Abstract
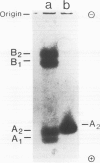
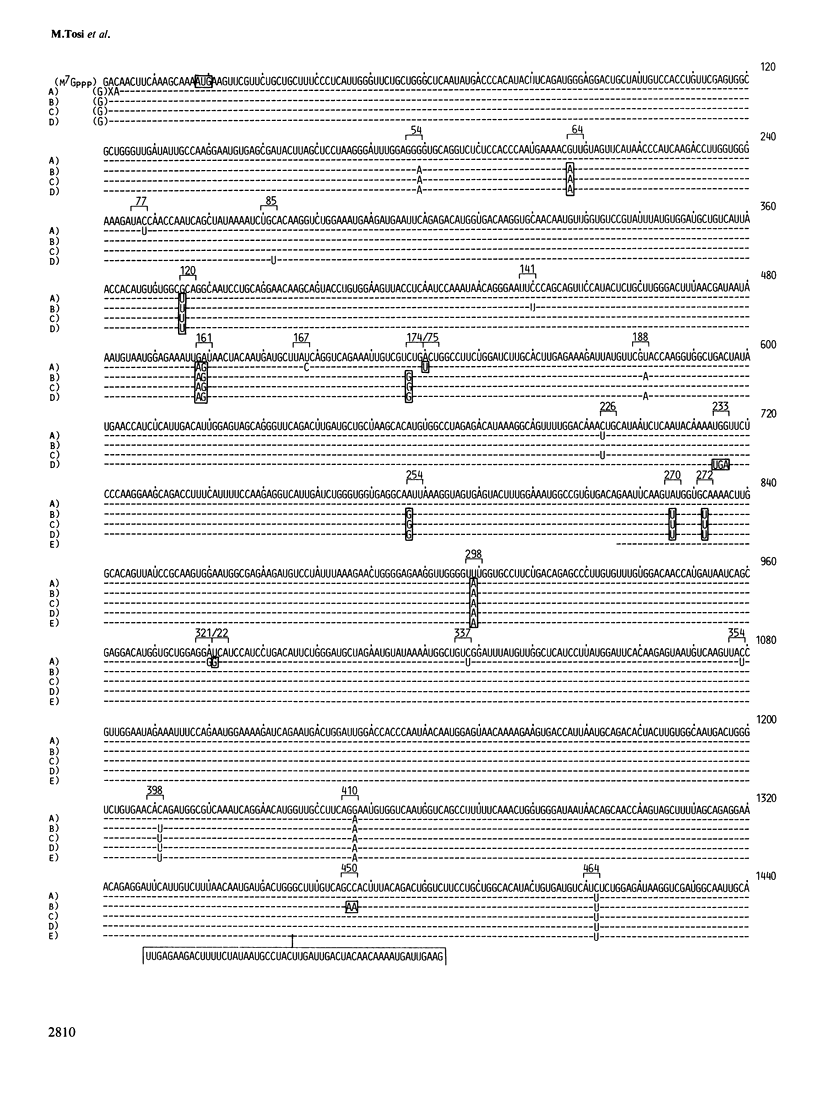
The number of active Amy-2 genes has been estimated in strain CE/J mice which produce four distinct electrophoretic forms of alpha-amylase in their pancreas. cDNA cloning and DNA sequence analysis discloses five distinct mRNA sequences which differ by approximately 1% of their nucleotides. Two of these mRNAs specify the same protein. Changes in the nucleotide sequences result in amino acid replacements that alter the net charges of the deduced proteins. This has allowed a tentative assignment of individual mRNAs to isozymes detected by electrophoresis. Quantitative Southern blot hybridization using a DNA probe specific for the first exon of Amy-2 reveals the presence of greater than 10 Amy-2 related sequences per haploid CE/J genome. Models which could account for the mouse strain-specific differences with respect to the number of pancreatic alpha-amylase isozymes and their variable but genetically determined quantitative ratios are discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloor J. H., Meisler M. H., Nielsen J. T. Genetic determination of amylase synthesis in the mouse. J Biol Chem. 1981 Jan 10;256(1):373–377. [PubMed] [Google Scholar]
- Deng G., Wu R. An improved procedure for utilizing terminal transferase to add homopolymers to the 3' termini of DNA. Nucleic Acids Res. 1981 Aug 25;9(16):4173–4188. doi: 10.1093/nar/9.16.4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eicher E. M., Lane P. W. Assignment of LH XVI to chromosome 3 in the mouse. J Hered. 1980 Sep-Oct;71(5):315–318. doi: 10.1093/oxfordjournals.jhered.a109378. [DOI] [PubMed] [Google Scholar]
- Groner B., Hynes N. E. Number and location of mouse mammary tumor virus proviral DNA in mouse DNA of normal tissue and of mammary tumors. J Virol. 1980 Mar;33(3):1013–1025. doi: 10.1128/jvi.33.3.1013-1025.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenbüchle O., Bovey R., Young R. A. Tissue-specific expression of mouse-alpha-amylase genes: nucleotide sequence of isoenzyme mRNAs from pancreas and salivary gland. Cell. 1980 Aug;21(1):179–187. doi: 10.1016/0092-8674(80)90125-7. [DOI] [PubMed] [Google Scholar]
- Hagenbüchle O., Tosi M., Schibler U., Bovey R., Wellauer P. K., Young R. A. Mouse liver and salivary gland alpha-amylase mRNAs differ only in 5' non-translated sequences. Nature. 1981 Feb 19;289(5799):643–646. doi: 10.1038/289643a0. [DOI] [PubMed] [Google Scholar]
- Harding J. D., Przybyla A. E., MacDonald R. J., Pictet R. L., Rutter W. J. Effects of dexamethasone and 5-bromodeoxyuridine on the synthesis of amylase mRNA during pancreatic development in vitro. J Biol Chem. 1978 Oct 25;253(20):7531–7537. [PubMed] [Google Scholar]
- Hjorth J. P. Altered salivary amylase gene in the mouse strain BXD-16. Heredity (Edinb) 1982 Feb;48(Pt 1):127–135. doi: 10.1038/hdy.1982.13. [DOI] [PubMed] [Google Scholar]
- Hjorth J. P. Genetic variation in mouse salivary amylase rate of synthesis. Biochem Genet. 1979 Aug;17(7-8):665–682. doi: 10.1007/BF00502125. [DOI] [PubMed] [Google Scholar]
- Hjorth J. P., Lusis A. J., Nielsen J. T. Multiple structural genes for mouse amylase. Biochem Genet. 1980 Apr;18(3-4):281–302. doi: 10.1007/BF00484242. [DOI] [PubMed] [Google Scholar]
- Hubacek J., Glover S. W. Complementation analysis of temperature-sensitive host specificity mutations in Escherichia coli. J Mol Biol. 1970 May 28;50(1):111–127. doi: 10.1016/0022-2836(70)90108-7. [DOI] [PubMed] [Google Scholar]
- Karn R. C., Petersen T. E., Hjorth J. P., Nieles J. T., Roepstorff P. Characterization of the amino termini of mouse salivary and pancreatic amylases. FEBS Lett. 1981 Apr 20;126(2):293–296. doi: 10.1016/0014-5793(81)80264-5. [DOI] [PubMed] [Google Scholar]
- Land H., Grez M., Hauser H., Lindenmaier W., Schütz G. 5'-Terminal sequences of eucaryotic mRNA can be cloned with high efficiency. Nucleic Acids Res. 1981 May 25;9(10):2251–2266. doi: 10.1093/nar/9.10.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- Nielsen J. T., Sick K. Gentic polymorphism of amylase isoenzymes in feral populations of the house mouse. Hereditas. 1975;79(2):279–286. [PubMed] [Google Scholar]
- Nielsen J. T. Variation in amylase haplotypes among congenic lines of the house mouse. Genetics. 1982 Nov;102(3):571–582. doi: 10.1093/genetics/102.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owerbach D., Nielsen J. T., Rutter W. J. On the mechanism of variation of pancreatic amylase levels in mouse strains. J Biol Chem. 1981 Jun 25;256(12):6502–6506. [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E., Schibler U. Comparison of mRNA precursors in plasmacytomas producing closely related kappa chains. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3678–3682. doi: 10.1073/pnas.76.8.3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schibler U., Hagenbüchle O., Wellauer P. K., Pittet A. C. Two promoters of different strengths control the transcription of the mouse alpha-amylase gene Amy-1a in the parotid gland and the liver. Cell. 1983 Jun;33(2):501–508. doi: 10.1016/0092-8674(83)90431-2. [DOI] [PubMed] [Google Scholar]
- Schibler U., Pittet A. C., Young R. A., Hagenbüchle O., Tosi M., Gellman S., Wellauer P. K. The mouse alpha-amylase multigene family. Sequence organization of members expressed in the pancreas, salivary gland and liver. J Mol Biol. 1982 Mar 5;155(3):247–266. doi: 10.1016/0022-2836(82)90004-3. [DOI] [PubMed] [Google Scholar]
- Schibler U., Tosi M., Pittet A. C., Fabiani L., Wellauer P. K. Tissue-specific expression of mouse alpha-amylase genes. J Mol Biol. 1980 Sep 5;142(1):93–116. doi: 10.1016/0022-2836(80)90208-9. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Strahler J. R., Meisler M. Two distinct pancreatic amylase genes are active in YBR mice. Genetics. 1982 May;101(1):91–102. doi: 10.1093/genetics/101.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosi M., Young R. A., Hagenbüchle O., Schibler U. Multiple polyadenylation sites in a mouse alpha-amylase gene. Nucleic Acids Res. 1981 May 25;9(10):2313–2323. doi: 10.1093/nar/9.10.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner L. A., Neel J. V., Meisler M. H. Separation of allelic variants by two-dimensional electrophoresis. Am J Hum Genet. 1982 Mar;34(2):209–215. [PMC free article] [PubMed] [Google Scholar]
- Wellauer P. K., Dawid I. B. The structural organization of ribosomal DNA in Drosophila melanogaster. Cell. 1977 Feb;10(2):193–212. doi: 10.1016/0092-8674(77)90214-8. [DOI] [PubMed] [Google Scholar]
- Young R. A., Hagenbüchle O., Schibler U. A single mouse alpha-amylase gene specifies two different tissue-specific mRNAs. Cell. 1981 Feb;23(2):451–458. doi: 10.1016/0092-8674(81)90140-9. [DOI] [PubMed] [Google Scholar]