Abstract
Enzyme-sensitive hydrogels are a promising class of materials for cell encapsulation and tissue engineering because their ability to be degraded by cell-secreted factors. However, it is well known that nearly all synthetic biomaterials elicit a foreign body response upon implantation. Therefore, this study aimed to evaluate the in vitro and in vivo response to an enzyme-sensitive hydrogel. Hydrogels were formed from poly(ethylene glycol) with the peptide crosslinker, C-VPLS↓LYSG-C, which is susceptible to matrix metalloproteinases 2 and 9. We evaluated the hydrogel by exogenously delivered enzymes, encapsulated mesenchymal stem cells as a tissue engineering relevant cell type, and by macrophage-secreted factors in vitro and for the foreign body response through macrophage attachment in vitro and in a subcutaneous mouse model. These hydrogels rapidly degraded upon exposure to exogenous MMP-2 and to lesser degree with MMP-9. Encapsulated mesenchymal stem cells were capable of degrading the hydrogels via matrix metalloproteinases. Inflammatory macrophages were confirmed to attach to the hydrogels, but were not capable of rapidly degrading the hydrogels. In vivo, these hydrogels remained intact after 4 weeks and exhibited a classic foreign body response with inflammatory cells at the hydrogel surface and a fibrous capsule. In summary, these findings suggest that while this MMP-2/9 sensitive hydrogel is readily degraded in vitro, it does not undergo rapid degradation by the foreign body response. Thus, the long term stability of these hydrogels in vivo coupled with the ability for encapsulated cells to degrade the hydrogel makes them promising materials for tissue engineering.
Keywords: Enzyme-sensitive hydrogel, cell encapsulation, mesenchymal stem cell, macrophage, foreign body response
INTRODUCTION
The goal of tissue engineering is to create new living tissue that is capable of restoring function after injury or disease. One promising approach involves the use of hydrogels that enable cell encapsulation and in situ formation.43 Hydrogels that are designed with crosslinks (e.g., peptides) susceptible to cell-secreted enzymes allow cells to degrade the hydrogel in a fashion similar to native tissues.34,36 Furthermore, the chemistry of the crosslinks can be designed to a particular enzyme or group of enzymes and through slight changes in the amino acids and/or sequence, degradation can be further tuned to achieve the desired degradation kinetics.35,41,59 As a result, enzyme-sensitive hydrogels offer a great deal of tunability for many cell types and tissue engineering applications.
Enzyme-sensitive hydrogels have shown promise in a wide range of tissue engineering applications.16,20,36,40,54,60 For example, enzyme-sensitive crosslinks have been shown to facilitate (1) cell infiltration and neo-bone formation when hydrogels are implanted into bone defects in vivo,16,24,36 (2) neo-cartilage matrix deposition from encapsulated chondrocytes,54 and (3) neurite outgrowth from encapsulated embryonic stem cell derived motor neurons.40 These hydrogels have also been utilized as microcarriers for controlled release and drug delivery.1,30,31 While many studies have utilized a peptide sequence derived from collagen type I, which degrades in response to generic pan-MMPs (e.g.,34), this platform can be further tuned to target degradation by a specific MMP or cell type19 thus controlling when and what degrades the hydrogel.9 For example, hydrogels sensitive to MMP-13, which is up-regulated during bone injury and secreted by MSCs, have been shown to support osteogenic differentiation of mesenchymal stem cells in vitro23,27 and regenerate bone-like tissue in vivo.16 Hydrogels sensitive to MMP-7, which was detected in mesenchymal stem cells during chondrogenesis, enabled tuning of degradation to the onset of differentiation.7
While promising, synthetic materials are well-known for initiating a foreign body response (FBR) once implanted into higher organisms.11 This response is characterized by the infiltration of neutrophils and then macrophages, which release pro-inflammatory cytokines, reactive oxygen and nitrogen species, and matrix degrading enzymes in their attempt to remove the foreign material.2 Eventually, the response transitions to a ‘resolution phase’, which is characterized by the formation of a fibrous capsule that walls off the implant from the surrounding host tissue. Macrophages, however, remain at the surface of the implant, eliciting a chronic inflammatory response that persists for the lifetime of the implant. Given the inflammatory environment and the presence of matrix degrading enzymes, questions arise as to whether in vivo implantation and the resulting FBR accelerate degradation of enzyme sensitive hydrogels.
The overall goals of this study were two-fold: (1) investigate the degradation of enzyme-sensitive hydrogels in response to encapsulated cells and interrogating macrophages in an in vitro culture system and (2) characterize the FBR to enzyme-sensitive hydrogels in vivo. To this end, we designed poly(ethylene glycol) (PEG) hydrogels formed with a peptide crosslinker based on the amino acid sequence, VPLS↓LYSG, which is susceptible to MMP-2 and MMP-9.59 PEG hydrogels were chosen because of the ease with which peptide crosslinkers can be incorporated32 and their wide use in tissue engineering research.43 In addition, we have shown that although PEG hydrogels are highly hydrophilic, they elicit a FBR when implanted into immunocompetent mice.38,56 Crosslinks sensitive to MMP-2 and MMP-9 were chosen because macrophages have been shown to secrete these enzymes,4,14,25,33 MMP-2/9 are well known for their involvement in wound healing and fibrosis,18 and MMP-9 in particular has been shown to be critical to the ‘resolution phase’ of the FBR that involves macrophage fusion and the formation of the fibrous capsule.57 Specifically in this work, hydrogel degradation was evaluated by exogenously delivered enzymes, encapsulated mesenchymal stem cells as a tissue engineering relevant cell type and by enzymes secreted from macrophages. The FBR was characterized in vitro by macrophage attachment and then four weeks after subcutaneous implantation in mice.
MATERIALS AND METHODS
Macromer Synthesis and Hydrogel Formation
The macromer, 8-arm poly(ethylene glycol) (PEG) functionalized with norbornene, was synthesized following previously established protocols.17,50 Briefly, 8-arm PEG-NH2 (20 kDa, JenKemUSA) was dissolved in a sparing amount of dimethylformamide (Sigma) and combined with 6 M excess of 5-norbornene-2-carboxylic acid (Sigma), 3 M excess of 2-(1H-7-Azabenzotriazol-1-yl) -1,1,3,3-tetramethyl uronium hexafluorophosphate methanaminium (AKSci), and a 3M excess of N,N-Diisopropylethylamine (Sigma), and reacted overnight. The product was recovered by precipitation in ice-cold diethyl ether, and subsequently dialyzed against de-ionized H2O, sterile filtered, and lyophilized. Functionalization of PEG with norbornene, referred to as 8-arm PEG-NB, was determined using 1H NMR and confirmed to be ~100% (i.e., each arm of a PEG molecule was functionalized with a norbornene).
Hydrogels were formed from a pre-polymer solution containing 6 % (g/g) 8-arm PEG-NB, and either PEG-dSH (MW 1000, Sigma) or a bis-cysteine crosslinker (CVPLS↓LYSGC) (GenScript) at a 0.5:1 thiol:ene ratio and 0.05% (g/g) photoinitiator (Irgacure 2959) in phosphate buffer saline, to form stable (i.e., enzyme-insensitive) or enzyme-sensitive hydrogels. All experiments used this hydrogel formulation unless otherwise noted. In some cases 2.5 mM of a cell adhesion peptide (CRGDS) (GenScript) was added to the aforementioned mixture. Cylindrical hydrogels (2 mm in height and 4.5 mm in diameter) were formed by polymerizing this solution with 352 nm light at ~6 mW/cm2 for 6 minutes. These photopolymerization conditions have been shown to be cytocompatible10 and widely used in studies for cell encapsulation.32
Hydrogel Degradation by Exogenous Enzymes
Cylindrical hydrogels (~3 mm in height and 3.5 mm in diameter) were formed from a pre-polymer solution as described above, but containing 5 % (g/g) 8-arm PEG-NB in Milli-Q water. For the degradation experiment with exogenous enzymes, hydrogels were placed in a buffer solution for either MMP-2 (50 mM HEPES, 10 mM CaCl2, 20% glycerol, and 0.005% BRIJ-35) or MMP-9 (300 mM NaCl, 50 mM Tris-HCl, 5 mM CaCl2, and 20 μM ZnCl2) and allowed to swell to equilibrium for 24 hours. After 24 hours the buffer solution was replaced with the aforementioned buffer solutions containing either 20 nM MMP-2 (Calbiochem) or 40 nM MMP-9 (Anaspec) and incubated at 37°C. Samples were collected at 24, 48, and 72 hours, with enzyme solutions refreshed every 24 hours. After each sample collection, hydrated samples were weighed (wet weight, mw), the tangent compressive modulus measured under unconfined compression (MTS Synergie 100) at a constant strain rate (0.5 mm/min), and then lyophilized to obtain polymer dry weight. After lyophilization, the dry mass consisted of mass resulting from the lyophilized buffers as well as the polymer mass. A standard curve was generated for each buffer to account for the mass that was associated with the volume of buffer removed during lyophilization. An effective dry polymer mass was determined (md). Equilibrium mass swelling ratio (q) was determined by mw/md.
MSC Culture and Encapsulation
Primary murine MSCs from C57BL/6 mice were obtained from Texas A&M University Health Science Center and College of Medicine Institute for Regenerative Medicine. The MSCs were grown in Iscove’s Modified Dulbecco’s Medium (IMDM) (Life Technologies) supplemented with 10% fetal bovine serum (FBS) (Atlanta Biologicals), 10% horse serum (Hyclone), penicillin/streptomycin/amphotericin B (Life Technologies), and 1% 200mM L-glutamine (Life Technologies) before being collected for encapsulation. MSCs at a concentration of 2 × 106 cells/mL were combined with the aforementioned pre-polymer solution containing CRGDS and polymerized as described above. MSC-laden hydrogels were cultured in MSC growth media for up to 7 days, or in MSC growth media containing 10 μM of the broad MMP inhibitor batimastat (Santa Cruz Biotechnology). MSC-laden hydrogels were fixed in 10% neutral buffered formalin for 30 minutes at room temperature and incubated with Alexfluor 488 Phalloidin in 1:30 in TBS for 30 minutes. Full intact hydrogels were imaged on a two photon microscope (A1R Upright Multiphoton Confocal).
Hydrogel Degradation by Macrophages
RAW 264.7 macrophages were purchased from American Type Culture Collection (ATCC) and cultured in DMEM (ATCC) supplemented with 10% FBS (Atlanta Biologicals) and penicillin/streptomycin/fungizone (PSF). RAW 264.7 macrophages plated at an initial density of ~ 2 × 105 cells/cm2 and allowed to adhere overnight before replacing the media with DMEM + 1% FBS and 100 ng/ml LPS (Salmonella enterica, Sigma Aldrich) for 24 hours. A freshly seeded group of RAW 264.7 cells were prepared each day such that macrophages were only stimulated with LPS for a total of 24 hours. Hydrogels were placed in separate wells and incubated in DMEM (ATCC) + 1% FBS (Atlanta Biologicals) and allowed to swell to equilibrium for 24 hours. After 24 hours the media in the remaining samples were replaced with LPS-stimulated RAW 264.7 conditioned media and incubated at 37°C. The media was refreshed every 24 hours. Hydrogel samples were collected at 24, 48, and 72 hours and their wet weight, tangent compressive modulus, and dry weights measured as described above.
Primary Macrophages
Bone marrow derived monocytes were isolated from the femurs and tibia of 6 week old C57BL/6 mice (Charles River Laboratories) as described previously26 and then differentiated into macrophages following well-established protocols.26 Briefly, bone marrow was flushed and collected in IMDM with 10% FBS and PSF, and layered in Lympholyte M (Accurate Chemicals). Mononuclear cells were obtained following centrifugation and then plated on non-tissue culture treated polystyrene and differentiated into macrophages following a 10 day protocol in medium containing IMDM, 20% FBS, 2 mM L-glutamine, PSF, 1.5 ng/ml human macrophage colony stimulating factory (R&D systems) and 100 ng/ml huFLT-3 (R&D systems). Primary macrophages were collected by scraping and then seeded on the surface of hydrogels or on TCPS at 2.5 × 105 macrophages/cm2 in medium containing IMDM, 20% fetal bovine serum (FBS), 2 mM L-glutamine, PSF, and 100 ng/ml LPS for 24 hours. Hydrogels were formed from pre-polymer solutions as described above with either stable, enzyme insensitive PEG-dSH or the enzyme-sensitive crosslinker with CRGDS.
After 24 hours, supernatants were collected and flash frozen in liquid nitrogen until the time of assay. The amounts of active MMP-2 and MMP-9 in the culture supernatant was measured using fluorometric assay kits (Anaspec) according to manufacturer guidelines, but without the addition of an activator. After 24 hours, hydrogel samples were fixed in 10% neutral buffered formalin for 30 minutes at room temperature and stained for F-actin and MMP-9. In brief, samples were blocked with TBS containing 5% dry milk + 1% BSA for 2 hr. Samples were then incubated with polyclonal goat-anti-mouse MMP9 (Santa Cruz Biotechnology) 1:100 in TBS with 1% BSA overnight at 4°C. The following day, samples were washed with Tris Buffered Saline (TBS) with 0.025% (v/v) Triton-X-100 and incubated with Alexafluor 546 goat anti rabbit antibody (Life Technologies) in 1:300 in TBS + 1% BSA for 2 hours followed by incubation with Alexafluor 488 Phalloidin (Life Technologies) in 1:30 in TBS for 30 minutes. Finally, the samples were counterstained with 4,6-diamidino-2-phenylindole (DAPI, Life Technologies) to visualize the cell nuclei. Samples were imaged by laser scanning confocal microscopy (Zeiss LSM5 Pascal).
Implantation Study
Hydrogels were formed as described above from a pre-polymer solution containing 6% (g/g) 8-arm PEG norbornene. In a separate set of samples, CRGDS was incorporated into the pre-polymer mixture at 2.5 mM. Hydrogel disks (~2.5 mm in height and ~4 mm in diameter) were implanted into dorsal subcutaneous pockets of 7-week old male C57BL/6 mice (Charles River Laboratories) for 4 weeks. Mice were euthanized via CO2 asphyxiation and cervical dislocation. All animal protocols follow were approved by the University of Colorado at Boulder Institutional Animal Care and Use Committee and follow the NIH guidelines for animal care.
After explanation from the implantation study, hydrogels and surrounding tissue were fixed in 10% buffered formalin for 4 hours. Following dehydration and embedding in paraffin wax, samples were sectioned in 10 μm thick slices, and stained with Masson’s Trichrome and hematoxylin as a nuclear counterstain.
Statistical Analysis
All data are from 3–5 replicates and presented as the mean with standard deviation as error bars. Statistical analysis was performed in KaleidaGraph and determined by ANOVA and Tukey’s post hoc analysis with α = 0.05. P-values are reported.
RESULTS
Enzyme-sensitive hydrogel formation and characterization
Enzyme-sensitive and enzyme-insensitive (i.e., stable) hydrogels were formed by a photoclickable reaction between an 8-arm PEG monomer functionalized with norbornene and either the bis-cysteine peptide, CVPLS↓LYSGC, or PEG dithiol crosslinker, respectively (Fig. 1A). By varying the concentration of the multi-arm PEG nobornene monomer and/or the thiol:ene ratio, the macroscopic properties of the enzyme-sensitive hydrogel were systematically controlled (Fig. 1B). Robust enzyme-sensitive hydrogels were formed, which maintained their shape (Fig. 1C).
Figure 1.
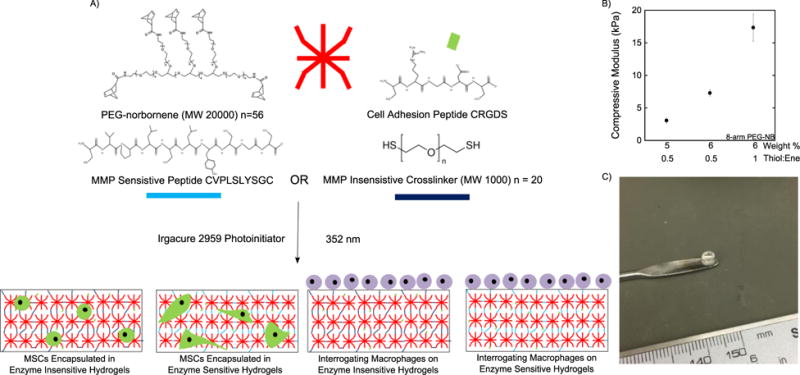
A) Schematic depicting the formation of enzyme sensitive PEG hydrogels which allow for spreading of encapsulated MSCs or interrogation by macrophages. B) Mechanical properties of the hydrogel can be varied by changing the PEG weight % or thiol:ene ratio. C) Hydrogels formed are viscoelastic and highly water swollen after polymerization but retain the shape of mold used for polymerization.
Degradation of hydrogels to exogenous enzymes
PEG hydrogels (5% 8-arm PEG-NB, 0.5 thiol:ene) were incubated with solutions containing either active MMP-2 or MMP-9 and their degradation assessed over time by polymer dry weight (Fig. 2A,D), mass swelling ratio (Fig. 2B,E), and compressive modulus (Fig. 2C,F). The soft formulation used for these studies (5% 8-arm PEG-NB, 0.5 thiol:ene) was chosen to test if these hydrogels were degradable by MMP-2 and MMP-9. Upon exposure to 20 nM MMP-2, the enzyme-sensitive hydrogels were fully degraded by day 3. From day 1 to day 2, the dry weights decreased (p=0.01) by 8-fold and the compressive modulus decreased (p=0.0006) by 5-fold, but the mean mass swelling ratio was only slightly higher (p=0.40). In addition, the dry weight was lower (p=0.015) and as well the compressive modulus was lower (p=0.0006) for the enzyme-sensitive hydrogels exposed to MMP-2 when compared to those in buffer alone. The dry weights were higher than the predicted dry mass based on the formulation. This observation is attributed to the presence of glycerol in the buffer, which prevented complete lyophilization. It should also be noted that after two days, hydrogels in the presence of MMP-2 were near their reverse gelation point, where small differences in crosslink density result in large differences in the hydrogel properties and thus the large error bars. Hydrogels exposed to 40 nM MMP-9 displayed slower degradation kinetics and were still intact at day 3. At day 3, dry weights were lower (p=0.20), mass swelling ratio was higher (p=0.20), but modulus was similar (p=0.52) when comparing the hydrogels exposed to MMP-9 to those not exposed to MMPs.
Figure 2.
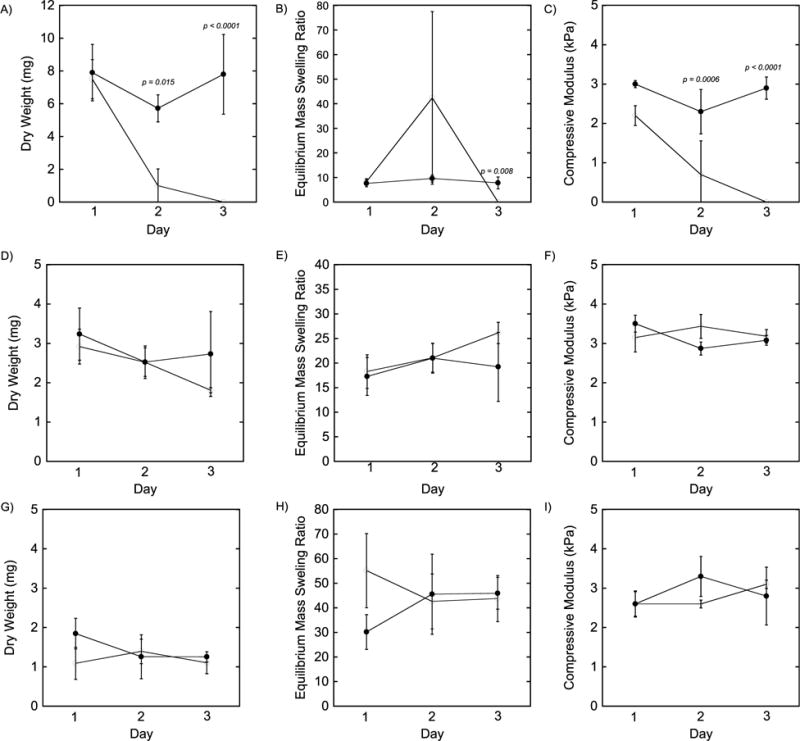
Hydrogel characterization for samples cultured in A–C) 20 nM MMP-2 D–F) 40 nM MMP-9 and G–I) Macrophage conditioned media indicating the polymer dry weight (A,D,G) equilibrium mass swelling ratio (B,E,H) and compressive modulus (C,F,I). Filled black circles represent samples cultured in buffer alone, while open circles represent samples cultured with enzyme or conditioned media. Data are presented as mean with standard deviation as error bars (n=3–4). * = p < 0.05 ** = p < 0.01 compare the buffer alone to the buffer with enzyme.
MSC spreading in enzyme-sensitive hydrogels
Mesenchymal stem cells (MSCs) were encapsulated in PEG hydrogels (6% 8-arm PEG-NB, 0.5 thiol:ene) containing either the enzyme-insensitive PEG crosslinker or the enzyme-sensitive peptide crosslinker and their morphology assessed over time in vitro. The MSCs were stained for F-actin to visualize their cytoskeleton. Immediately after encapsulation, MSCs adopted a round morphology in the enzyme-sensitive hydrogels (Fig. 3A) with similar morphology in the enzyme-insensitive hydrogels (data not shown). After 7 days, MSCs retained their round morphology in the enzyme-insensitive hydrogel (Fig. 3B). On the contrary, MSCs displayed a spindle-like morphology with extended processes in three dimensions within the enzyme-sensitive hydrogels after 7 days (Fig. 3C). However, when an MMP inhibitor was added to the culture medium, MSC spreading was reduced in the enzyme sensitive hydrogels and the cells adopted a round morphology (Fig. 3D). Although the intensity of the stain was lower when an MMP inhibitor was added to the culture medium, cell number did not appear to be affected.
Figure 3.
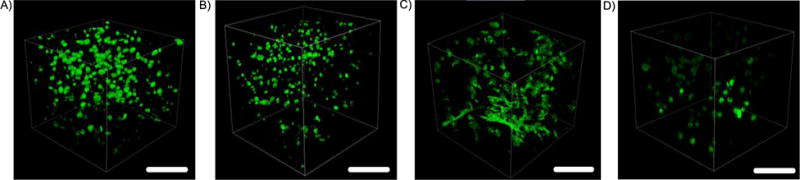
Murine MSCs encapsulated in enzyme-sensitive and enzyme-insensitive PEG hydrogels containing RGD and stained for F-actin. Images were taken A) the day of encapsulation in enzyme sensitive hydrogels and after 7 days in B) enzyme-insensitive and C) enzyme-sensitive hydrogels and D) enzyme-sensitive hydrogels with 10 μM batimastat. Scale bar = 250 μm.
Degradation of hydrogels to macrophage conditioned media
Hydrogels (5% 8-arm PEG-NB, 0.5 thiol:ene) were cultured in the presence of media that was conditioned for 24 hours by RAW 264.7 macrophages stimulated with LPS. Hydrogel degradation was assessed by polymer dry weight (Fig. 2G), mass swelling ratio (Fig. 2H), and compressive modulus (Fig. 2I). Over the course of three days, no changes in polymer dry weight (p=0.40), equilibrium mass swelling ratio (p=0.97), or compressive modulus (p=0.61) were observed.
Macrophage interrogation of hydrogels in vitro
Bone marrow derived macrophages were cultured on the surface of PEG hydrogels (6% 8-arm PEG-NB, 0.5 thiol:ene) and evaluated for their cytoskeleton by F-actin staining (Fig. 4A–D) and for the presence of MMP-9 (Fig. 4E–H). In all hydrogel formulations, with or without RGD and with enzyme-insensitive or enzyme-sensitive crosslinks, macrophages adhered to the hydrogel surfaces. Macrophages assumed a round morphology exhibiting a localized and dense F-actin organization. However on the enzyme-sensitive hydrogels with RGD, several cells exhibited signs of spreading with filopodia extending outward from the cell and a more defined F-actin structure (Fig. 4D).
Figure 4.
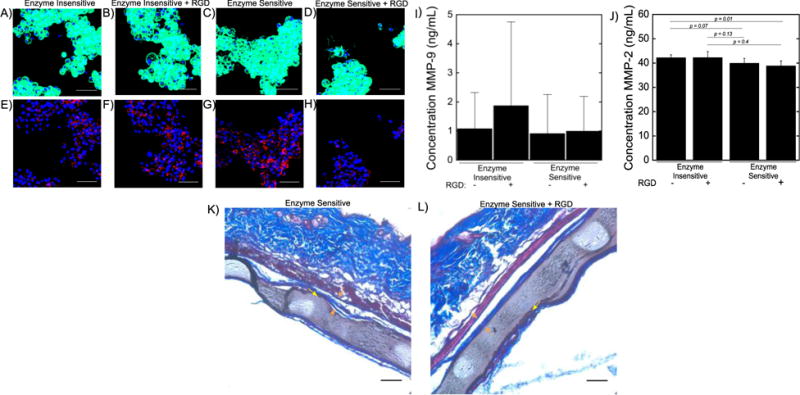
Primary macrophages cultured on PEG hydrogels produce MMP-9. Staining is visualized for F-actin cytoskeletal staining (top) and MMP-9 staining (bottom) for A,E) enzyme insensitive hydrogels B,F) enzyme insensitive hydrogels + RGD C,G) enzyme sensitive hydrogels and D,H) enzyme sensitive hydrogels + RGD. Scale bar = 50 μm. PEG hydrogels with enzyme sensitive crosslinkers were subcutaneously implanted into dorsal pockets in C57BL/6 mice for 28 days. Low levels of active MMP-9 (I) and MMP-2 (J) are produced by primary macrophages cultured on PEG hydrogels for 24 hours. The production of MMP-9 was not statistically different for the different hydrogel chemistries employed, while MMP-2 production was lower in macrophages on the enzyme sensitive hydrogels. Data presented as mean with standard deviation as error bars (n = 4–5). Sections were stained with Masson’s trichrome and shown for K) enzyme sensitive hydrogels and L) enzyme sensitive hydrogels with RGD which show no obvious signs of degradation in vivo. A collagenous fibrous capsule surrounding the hydrogel implants can be seen in both cases shown by the orange arrows. A thin layer of inflammatory cells is also present at the surface of the hydrogel and indicated by yellow arrows. White areas are artifacts that occurred during tissue processing. Scale bar = 50 μm.
Macrophages stained positive for MMP-9 in all hydrogel formulations. Qualitatively, macrophages on the enzyme-insensitive hydrogels exhibited similar MMP-9 staining with no observable differences with or without the presence of RGD (Fig. 4E and Fig. 4F). However, there appeared to be reduced MMP-9 staining in the enzyme-sensitive hydrogels containing RGD (Fig. 4H) when compared to their counterpart hydrogels without RGD (Fig. 4G). Low levels of active MMP-9 were found in the culture supernatant in all conditions, with values ranging from ~1–2 ng/mL (Fig. 4I). Active MMP-2 was also found in the culture supernatant, with slightly lower amounts from macrophages on enzyme sensitive hydrogels with RGD when compared (p = 0.01) to enzyme insensitive hydrogels and when compared (p = 0.04) to enzyme insensitive hydrogels with RGD (Fig. 4J).
Characterization of hydrogels after subcutaneous implantation
To evaluate the FBR to these hydrogels in vivo, enzyme-sensitive hydrogels (6% 8-arm PEG-NB, 0.5 thiol:ene) containing either no RGD or RGD were implanted subcutaneously in mice for 28 days (Fig. 4K,L). The compressive modulus of the hydrogel prior to implantation was ~8 kPa (Fig. 1B). Histological examination of the implanted hydrogels revealed that the hydrogels were still intact after 28 days. Regardless of the presence of RGD, a characteristic fibrous capsule was apparent that was ~ 20 microns in thickness. Additionally, a thin layer of inflammatory cells was present at the surface of the implant. There were no observable differences in the FBR between the two hydrogel formations with and without RGD.
DISCUSSION
Towards developing MMP-sensitive hydrogels for in vivo implantation and tissue engineering, we investigated a PEG based hydrogel platform with peptide crosslinkers that are susceptible to MMP-2 and 9. In this work, we demonstrate that PEG hydrogels formed with this MMP-2/9 sensitive crosslinker readily form 3D hydrogels, are susceptible to enzymatic degradation by MMP-2 and to a lesser degree by MMP-9, support encapsulation of MSCs and undergo MSC-mediated degradation. However, inflammatory macrophages did not readily degrade the hydrogels in vitro. In vivo the hydrogel elicited a classic FBR, but remained intact after four weeks. Overall these findings suggest that while this MMP-2/9 sensitive hydrogel is readily degraded in vitro, it does not undergo rapid degradation by the FBR.
The specific peptide sequence, VPLS↓LYSG, that was used in this study to form the peptide crosslinker has been reported to follow Michaelis-Menten kinetics and have high specificity for both MMP-2 and MMP-9 when compared to other MMPs with kcat/Km values of 61,000 and 49,000 M−1s−1, respectively.59 We observed rapid degradation of low crosslinked hydrogels with an initial modulus of ~2 kPa in the presence of MMP-2 within three days, which is agreement with previous reports.44 This finding is attributed to a high value for kcat (e.g., 3 s−1) for the peptide in solution.44 Studies have also shown that for other peptides kcat is higher when incorporated into a hydrogel,34 suggesting that kcat may be even greater for MMP-2 and this hydrogel. Surprisingly, we observed much slower degradation with MMP-9, despite using a two-fold higher concentration of MMP-9 compared to MMP-2, which is consistent with other reports.53 We attribute this finding to a lower value for kcat, where others have reported significantly lower activity for MMP-9 compared to MMP-2.61 Furthermore, the activity of circulating MMP-2 is reported to be over 10 times greater than circulating MMP-9.15 It is also possible that Km may be playing a role. If Km is on the same order of magnitude as the crosslink density of the hydrogel as measured in solution (i.e., corresponding to substrate concentration), then Km can have a significant contribution to lowering the rate of degradation. Indeed, we have previously observed that for loosely crosslinked hydrogels, Km influences the degradation of enzyme-sensitive hydrogels.52
Mesenchymal stem cells were evaluated given their promise in tissue engineering. When encapsulated in these MMP-2/9-sensitive PEG hydrogels, MSCs were capable of spreading in three dimensions. A broad spectrum MMP inhibitor confirmed that spreading was mediated by MMP degradation of the hydrogel. This indicates that the concentration of cell secreted MMPs were sufficient to degrade the hydrogel locally and enable cellular processes to extend from the cells. It should be noted that these hydrogels contained cell adhesion peptides which are necessary to facilitate cell spreading in enzyme sensitive hydrogels.46 Although these hydrogels were relatively soft, MSC spreading was not observed in the enzyme-insensitive hydrogels containing RGD indicating that RGD alone was insufficient to support cell spreading in these materials. While we did not confirm which MMP was mediating degradation, MSCs have been shown to constitutively express high levels of MMP-2, but not MMP-9,49 suggesting that degradation was likely mediated by MMP-2.
Macrophages are also known to constitutively express MMP-213,42 with increased expression in response to inflammatory stimuli45 while MMP-9 expression is induced in macrophages during inflammation28,48 and the ‘resolution’ phase of the FBR.4,14,25,33 Interestingly, conditioned media from LPS-stimulated RAW 264.7 macrophages was not sufficient to cause any discernable degradation of the hydrogel within three days. It is possible that the concentration of MMPs was too low to result in degradation of the hydrogel within this time frame. In addition, the presence of serum, even at a low concentration, could have sufficiently highly levels of tissue inhibitor of metalloproteinases (TIMPs)21,22 to inhibit and/or slow degradation.
To evaluate the FBR to this MMP-2/9 sensitive PEG hydrogel we first investigated primary murine macrophage interrogation of the hydrogel using a controlled in vitro platform. In vitro macrophages adhered to the hydrogel surfaces regardless of the presence of a cell adhesion peptide, which is consistent with our prior studies.39 We have previously confirmed that proteins adsorb to PEG hydrogels in vitro and in vivo despite being highly hydrophilic and that macrophage attachment in vitro to PEG hydrogels lacking any cell adhesion moiety is mediated by serum-adsorbed proteins.55 In this work in LPS-stimulated macrophages, MMP-9 was expressed intracellular and active MMP-2 and MMP-9 proteins were present in the culture medium. The amount of active MMP-2 was substantially higher than MMP-9, albeit both at low levels. These findings confirm that macrophages are capable of synthesizing both MMPs when adhered to the hydrogel surfaces. Qualitatively, the hydrogels appeared intact suggesting that the low levels of MMPs coupled with the presence of serum in the culture medium were not sufficient to induce any significant degradation of the hydrogel, at least on the time scale of the experiment, which is consistent with the findings with the RAW 264.7 macrophages. The levels of active MMP-2 were slightly lower for macrophages cultured on the enzyme sensitive hydrogels, although no differences were observed in the levels of active MMP-9. Despite the lack of macroscopic signs of degradation, it is possible that macrophages could cleave the underlying substrate leading to softer substrates. Previous studies have shown that substrate stiffness influences macrophage activation with softer PEG hydrogels leading to reduced macrophage activation in vitro and the FBR in vivo8; although additional studies are needed to confirm the presence of local hydrogel degradation.
In vivo, the hydrogels elicited a classic FBR with the presence of inflammatory cells at the hydrogel surface and the formation of a fibrous capsule, which is consistent with our previous reports showing mac-3 positive macrophages at the hydrogel surface.8,38,39 Interestingly, the hydrogels were intact after four weeks in vivo, indicating that the FBR did not result in high levels of MMPs to cause significant degradation and dissolution of the hydrogel. In addition, there were no signs of cell infiltration into the hydrogels even with the presence of RGD. In the lymphatic system, cell migration is driven by chemoattractant molecules, known as chemokines.12 The hydrogels implanted for this study, however, did not contain any chemokines upon implantation, and thus it is not surprising that there was minimal cell migration into the material. Several hypotheses exist as to why these hydrogels show minimal signs of degradation and infiltration in vivo. MMPs are known to be tightly regulated such that tissue degradation is minimized.5 While it is likely that inflammatory cells present at and near the implant site secrete matrix metalloproteinases37 which are necessary for cell migration to the implant site and the formation of the fibrous capsule, these molecules may be inhibited by other proteins present at the implant surface. For example, we have identified several proteins by proteomics, which adsorb to PEG hydrogels immediately upon implantation, and which have known roles in regulating MMPs. These proteins include alpha-2-macroglobulin and murinoglobulin-1, which are broad protease inhibitors,47 Factor Xa and thrombin, which have been shown to regulate MMP-2 activation in vivo,29 and vitamin D binding protein that sequesters vitamin D, which has been correlated with low levels of MMP-2 and MMP-9 in serum58 and shown to inhibit MMP-9 activity.6 Furthermore, macrophages have been shown to secrete TIMPs3 and therefore may have mechanisms to self-regulate MMP activity. Although, stiffer hydrogels (~8 kPa) were used in these in vivo studies compared to the in vitro degradation studies (~ 2 kPa), we have previously shown that similar MMP-2/9 sensitive hydrogels over a range of stiffness (~1–200 kPa) are fully degradable by exogenous enzymes.1,51 Thus, these findings are attributed to low levels of MMP-2 and MMP-9, which are not sufficient to induce significant degradation of the hydrogel, and/or to the presence of MMP inhibitors.
In conclusion, this study demonstrates that a MMP-sensitive hydrogel, specifically a PEG hydrogel containing the MMP-2/9 enzyme sensitive crosslinker, C-VPLS↓LYSG-C, readily degrades by exogenous enzymes and encapsulated MSCs, but does not undergo rapid degradation by macrophages in vitro or by the FBR in vivo. Our findings suggest that while MMPs are likely present in vivo as part of the FBR, the MMPs may be tightly regulated thus preventing global degradation of the surrounding tissue and hydrogel. The stability of these hydrogels in the context of the FBR coupled with their ability to be degraded by encapsulated cells make them promising materials for tissue engineering.
Acknowledgments
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number 1R21AR064436 and by and by the Department of Education’s Graduate Assistantships in Areas of National Need fellowship to LA.
References
- 1.Amer LD, Holtzinger A, Keller G, Mahoney MJ, Bryant SJ. Enzymatically degradable poly(ethylene glycol) hydrogels for the 3D culture and release of human embryonic stem cell derived pancreatic precursor cell aggregates. Acta Biomater. 2015;22:103–110. doi: 10.1016/j.actbio.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson J. Biological responses to materials. Annu Rev Mater Res. 2001;31:81–110. [Google Scholar]
- 3.Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20:86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aoki T, Kataoka H, Morimoto M, Nozaki K, Hashimoto N. Macrophage-derived matrix metalloproteinase-2 and -9 promote the progression of cerebral aneurysms in rats. Stroke. 2007;38:162–169. doi: 10.1161/01.STR.0000252129.18605.c8. [DOI] [PubMed] [Google Scholar]
- 5.Arpino V, Brock M, Gill SE. The role of TIMPs in regulation of extracellular matrix proteolysis. Matrix Biol. 2015:44–46. 247–254. doi: 10.1016/j.matbio.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 6.Bahar-Shany K, Ravid A, Koren R. Upregulation of MMP-9 production by TNFalpha in keratinocytes and its attenuation by vitamin D. J Cell Physiol. 2010;222:729–37. doi: 10.1002/jcp.22004. [DOI] [PubMed] [Google Scholar]
- 7.Bahney CS, Hsu C-W, Yoo JU, West JL, Johnstone B. A bioresponsive hydrogel tuned to chondrogenesis of human mesenchymal stem cells. FASEB J. 2011;25:1486–1496. doi: 10.1096/fj.10-165514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blakney AK, Swartzlander MD, Bryant SJ. The effects of substrate stiffness on the in vitro activation of macrophages and in vivo host response to poly(ethylene glycol)-based hydrogels. J Biomed Mater Res A. 2012;100:1375–86. doi: 10.1002/jbm.a.34104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bracher M, Bezuidenhout D, Lutolf MP, Franz T, Sun M, Zilla P, Davies NH. Cell specific ingrowth hydrogels. Biomaterials. 2013;34:6797–6803. doi: 10.1016/j.biomaterials.2013.05.057. [DOI] [PubMed] [Google Scholar]
- 10.Bryant SJ, Nuttelman CR, Anseth KS. Cytocompatibility of UV and visible light photoinitiating systems on cultured NIH/3T3 fibroblasts in vitro. J Biomater Sci Polym Ed. 2012;11:439–457. doi: 10.1163/156856200743805. [DOI] [PubMed] [Google Scholar]
- 11.Bryant SJ, Ratner BD. Biomaterials: Where We Have Been and Where We Are Going. Annu Rev Biomed Eng. 2004;6:41–75. doi: 10.1146/annurev.bioeng.6.040803.140027. [DOI] [PubMed] [Google Scholar]
- 12.Campbell DJ, Kim CH, Butcher EC. Chemokines in the systemic organization of immunity. Immunol Rev. 2003;195:58–71. doi: 10.1034/j.1600-065x.2003.00067.x. [DOI] [PubMed] [Google Scholar]
- 13.Cauwenberghs S, Feijge MA, Harper AG, Sage SO, Curvers J, Heemskerk JW. Macrophage matrix metalloproteinase-2/-9 gene and protein expression following adhesion to ECM-derived multifunctional matrices via integrin complexation. FEBS Lett. 2006;580:5313–20. Epub 2006 Sep 12. [Google Scholar]
- 14.Chellat F, Grandjean-Laquerriere A, Le Naour R, Fernandes J, Yahia LL, Guenounou M, Laurent-Maquin D, Le Naour R, Fernandes J, Yahia LL, Guenounou M, Laurent-Maquin D. Metalloproteinase and cytokine production by THP-1 macrophages following exposure to chitosan-DNA nanoparticles. Biomaterials. 2005;26:961–970. doi: 10.1016/j.biomaterials.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 15.Cheng KS, Liao Y-C, Chen MY, Kuan T-C, Hong Y-H, Ko L, Hsieh W-Y, Wu C-L, Chen M-R, Lin C-S. Circulating Matrix Metalloproteinase-2 and -9 Enzyme Activities in the Children with Ventricular Septal Defect. Int J Biol Sci. 2013;9:557–563. doi: 10.7150/ijbs.6398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chung E, Healy K. Biomimetic artificial ECMs stimulate bone regeneration. J Biomed Mater Res A. 2006;81:815–826. doi: 10.1002/jbm.a.30809. [DOI] [PubMed] [Google Scholar]
- 17.Fairbanks BD, Schwartz MP, Halevi AE, Nuttelman CR, Bowman CN, Anseth KS. A Versatile Synthetic Extracellular Matrix Mimic via Thiol-Norbornene Photopolymerization. Adv Mater. 2009;21:5005–5010. doi: 10.1002/adma.200901808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giannandrea M, Parks WC. Diverse functions of matrix metalloproteinases during fibrosis. Dis Model Mech. 2014;7:193–203. doi: 10.1242/dmm.012062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goetsch KP, Bracher M, Bezuidenhout D, Zilla P, Davies NH. Regulation of tissue ingrowth into proteolytically degradable hydrogels. Acta Biomater. 2015;24:44–52. doi: 10.1016/j.actbio.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 20.Greggio C, De Franceschi F, Figueiredo-Larsen M, Gobaa S, Ranga A, Semb H, Lutolf M, Grapin-Botton A. Artificial three-dimensional niches deconstruct pancreas development in vitro. Development. 2013;140:4452–62. doi: 10.1242/dev.096628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayakawa T, Yamashita K, Ohuchi E, Shinagawa A. Cell growth-promoting activity of tissue inhibitor of metalloproteinases-2 (TIMP-2) J Cell Sci. 1994;107:2373–2379. doi: 10.1242/jcs.107.9.2373. [DOI] [PubMed] [Google Scholar]
- 22.Hayakawa T, Yamashita K, Tanzawa K, Iwata K. Growth-promoting activity of tissue inhibitor of metalloproteinases-1 (TIMP-1) for a wide range of cells. FEBS Lett. 1992;298:29–32. doi: 10.1016/0014-5793(92)80015-9. [DOI] [PubMed] [Google Scholar]
- 23.He X, Jabbari E. Material Properties and Cytocompatibility of Injectable MMP Degradable Poly (lactide ethylene oxide fumarate) Hydrogel as a Carrier for Marrow Stromal Cells. Biomacromolecules. 2007:780–792. doi: 10.1021/bm060671a. [DOI] [PubMed] [Google Scholar]
- 24.Holloway JL, Ma H, Rai R, Burdick JA. Modulating hydrogel crosslink density and degradation to control bone morphogenetic protein delivery and in vivo bone formation. J Control Release. 2014;191:63–70. doi: 10.1016/j.jconrel.2014.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang WC, Sala-Newby GB, Susana A, Johnson JL, Newby AC. Classical macrophage activation up-regulates several matrix metalloproteinases through mitogen activated protein kinases and nuclear factor-κB. PLoS One. 2012;7:e42507. doi: 10.1371/journal.pone.0042507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jay SM, Skokos E, Laiwalla F, Krady M-M, Kyriakides TR. Foreign body giant cell formation is preceded by lamellipodia formation and can be attenuated by inhibition of Rac1 activation. Am J Pathol. 2007;171:632–640. doi: 10.2353/ajpath.2007.061213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jha AK, Jackson WM, Healy KE. Controlling osteogenic stem cell differentiation via soft bioinspired hydrogels. PLoS One. 2014;9:1–11. doi: 10.1371/journal.pone.0098640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim SY, Lee J-G, Cho WS, Cho K-H, Sakong J, Kim J-R, Chin B-R, Baek S-H. Role of NADPH oxidase-2 in lipopolysaccharide-induced matrix metalloproteinase expression and cell migration. Immunol Cell Biol. 2010;88:197–204. doi: 10.1038/icb.2009.87. [DOI] [PubMed] [Google Scholar]
- 29.Koo BH, Park MY, Jeon OH, Kim D-S. Regulatory mechanism of matrix metalloprotease-2 enzymatic activity by factor Xa and thrombin. J Biol Chem. 2009;284:23375–85. doi: 10.1074/jbc.M109.036848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee ST, Yun JI, Jo YS, Mochizuki M, van der Vlies AJ, Kontos S, Ihm JE, Lim JM, Hubbell JA. Engineering integrin signaling for promoting embryonic stem cell self-renewal in a precisely defined niche. Biomaterials. 2010;31:1219–26. doi: 10.1016/j.biomaterials.2009.10.054. [DOI] [PubMed] [Google Scholar]
- 31.Li H, Giorgio T. Matrix Metalloproteinase Responsive, Proximity-activated Polymeric Nanoparticles for siRNA Delivery. Adv Funct Mater. 2012;29:997–1003. doi: 10.1002/adfm.201202215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin CC, Ki CS, Shih H. Thiol-norbornene photoclick hydrogels for tissue engineering applications. J Appl Polym Sci. 2015;132 doi: 10.1002/app.41563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luan Z, Chase AJ, Newby AC. Statins inhibit secretion of metalloproteinases-1, -2, -3, and -9 From vascular smooth muscle cells and macrophages. Arter Thromb Vasc Biol. 2003;23:769–775. doi: 10.1161/01.ATV.0000068646.76823.AE. [DOI] [PubMed] [Google Scholar]
- 34.Lutolf MP, Lauer-Fields JL, Schmoekel HG, Metters AT, Weber FE, Fields GB, Hubbell JA. Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: engineering cell-invasion characteristics. Proc Natl Acad Sci U S A. 2003;100:5413–8. doi: 10.1073/pnas.0737381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lutolf MP, Tirelli N, Cerritelli S, Cavalli L, Hubbell JA. Systematic modulation of Michael-type reactivity of thiols through the use of charged amino acids. Bioconjugate Chem. 2001;12:1051–1056. doi: 10.1021/bc015519e. [DOI] [PubMed] [Google Scholar]
- 36.Lutolf MP, Weber FE, Schmoekel HG, Schense JC, Kohler T, Müller R, Hubbell JA. Repair of bone defects using synthetic mimetics of collagenous extracellular matrices. Nat Biotechnol. 2003;21:513–8. doi: 10.1038/nbt818. [DOI] [PubMed] [Google Scholar]
- 37.Luttikhuizen DT, van Amerongen MJ, de Feijter PC, Petersen AH, Harmsen MC, van Luyn MJA. The correlation between difference in foreign body reaction between implant locations and cytokine and MMP expression. Biomaterials. 2006;27:5763–5770. doi: 10.1016/j.biomaterials.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 38.Lynn AD, Blakney AK, Kyriakides TR, Bryant SJ. Temporal progression of the host response to implanted poly(ethylene glycol)-based hydrogels. J Biomed Mater Res A. 2011;96:621–31. doi: 10.1002/jbm.a.33015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lynn AD, Kyriakides TR, Bryant SJ. Characterization of the in vitro macrophage response and in vivo host response to poly(ethylene glycol)-based hydrogels. J Biomed Mater Res A. 2010;93:941–53. doi: 10.1002/jbm.a.32595. [DOI] [PubMed] [Google Scholar]
- 40.McKinnon DD, Kloxin AM, Anseth KS. Synthetic hydrogel platform for three-dimensional culture of embryonic stem cell-derived motor neurons. Biomater Sci. 2013;1:460. doi: 10.1039/c3bm00166k. [DOI] [PubMed] [Google Scholar]
- 41.Nagase H, Fields GB. Human matrix metalloproteinase specificity studies using collagen sequence-based synthetic peptides. Biopolymers. 1996;40:399–416. doi: 10.1002/(SICI)1097-0282(1996)40:4%3C399::AID-BIP5%3E3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 42.Newby AC. Metalloproteinase expression in monocytes and macrophages and its relationship to atherosclerotic plaque instability. Arterioscler Thromb Vasc Biol. 2008;28:2108–2114. doi: 10.1161/ATVBAHA.108.173898. [DOI] [PubMed] [Google Scholar]
- 43.Nicodemus GD, Bryant SJ. Cell encapsulation in biodegradable hydrogels for tissue engineering applications. Tissue Eng Part B Rev. 2008;14:149–65. doi: 10.1089/ten.teb.2007.0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patterson J, Hubbell JA. Enhanced proteolytic degradation of molecularly engineered PEG hydrogels in response to MMP-1 and MMP-2. Biomaterials. 2010;31:7836–45. doi: 10.1016/j.biomaterials.2010.06.061. [DOI] [PubMed] [Google Scholar]
- 45.Quiding-Järbrink M, Smith DA, Bancroft GJ. Production of matrix metalloproteinases in response to mycobacterial infection. Infect Immun. 2001;69:5661–70. doi: 10.1128/IAI.69.9.5661-5670.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raza A, Lin CC. The influence of matrix degradation and functionality on cell survival and morphogenesis in PEG-based hydrogels. Macromol Biosci. 2013;13:1048–1058. doi: 10.1002/mabi.201300044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rehman AA, Ahsan H, Khan FH. Alpha-2-macroglobulin: A physiological guardian. J Cell Physiol. 2013;228:1665–1675. doi: 10.1002/jcp.24266. [DOI] [PubMed] [Google Scholar]
- 48.Rhee JW, Lee K-W, Kim D, Lee Y, Jeon O-H. NF-kappaB-dependent regulation of matrix metalloproteinase-9 gene expression by lipopolysaccharide in a macrophage cell line RAW 264.7. J Biochem Mol Biol. 2007;40:88–94. doi: 10.5483/bmbrep.2007.40.1.088. [DOI] [PubMed] [Google Scholar]
- 49.Ries C, Egea V, Karow M, Kolb H, Jochum M, Neth P. MMP-2, MT1-MMP, and TIMP-2 are essential for the invasive capacity of human mesenchymal stem cells: differential regulation by inflammatory cytokines. Blood. 2007;109:4055–4063. doi: 10.1182/blood-2006-10-051060. [DOI] [PubMed] [Google Scholar]
- 50.Roberts JJ, Bryant SJ. Comparison of photopolymerizable thiol-ene PEG and acrylate-based PEG hydrogels for cartilage development. Biomaterials. 2013;34:9969–79. doi: 10.1016/j.biomaterials.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Skaalure SC, Akalp U, Vernerey FJ, Bryant SJ. Tuning Reaction and Diffusion Mediated Degradation of Enzyme-Sensitive Hydrogels. Adv Healthc Mater. 2016:432–438. doi: 10.1002/adhm.201500728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Skaalure SC, Akalp U, Vernerey FJ, Bryant SJ. Tuning Reaction and Diffusion Mediated Degradation of Enzyme-Sensitive Hydrogels. Adv Healthc Mater. doi: 10.1002/adhm.201500728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sokic S. Enhanced degradation and peptide specificity of MMP-sensitive scaffolds for neovascularization of engineered tissues. ProQuest Diss Publ. 2013 [Google Scholar]
- 54.Sridhar B, Anseth KS. Development of a cellularly degradable PEG hydrogel to promote articular cartilage extracellular matrix deposition. Adv Healthc Mater. 2015;4:702–713. doi: 10.1002/adhm.201400695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Swartzlander MD, Barnes CA, Blakney AK, Kaar JL, Kyriakides TR, Bryant SJ. Linking the foreign body response and protein adsorption to PEG-based hydrogels using proteomics. Biomaterials. 2015;41:26–36. doi: 10.1016/j.biomaterials.2014.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Swartzlander MD, Lynn AD, Blakney AK, Kyriakides TR, Bryant SJ. Understanding the host response to cell-laden poly(ethylene glycol)-based hydrogels. Biomaterials. 2013;34:952–64. doi: 10.1016/j.biomaterials.2012.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tian W, Kyriakides TR. Matrix metalloproteinase-9 deficiency leads to prolonged foreign body response in the brain associated with increased IL-1beta levels and leakage of the blood-brain barrier. Matrix Biol. 2009;28:148–59. doi: 10.1016/j.matbio.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Timms PM, Mannan N, Hitman GA, Noonan K, Mills PG, Syndercombe-Court D, Aganna E, Price CP, Boucher BJ. Circulating MMP9, vitamin D and variation in the TIMP-1 response with VDR genotype: Mechanisms for inflammatory damage in chronic disorders? QJM - Mon J Assoc Physicians. 2002;95:787–796. doi: 10.1093/qjmed/95.12.787. [DOI] [PubMed] [Google Scholar]
- 59.Turk BE, Huang LL, Piro ET, Cantley LC. Determination of protease cleavage site motifs using mixture-based oriented peptide libraries. 2001;19:661–667. doi: 10.1038/90273. [DOI] [PubMed] [Google Scholar]
- 60.Turturro MV, Christenson MC, Larson JC, Young DA, Brey EM, Papavasiliou G. MMP-sensitive PEG diacrylate hydrogels with spatial variations in matrix properties stimulate directional vascular sprout formation. PLoS One. 2013;8:e58897. doi: 10.1371/journal.pone.0058897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang H, Udukala DN, Samarakoon TN, Basel MT, Kalita M, Abayaweera G, Manawadu H, Malalasekera A, Robinson C, Villanueva D, Maynez P, Bossmann L, Riedy E, Barriga J, Wang N, Li P, Higgins DA, Zhu G, Troyer DL, Bossmann SH. Nanoplatforms for highly sensitive fluorescence detection of cancer-related proteases. Photochem Photobiol Sci. 2014;13:231. doi: 10.1039/c3pp50260k. [DOI] [PubMed] [Google Scholar]


