Abstract
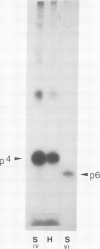
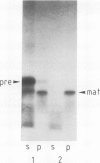
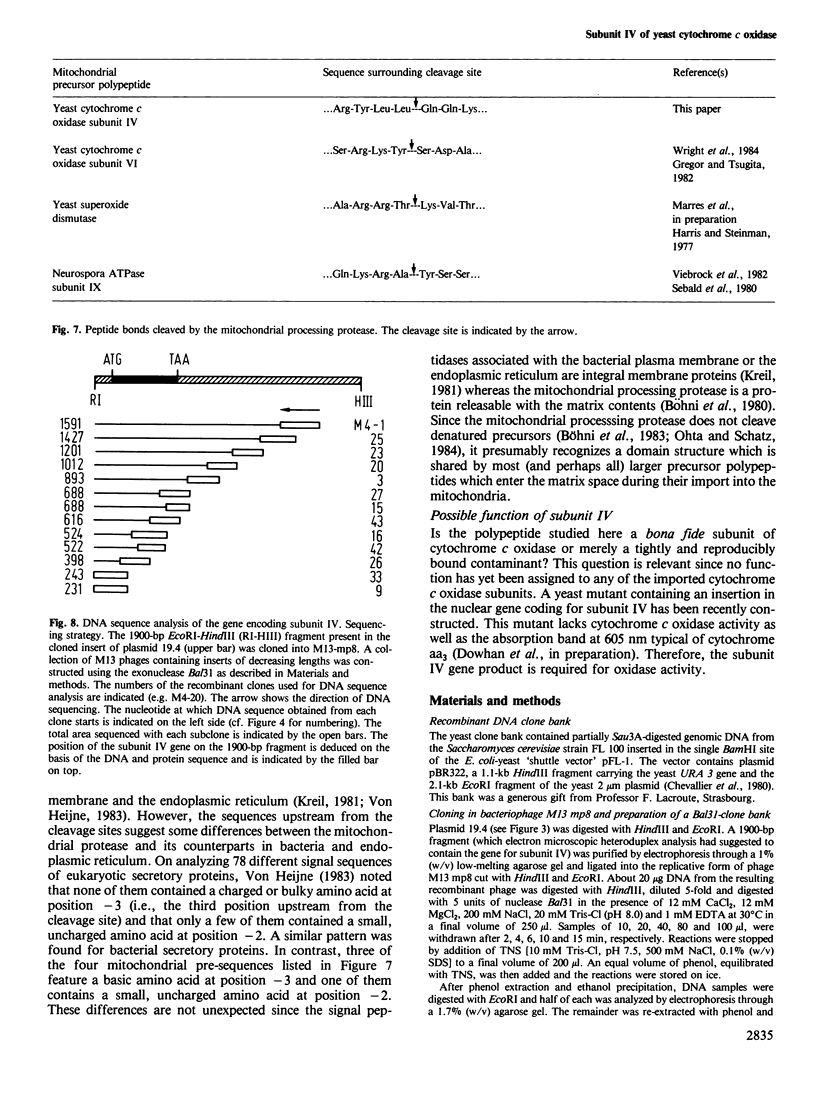
The six small subunits (IV-VII, VIIa, VIII) of yeast cytochrome c oxidase are encoded by nuclear genes and imported into the mitochondria. We have isolated the gene for subunit IV from a yeast genomic clone bank and determined its complete nucleotide sequence. We have also isolated subunit IV from purified yeast cytochrome c oxidase and determined most of its amino acid sequence which confirms the positioning of approximately 90% of the amino acid residues. The sequence comparison shows that the coding sequence of the gene lacks introns and that subunit IV is made as a precursor with an amino-terminal extension of 25 residues, five of which are basic and none of them acidic. Precursor processing involves cleavage of a Leu-Gln bond.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biewald R., Buse G. Studies on cytochrome c oxidase, IX. The primary structure of polypeptide VIa. Hoppe Seylers Z Physiol Chem. 1982 Oct;363(10):1141–1153. doi: 10.1515/bchm2.1982.363.2.1141. [DOI] [PubMed] [Google Scholar]
- Birchmeier W., Kohler C. E., Schatz G. Interaction of integral and peripheral membrane proteins: affinity labeling of yeast cytochrome oxidase by modified yeast cytochrome c. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4334–4338. doi: 10.1073/pnas.73.12.4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buse G., Steffens G. J. Studies on cytochrome c oxidase, II. The chemical constitution of a short polypeptide from the beef heart enzyme. Hoppe Seylers Z Physiol Chem. 1978 Aug;359(8):1005–1009. doi: 10.1515/bchm2.1978.359.2.1005. [DOI] [PubMed] [Google Scholar]
- Böhni P. C., Daum G., Schatz G. Import of proteins into mitochondria. Partial purification of a matrix-located protease involved in cleavage of mitochondrial precursor polypeptides. J Biol Chem. 1983 Apr 25;258(8):4937–4943. [PubMed] [Google Scholar]
- Cabral F., Schatz G. Identification of cytochrome c oxidase subunits in nuclear yeast mutants lacking the functional enzyme. J Biol Chem. 1978 Jun 25;253(12):4396–4401. [PubMed] [Google Scholar]
- Capaldi R. A., Vanderkooi G. The low polarity of many membrane proteins. Proc Natl Acad Sci U S A. 1972 Apr;69(4):930–932. doi: 10.1073/pnas.69.4.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Chevallier M. R., Bloch J. C., Lacroute F. Transcriptional and translational expression of a chimeric bacterial-yeast plasmid in yeasts. Gene. 1980 Oct;11(1-2):11–19. doi: 10.1016/0378-1119(80)90082-7. [DOI] [PubMed] [Google Scholar]
- Cumsky M. G., McEwen J. E., Ko C., Poyton R. O. Nuclear genes for mitochondrial proteins. Identification and isolation of a structural gene for subunit V of yeast cytochrome c oxidase. J Biol Chem. 1983 Nov 25;258(22):13418–13421. [PubMed] [Google Scholar]
- Daum G., Gasser S. M., Schatz G. Import of proteins into mitochondria. Energy-dependent, two-step processing of the intermembrane space enzyme cytochrome b2 by isolated yeast mitochondria. J Biol Chem. 1982 Nov 10;257(21):13075–13080. [PubMed] [Google Scholar]
- Davis R. W., Davidson N. Electron-microscopic visualization of deletion mutations. Proc Natl Acad Sci U S A. 1968 May;60(1):243–250. doi: 10.1073/pnas.60.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Haan M., van Loon A. P., Kreike J., Vaessen R. T., Grivell L. A. The biosynthesis of the ubiquinol-cytochrome c reductase complex in yeast. DNA sequence analysis of the nuclear gene coding for the 14-kDa subunit. Eur J Biochem. 1984 Jan 2;138(1):169–177. doi: 10.1111/j.1432-1033.1984.tb07896.x. [DOI] [PubMed] [Google Scholar]
- Deters D., Müller U., Homberger H. Bulk isolation of yeast mitochondria. Methods Cell Biol. 1978;20:107–112. doi: 10.1016/s0091-679x(08)62012-9. [DOI] [PubMed] [Google Scholar]
- Gasser S. M., Daum G., Schatz G. Import of proteins into mitochondria. Energy-dependent uptake of precursors by isolated mitochondria. J Biol Chem. 1982 Nov 10;257(21):13034–13041. [PubMed] [Google Scholar]
- Gasser S. M. Import of polypeptides into isolated yeast mitochondria. Methods Enzymol. 1983;97:329–336. doi: 10.1016/0076-6879(83)97145-8. [DOI] [PubMed] [Google Scholar]
- Gasser S. M., Schatz G. Import of proteins into mitochondria. In vitro studies on the biogenesis of the outer membrane. J Biol Chem. 1983 Mar 25;258(6):3427–3430. [PubMed] [Google Scholar]
- Gregor I., Tsugita A. The amino acid sequence of cytochrome c oxidase subunit VI from Saccharomyces cerevisiae. J Biol Chem. 1982 Nov 10;257(21):13081–13087. [PubMed] [Google Scholar]
- Jeffreys A. J., Flavell R. A. A physical map of the DNA regions flanking the rabbit beta-globin gene. Cell. 1977 Oct;12(2):429–439. doi: 10.1016/0092-8674(77)90119-2. [DOI] [PubMed] [Google Scholar]
- Kadenbach B., Jarausch J., Hartmann R., Merle P. Separation of mammalian cytochrome c oxidase into 13 polypeptides by a sodium dodecyl sulfate-gel electrophoretic procedure. Anal Biochem. 1983 Mar;129(2):517–521. doi: 10.1016/0003-2697(83)90586-9. [DOI] [PubMed] [Google Scholar]
- Kreil G. Transfer of proteins across membranes. Annu Rev Biochem. 1981;50:317–348. doi: 10.1146/annurev.bi.50.070181.001533. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lewin A. S., Gregor I., Mason T. L., Nelson N., Schatz G. Cytoplasmically made subunits of yeast mitochondrial F1-ATPase and cytochrome c oxidase are synthesized as individual precursors, not as polyproteins. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3998–4002. doi: 10.1073/pnas.77.7.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccecchini M. L., Rudin Y., Blobel G., Schatz G. Import of proteins into mitochondria: precursor forms of the extramitochondrially made F1-ATPase subunits in yeast. Proc Natl Acad Sci U S A. 1979 Jan;76(1):343–347. doi: 10.1073/pnas.76.1.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAda P. C., Douglas M. G. A neutral metallo endoprotease involved in the processing of an F1-ATPase subunit precursor in mitochondria. J Biol Chem. 1982 Mar 25;257(6):3177–3182. [PubMed] [Google Scholar]
- Meinecke L., Steffens G. J., Buse G. Studies on cytochrome c oxidase, X. Isolation and amino-acid sequence of polypeptide VIIIb. Hoppe Seylers Z Physiol Chem. 1984 Mar;365(3):313–320. doi: 10.1515/bchm2.1984.365.1.313. [DOI] [PubMed] [Google Scholar]
- Mihara K., Blobel G. The four cytoplasmically made subunits of yeast mitochondrial cytochrome c oxidase are synthesized individually and not as a polyprotein. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4160–4164. doi: 10.1073/pnas.77.7.4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta S., Schatz G. A purified precursor polypeptide requires a cytosolic protein fraction for import into mitochondria. EMBO J. 1984 Mar;3(3):651–657. doi: 10.1002/j.1460-2075.1984.tb01862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Power S. D., Lochrie M. A., Patterson T. E., Poyton R. O. The nuclear-coded subunits of yeast cytochrome c oxidase. II. The amino acid sequence of subunit VIII and a model for its disposition in the inner mitochondrial membrane. J Biol Chem. 1984 May 25;259(10):6571–6574. [PubMed] [Google Scholar]
- Power S. D., Lochrie M. A., Poyton R. O. The nuclear-coded subunits of yeast cytochrome c oxidase. III. Identification of homologous subunits in yeast, bovine heart, and Neurospora crassa cytochrome c oxidases. J Biol Chem. 1984 May 25;259(10):6575–6578. [PubMed] [Google Scholar]
- Power S. D., Lochrie M. A., Sevarino K. A., Patterson T. E., Poyton R. O. The nuclear-coded subunits of yeast cytochrome c oxidase. I. Fractionation of the holoenzyme into chemically pure polypeptides and the identification of two new subunits using solvent extraction and reversed phase high performance liquid chromatography. J Biol Chem. 1984 May 25;259(10):6564–6570. [PubMed] [Google Scholar]
- Poyton R. O., Schatz G. Cytochrome c oxidase from bakers' yeast. III. Physical characterization of isolated subunits and chemical evidence for two different classes of polypeptides. J Biol Chem. 1975 Jan 25;250(2):752–761. [PubMed] [Google Scholar]
- Riezman H., Hase T., van Loon A. P., Grivell L. A., Suda K., Schatz G. Import of proteins into mitochondria: a 70 kilodalton outer membrane protein with a large carboxy-terminal deletion is still transported to the outer membrane. EMBO J. 1983;2(12):2161–2168. doi: 10.1002/j.1460-2075.1983.tb01717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riezman H., Hay R., Gasser S., Daum G., Schneider G., Witte C., Schatz G. The outer membrane of yeast mitochondria: isolation of outside-out sealed vesicles. EMBO J. 1983;2(7):1105–1111. doi: 10.1002/j.1460-2075.1983.tb01553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacher R., Steffens G. J., Buse G. Studies on cytochrome c oxidase, VI. Polypeptide IV. the complete primary structure. Hoppe Seylers Z Physiol Chem. 1979 Oct;360(10):1385–1392. doi: 10.1515/bchm2.1979.360.2.1385. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebald W., Machleidt W., Wachter E. N,N'-dicyclohexylcarbodiimide binds specifically to a single glutamyl residue of the proteolipid subunit of the mitochondrial adenosinetriphosphatases from Neurospora crassa and Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1980 Feb;77(2):785–789. doi: 10.1073/pnas.77.2.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens G. C., Steffens G. J., Buse G. Studies on cytochrome c oxidase, VIII. The amino acid sequence of polypeptide VII. Hoppe Seylers Z Physiol Chem. 1979 Nov;360(11):1641–1650. doi: 10.1515/bchm2.1979.360.2.1641. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Haniu M., Yasunobu K. T., Yu C. A., Yu L., Wei Y. H., King T. E. Amino acid sequence of subunit V of bovine heart cytochrome oxidase, the heme alpha-containing subunit. J Biol Chem. 1979 May 25;254(10):3879–3885. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Loon A. P., Van Eijk E., Grivell L. A. Biosynthesis of the ubiquinol-cytochrome c reductase complex in yeast. Discoordinate synthesis of the 11-kd subunit in response to increased gene copy number. EMBO J. 1983;2(10):1765–1770. doi: 10.1002/j.1460-2075.1983.tb01655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viebrock A., Perz A., Sebald W. The imported preprotein of the proteolipid subunit of the mitochondrial ATP synthase from Neurospora crassa. Molecular cloning and sequencing of the mRNA. EMBO J. 1982;1(5):565–571. doi: 10.1002/j.1460-2075.1982.tb01209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loon A. P., Maarse A. C., Riezman H., Grivell L. A. Isolation, characterization and regulation of expression of the nuclear genes for the core II and Rieske iron-sulphur proteins of the yeast ubiquinol-cytochrome c reductase. Gene. 1983 Dec;26(2-3):261–272. doi: 10.1016/0378-1119(83)90196-8. [DOI] [PubMed] [Google Scholar]
- van Loon A. P., de Groot R. J., van Eyk E., van der Horst G. T., Grivell L. A. Isolation and characterization of nuclear genes coding for subunits of the yeast ubiquinol-cytochrome c reductase complex. Gene. 1982 Dec;20(3):323–337. doi: 10.1016/0378-1119(82)90201-3. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem. 1983 Jun 1;133(1):17–21. doi: 10.1111/j.1432-1033.1983.tb07424.x. [DOI] [PubMed] [Google Scholar]