD had lower quality of life than women in the Healthy group at baseline but no significant differences were found in depressive caseness at any time.
Our finding of no gender differences in quality of life at baseline is in agreement with two earlier studies of myocardial infarction patients (Kristofferzon et al., 2005; Mendes de Leon et al., 2001). Our study is the first to show that women’s quality of life is better than men’s quality of life in the two and four years following a CHD event. To our knowledge, this study is also the first to show that women’s quality of life does not deteriorate in the first two years following a CHD event, while men’s quality of life deteriorated progressively over time.
Our observation that women are more likely than men to report depressive caseness following a CHD event is well-known (Forrester et al., 1992; Mallik et al., 2006) However, this study is the first to show that in unadjusted analyses gender differences are present at baseline and at two year follow-up but not at four year follow-up. The gender difference in depressive caseness disappeared after adjustment for covariates, implying that it was other characteristics rather than gender per se that led to the observed difference in crude analysis. Only one previous study reported that gender differences in the mental health dimension of health-related quality of life found at one year post-myocardial infarction did not persist once the model was adjusted for demographic, clinical, comorbid and psychosocial covariates (Norris et al., 2007). Our finding of an improvement in depressive caseness in women is somewhat in line with the study of Bjerkeset et al., (2005) which showed that women had a significant decrease in the risk of depressive symptoms (measured using a cut-off of 8 and more symptoms on the Hospital Anxiety and Depression rating Scale) two year post-myocardial infarction.
We found that among people with CHD, depressive caseness was constant over time among men while women reported an improvement. Quality of life decreased in both men and women in the long term, although for women the decrease was less important. It is possible that depressive caseness reflected the immediate psychological reaction to the cardiac event, while the long term decline in quality of life was the consequence of the burdens that the disease placed on the health and socioeconomic status of individuals. A CHD event often involves changes to an individual’s lifestyles, therefore recovery from poor quality of life might require a long time, especially in those who as a result of the disease have experienced loss of control and autonomy. Results of this study provided evidence of lower autonomy among men who experienced CHD compared to women. In this sample of older people, the long term decline in quality of life could also be partly a consequence of ageing. This is supported by our finding of a decline over time in quality of life among healthy individuals, and it is consistent with previous studies reporting a trend of worsening quality of life over time especially at older ages (Zaninotto et al., 2009). On the other hand, the improvement in depressive caseness seen in women might reflect a process of adaptation to the disease. It is thought that women may generally have more effective coping strategies for managing stressful life events than men (Hobfoll et al., 1994). In particular, after experiencing myocardial infarction women are more likely than men to adopt problem-focused and emotion-focused coping strategies (Bogg et al., 2000). Learning more about how women cope with CHD may provide lessons that will help men adopt more effective strategies for long-term recovery in their quality of life.
No previous studies have reported a decrease over time in the quality of life of women concomitant with a reduction in the risk of reporting 3 or more depressive symptoms. One possible explanation might be in the relatively long follow-up of our study. Another possible explanation might be that women reported depressive caseness as a consequence of the disease, therefore in the long term they might have adjusted to the CHD event and consequently their mental health improved.
Study strengths and limitations
One of the strengths of this study is the use of a large sample of older people living in private households in England. The study was designed to collect information on topics necessary to understand the economic, social, psychological and health elements of the ageing process. Some of the advantages of using this data set include adjustment for several important covariates; and the ability to compare the results for the CHD population with those of a Healthy group of people of similar age. This allowed us to determine whether patterns of change over time were specific to CHD, or reflected more general trends in men and women as they grow older.
Another strength of this study is the use of two distinct measures of well-being. By exploring both quality of life and depressive caseness after the CHD event it was possible to untangle aspects of people’s well-being never formally identified before. Results from this study highlight the difference between these two aspects of people’s well-being and contribute to the current debate on the importance of measuring them separately to develop a broader appreciation of people’s lives (Dolan et al., 2011; Kahneman et al., 2006).
The treatment of missing data constitutes a further strength of this analysis. Missing data often occur in epidemiological studies where non-response is a major problem. The development of sophisticated missing data techniques allows researchers to improve the validity of research results (Sterne et al., 2000).We used a technique to impute missing data particularly suitable for repeated measures, the recently developed two-fold fully conditional specification (Nevalainen et al., 2009).
One possible limitation of this analysis is the use of a self-reported measure of CHD. Although an objective measure of CHD would have been preferable, a number of validation studies have demonstrated that self-reported CHD is reasonably accurate when compared with medical records (Lampe et al., 1999; Baumeister et al., 2010). Furthermore, no allowance was made for the severity of cardiac illness. Some people will have more severe CHD than others, but we were unable to take this into account in the analyses. Also, no distinction was made between first and recurrent CHD events, so we implicitly assumed that a recent recurrence was as important as the first onset.
Our measure of depression is not designed to detect depressive illness; therefore we were unable to account for the severity or the chronicity of depression. However, we believe that reporting at least 3 symptoms is valid as a negative indicator of mental health.
Lastly, the changes in quality of life and depressive caseness were analysed according to disease status at baseline. About 3% of people in the Healthy group experienced a CHD event after the baseline interview and 7% developed other diseases (such as diabetes, stroke, pulmonary disease, Alzheimer, Parkinson’s and cancer). As a consequence, the healthy controls were not necessarily free of disease at follow-up. The decision not to exclude people that developed a chronic condition at follow-up was made to allow fair comparisons with the group of people with CHD, who at subsequent waves have also experienced a chronic condition and about 12% of them experienced a repeat CHD event might have affected their quality of life and depressive caseness.
CONCLUSIONS
We found that changes over time in quality of life differed from those for depressive caseness after a CHD event. Men’s quality of life declined over time and no changes in depressive caseness were found. Women’s quality of life declined slightly only between baseline and four year follow-up, while in the same period their risk of having depressive caseness diminished. In the long term women with CHD reported higher scores of quality of life than men, and when looking at each domain of quality of life it was found that they reported higher scores of Autonomy. No gender differences in depressive caseness were found.
Our findings might inform caregivers that after CHD the mental health of men in terms of depressive caseness does not necessarily deteriorate over time, and the mental health of women could even improve in the long term. On the other hand, men seem to be less able to cope with the disease in the long term with respect to their quality of life and in particular in the Autonomy domain of quality of life. Men’s quality of life should be monitored in the years following the event in order to reduce the risk of long term deterioration and to help them to maintain autonomy. Health professionals might advise patients and their immediate relatives on effective strategies for coping with cardiac events in order to help maintain good mental health and quality of life. The findings of this study indicate that the focus of such advice might differ between men and women.
Acknowledgments
Source of funding: The ELSA study is funded by National Institutes of Health/National Institute of Aging [5R01AG017644-08]; and the Office for National Statistics for UK Government departments [NT-4779A/01]. The findings and conclusions in this report are those of the authors and do not represent the views of the funders, who had no role in the design or publication of the manuscript.
Footnotes
Conflicts of interest: The authors have no conflicts of interest.
Contributor Information
Paola Zaninotto, Research Department of Epidemiology and Public Health, 1-19 Torrington Place, UCL, WC1E 7HB London, Phone 0044(0)2076791668.
Amanda Sacker, Department of Epidemiology and Public Health, 1-19 Torrington Place, UCL, WC1E 7HB London, a.sacker@ucl.ac.uk Phone 0044(0)2076791711.
Elizabeth Breeze, Research Department of Epidemiology and Public Health, 1-19 Torrington Place, UCL, WC1E 7HB London, e.breeze@ucl.ac.uk Phone 0044(0)2076791656.
Anne McMunn, Research Department of Epidemiology and Public Health, 1-19 Torrington Place, UCL, WC1E 7HB London, a.mcmunn@ucl.ac.uk Phone 0044(0)2076791730.
Andrew Steptoe, Research Department of Epidemiology and Public Health, 1-19 Torrington Place, UCL, WC1E 7HB London, a.steptoe@ucl.ac.uk Phone 0044(0)2076791804.
References
- 1.Baumeister H, Kriston L, Bengel J, Harter M. High agreement of self-report and physician-diagnosed somatic conditions yields limited bias in examining mental-physical comorbidity. J Clin Epidemiol. 2010 May;63(5):558–65. doi: 10.1016/j.jclinepi.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 2.Bjerkeset O, Nordahl HM, Mykletun A, Holmen J, Dahl AA. Anxiety and depression following myocardial infarction: gender differences in a 5-year prospective study. J Psychosom Res. 2005 Feb;58(2):153–61. doi: 10.1016/j.jpsychores.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 3.Blane D, Netuveli G, Montgomery SM. Quality of life, health and physiological status and change at older ages. Soc Sci Med. 2008;66:1579–1587. doi: 10.1016/j.socscimed.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 4.Bogg J, Thornton E, Bundred P. Gender variability in mood, quality of life and coping following primary myocardial infarction. Coronary Health Care. 2000;4:163–8. [Google Scholar]
- 5.Scarborough P, Bhatnagar P, Wickramasinghe K, Smolina K, Mitchell C, Rayner M, editors. British heart foundation. Coronary heart disease statistics 2010 edition. University of Oxford, Department of Public Health; 2010. [Google Scholar]
- 6.Brink E, Grankvist G, Karlson BW, Hallberg LR. Health-related quality of life in women and men one year after acute myocardial infarction. Qual Life Res. 2005 Apr;14(3):749–57. doi: 10.1007/s11136-004-0785-z. [DOI] [PubMed] [Google Scholar]
- 7.Bush DE, Ziegelstein RC, Tayback M, Richter D, Stevens S, Zahalsky H, Fauerbach JA. Even minimal symptoms of depression increase mortality risk after acute myocardial infarction. Am J Cardiol. 2001 Aug 15;88(4):337–41. doi: 10.1016/s0002-9149(01)01675-7. [DOI] [PubMed] [Google Scholar]
- 8.Carney RM, Blumenthal JA, Catellier D, Freedland KE, Berkman LF, Watkins LL, Czajkowski SM, Hayano J, Jaffe AS. Depression as a risk factor for mortality after acute myocardial infarction. Am J Cardiol. 2003 Dec 1;92(11):1277–81. doi: 10.1016/j.amjcard.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 9.Chou KL. Reciprocal relationship between pain and depression in older adults: evidence from the English Longitudinal Study of Ageing. Journal of Affective Disorders. 2007;103(1-3):115–123. doi: 10.1016/j.jad.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 10.Dolan P, Layard R, Metcalf R. London: Office for National Statistics; 2011. [8 May 2012]. Measuring subjective well-being for public policy. http://cep.lse.ac.uk/pubs/download/special/cepsp23.pdf. [Google Scholar]
- 11.Ferketich AK, Schwartzbaum JA, Frid DJ, Moeschberger ML. Depression as an antecedent to heart disease among women and men in the NHANES I study. National Health and Nutrition Examination Survey. Arch Intern Med. 2000 May 8;160(9):1261–8. doi: 10.1001/archinte.160.9.1261. [DOI] [PubMed] [Google Scholar]
- 12.Forrester AW, Lipsey JR, Teitelbaum ML, DePaulo JR, Andrzejewski PL. Depression following myocardial infarction. Int J Psychiatry Med. 1992;22(1):33–46. doi: 10.2190/CJ9D-32C2-8CM7-FT3D. [DOI] [PubMed] [Google Scholar]
- 13.Goldstein H. Multilevel statistical models. 3. London: Edward Arnold; 2003. [Google Scholar]
- 14.Hobfoll SE, Dunahoo CL, Benporath Y, Monnier J. Gender and Coping - the Dual-Axis Model of Coping. American Journal of Community Psychology. 1994 Feb;22(1):49–82. doi: 10.1007/BF02506817. [DOI] [PubMed] [Google Scholar]
- 15.Hyde M, Wiggins RD, Higgs P, Blane DB. A measure of quality of life in early old age: the theory, development and properties of a needs satisfaction model (CASP-19) Aging Ment Health. 2003 May;7(3):186–94. doi: 10.1080/1360786031000101157. [DOI] [PubMed] [Google Scholar]
- 16.Kahneman D, Krueger AB. Developments in the measurement of subjective well-being. Journal of Economic Perspectives. 2006;20(1):3–24. [Google Scholar]
- 17.Kristofferzon ML, Lofmark R, Carlsson M. Coping, social support and quality of life over time after myocardial infarction. J Adv Nurs. 2005 Oct;52(2):113–24. doi: 10.1111/j.1365-2648.2005.03571.x. [DOI] [PubMed] [Google Scholar]
- 18.Lampe FC, Walker M, Lennon LT, Whincup PH, Ebrahim S. Validity of a self-reported history of doctor-diagnosed angina. J Clin Epidemiol. 1999 Jan;52(1):73–81. doi: 10.1016/s0895-4356(98)00146-2. [DOI] [PubMed] [Google Scholar]
- 19.Lane D, Ring C, Lip GY, Carroll D. Depression, indirect clinical markers of cardiac disease severity, and mortality following myocardial infarction. Heart. 2005 Apr;91(4):531–2. doi: 10.1136/hrt.2004.036392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lauzon C, Beck CA, Huynh T, Dion D, Racine N, Carignan S, Diodati JG, Charbonneau F, Dupuis R, Pilote L. Depression and prognosis following hospital admission because of acute myocardial infarction. CMAJ. 2003 Mar 4;168(5):547–52. [PMC free article] [PubMed] [Google Scholar]
- 21.Lesperance F, Frasure-Smith N, Talajic M. Major depression before and after myocardial infarction: its nature and consequences. Psychosom Med. 1996 Mar;58(2):99–110. doi: 10.1097/00006842-199603000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Lesperance F, Frasure-Smith N. Depression in patients with cardiac disease: a practical review. J Psychosom Res. 2000 Apr;48(4-5):379–91. doi: 10.1016/s0022-3999(99)00102-6. [DOI] [PubMed] [Google Scholar]
- 23.Mallik S, Spertus JA, Reid KJ, Krumholz HM, Rumsfeld JS, Weintraub WS, Purva Agarwal MD, Santra M, Bidyasar S, Lichtman JH, Wenger NK, Vaccarino V. Depressive symptoms after acute myocardial infarction: evidence for highest rates in younger women. Arch Intern Med. 2006 Apr 24;166(8):876–83. doi: 10.1001/archinte.166.8.876. [DOI] [PubMed] [Google Scholar]
- 24.Mendes de Leon CF, Dilillo V, Czajkowski S, Norten J, Schaefer J, Catellier D, Blumenthal JA. Psychosocial characteristics after acute myocardial infarction: the ENRICHD pilot study. Enhancing Recovery in Coronary Heart Disease. J Cardiopulm Rehabil. 2001 Nov;21(6):353–62. doi: 10.1097/00008483-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Nevalainen J, Kenward MG, Virtanen SM. Missing values in longitudinal dietary data: a multiple imputation approach based on a fully conditional specification. Stat Med. 2009 Dec 20;28(29):3657–69. doi: 10.1002/sim.3731. [DOI] [PubMed] [Google Scholar]
- 26.Netuveli G, Wiggins RD, Hildon Z, Montgomery SM, Blane D. Quality of life at older ages: evidence from the English longitudinal study of aging (wave 1) J Epidemiol Community Health. 2006 Apr;60(4):357–63. doi: 10.1136/jech.2005.040071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Norris CM, Hegadoren K, Pilote L. Depression symptoms have a greater impact on the 1-year health-related quality of life outcomes of women post-myocardial infarction compared to men. Eur J Cardiovasc Nurs. 2007 Jun;6(2):92–8. doi: 10.1016/j.ejcnurse.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 28.Parashar S, Rumsfeld JS, Spertus JA, Reid KJ, Wenger NK, Krumholz HM, Amin A, Weintraub WS, Lichtman J, Dawood N, Vaccarino V. Time course of depression and outcome of myocardial infarction. Arch Intern Med. 2006 Oct 9;166(18):2035–43. doi: 10.1001/archinte.166.18.2035. [DOI] [PubMed] [Google Scholar]
- 29.Rice N, Bandinelli S, Corsi AM, Ferrucci L, Guralnik JM, et al. The paraoxonase (PON1) Q192R polymorphism is not associated with poor health status or depression in the ELSA or InCHIANTi studies. International Journal of Epidemiology. 2009;38:1374–1379. doi: 10.1093/ije/dyp265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 31.Rubin DB. Multiple imputation after 18+ years. Journal of the American Statistical Association. 1996 Jun;91(434):473–89. [Google Scholar]
- 32.Scholes S, Taylor R, Cheshire H, Cox K, Lessof C. Technical report (ELSA wave 3): living in the 21st century: older people in England. 2008 http://www.ifs.org.uk/elsa/report06/w2_tech.pdf.
- 33.Stafford M, McMunn A, Zaninotto P, Nazroo J. Positive and negative exchanges in social relationships as predictors of depression: evidence from the English Longitudinal Study of Aging. J Aging Health. 2011 Jun;23(4):607–28. doi: 10.1177/0898264310392992. [DOI] [PubMed] [Google Scholar]
- 34.Steffick D. HRS/AHEAD Documentation. Report DR-005. Survey Research Center, University of Michigan; Ann Arbor, MI: 2000. [2 February 2011]. Documentation of affective functioning measures in the Health and Retirement Study. http://hrsonline.isr.umich.edu/sitedocs/userg/dr-005.pdf. [Google Scholar]
- 35.Steptoe A, Breeze E, Banks J, Nazroo J. Cohort Profile: The English Longitudinal Study of Ageing. Int J Epidemiol. 2012 Nov 9; doi: 10.1093/ije/dys168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, Wood AM, Carptenter J. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. doi: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Westin L, Carlsson R, Erhardt L, Cantor-Graae E, McNeil T. Differences in quality of life in men and women with ischemic heart disease. A prospective controlled study. Scand Cardiovasc J. 1999;33(3):160–5. doi: 10.1080/14017439950141795. [DOI] [PubMed] [Google Scholar]
- 38.Wiggins RD, Netuveli G, Hyde M, Higgs P, Blane D. The evaluation of a self-enumerated scale of quality of life (CASP-19) in the context of research on ageing: A combination of exploratory and confirmatory approaches. Social Indicators Research. 2008 Oct;89(1):61–77. [Google Scholar]
- 39.Wiklund I, Herlitz J, Johansson S, Bengtson A, Karlson BW, Persson NG. Subjective symptoms and well-being differ in women and men after myocardial infarction. Eur Heart J. 1993 Oct;14(10):1315–9. doi: 10.1093/eurheartj/14.10.1315. [DOI] [PubMed] [Google Scholar]
- 40.Zaninotto P, Falaschetti E, Sacker A. Age trajectories of quality of life among older adults: results from the English Longitudinal Study of Ageing. Qual Life Res. 2009 Dec;18(10):1301–9. doi: 10.1007/s11136-009-9543-6. [DOI] [PubMed] [Google Scholar]
- 41.Ziegelstein RC, Fauerbach JA, Stevens SS, Romanelli J, Richter DP, Bush DE. Patients with depression are less likely to follow recommendations to reduce cardiac risk during recovery from a myocardial infarction. Arch Intern Med. 2000 Jun 26;160(12):1818–23. doi: 10.1001/archinte.160.12.1818. [DOI] [PubMed] [Google Scholar]
- 42.Ziegelstein RC. Depression in patients recovering from a myocardial infarction. JAMA. 2001 Oct 3;286(13):1621–7. doi: 10.1001/jama.286.13.1621. [DOI] [PubMed] [Google Scholar]


