Abstract
Objective
Aortic vascular stiffness has been implicated in the development of cardiovascular disease (CVD) and chronic kidney disease (CKD) in obese individuals. However, the mechanism promoting these adverse effects are unclear. In this context, promotion of obesity through consumption of a western diet (WD) high in fat and fructose leads to excess circulating uric acid. There is accumulating data implicating elevated uric acid in the promotion of CVD and CKD. Accordingly, we hypothesized that xanthine oxidase inhibition with allopurinol would prevent a rise in vascular stiffness and proteinuria in a translationally relevant model of WD-induced obesity.
Materials/Methods
Four-week-old C57BL6/J male mice were fed a WD with excess fat (46%) and fructose (17.5%) with or without allopurinol (125mg/L in drinking water) for 16 weeks. Aortic endothelial and extracellular matrix/vascular smooth muscle stiffness was evaluated by atomic force microscopy. Aortic XO activity, 3-nitrotyrosine and aortic endothelial sodium channel (EnNaC) expression were evaluated along with aortic expression of inflammatory markers. In the kidney, expression of toll like receptor 4 (TLR4) and fibronectin were assessed along with evaluation of proteinuria.
Results
XO inhibition significantly attenuated WD-induced increases in plasma uric acid, vascular XO activity and oxidative stress, in concert with reductions in proteinuria. Further, XO inhibition prevented WD-induced increases in aortic EnNaC expression and associated endothelial and subendothelial stiffness. XO inhibition also reduced vascular pro-inflammatory and maladaptive immune responses induced by consumption of a WD. XO inhibition also decreased WD-induced increases in renal TLR4 and fibronectin that associated proteinuria.
Conclusions
Consumption of a WD leads to elevations in plasma uric acid, increased vascular XO activity, oxidative stress, vascular stiffness, and proteinuria all of which are attenuated with allopurinol administration.
Keywords: Arterial stiffness, obesity, uric acid, xanthine oxidase, macrophage polarization, proteinuria
1. Introduction
The prevalence of obesity continues to increase in children and adults, in part, due to an increased consumption of diets high in saturated fats and refined carbohydrates [1, 2]. Further, obesity is associated with increased vascular stiffness, impaired endothelial mediated relaxation and proteinuria, which are strong markers for a heightened risk for cardiovascular disease (CVD) and chronic kidney disease (CKD) [2, 3]. In this regard, hyperuricemia resulting from consumption of a western diet (WD), high in saturated fat and fructose, is emerging as a risk factor for vascular and renal fibrosis, proteinuria and associated CVD and CKD [4, 5].
Arterial stiffness has emerged as a risk factor for the development and progression of CVD and CKD especially in those with obesity [6, 7]. Further, accumulating evidence suggests that increased serum uric acid levels are associated with endothelial dysfunction contributing to the development of vascular stiffness [8]. Moreover, the role of diet-induced enhancement of tissue xanthine oxidase (XO) activity, the enzyme responsible for uric acid production, has also been implicated in endothelial dysfunction in obesity [9]. In this regard, recent studies suggest a pivotal role for oxidative stress and pro-inflammatory immune responses in the development of endothelial dysfunction, vascular stiffness, kidney injury, and proteinuria associated with obesity [10–12]. However, the mechanisms involved in the development of arterial stiffness in association with elevated uric acid levels and enhanced vascular tissue XO activity are poorly understood. Accordingly, we hypothesized that consumption of a WD, high in fat and fructose, would increase hepatic production of uric acid and increase vascular tissue XO activity. We further posited that this would lead to enhanced vascular oxidative stress and vascular inflammatory and immune responses promoting vascular stiffness and proteinuria.
Our prior work has suggested that consumption of a WD promotes endothelial stiffness, which is driven, in part, by enhanced activation of the endothelial sodium channel (EnNaC), increased vascular oxidative stress and reduced bioavailable nitric oxide (NO) [11]. As bioavailable NO normally suppresses EnNaC activity, it is quite plausible that excessive production of reactive oxygen species (ROS) caused by WD feeding, mediates the destruction of NO which in turn drives the increased EnNaC-mediated vascular stiffness and associated inflammation and proteinuria. To test this hypothesis, we fed male mice a WD, with or without allopurinol for 16 weeks. We report here that allopurinol prevented the increases in uric acid, aortic XO activity and oxidative stress associated with the WD. In addition, allopurinol prevented WD-induced increases in endothelial cell (EC) and sub-endothelial cell stiffness, renal fibrosis and proteinuria.
2. Methods
2.1. Experimental design
Four-week-old C57BL6/J male mice were divided into three groups and fed for 16 weeks. Group 1 were fed control diet (CD, Test Diet 58Y2, Richmond, Indiana). Group 2 were fed a Western diet (WD, Test Diet 58Y1) with excess fat (46%) and high fructose corn syrup (17.5%) and sucrose (17.5%) ad libitum. Group 3 were fed a WD and allopurinol, a potent XO inhibitor [13], was administered to this group in drinking water (125 mg/L) at the start of WD feeding as a preventive strategy and continued for 16 weeks. At 20 weeks of age, mice were euthanized by exsanguination under isoflurane anesthesia and tissues were harvested for downstream analysis. Prior to sacrifice, mice were fasted for 4 hours. The experiments were done as two independent cohort with 4 to 5 animals in each cohort and both tissues samples and blood were collected from these animals. All animal protocols were approved by the University of Missouri Institutional Animal Care and Use Committee.
2.2. Body composition
The percent body fat was measured by a nuclear magnetic resonance imaging whole-body composition analyzer (Echo MRI 4in1/1100; Echo Medical Systems, Houston, TX). This noninvasive measure was performed on conscious mice.
2.3. Aortic endothelial cortical stiffness determination via atomic force microscopy (AFM)
To evaluate the stiffness of ECs in enface aortic preparations, a 2×2 mm segment of the thoracic aorta was obtained from mice after sacrifice as previously described [10]. The aorta was opened longitudinally and the adventitial surface of each explant was fastened to a glass cover slip using cell tak [10]. Stiffness of ECs within intact aortic explants was measured using a cell nano-indentation protocol with AFM according to previously described procedures [10, 14]. AFM measurements were conducted at room temperature. For stiffness measurements, an AFM cantilever was placed on the ex vivo aorta with or without denudation of EC layer as previously described [10].
2.4. Microvessel stiffness indexes
At euthanasia, 1A mesenteric resistance arteries were collected and their mechanical and elastic characteristics were determined ex vivo as a surrogate vessel for microvascular (ie. renal vascular stiffness) as previously described [15]. Briefly, mesenteric arteries were cannulated and pressurized to 70 mmHg while exposed to Ca-free buffer containing 2mM EGTA and 100 uM adenosine to induce passive conditions. Vessels were then subjected to consecutive changes in intraluminal pressure from 5–120 mmHg. At each pressure step, intraluminal diameter, as well as vascular wall thicknesses were measured using a video dimension analyzer. These measurements were used to calculate incremental moduli of elasticity and strain-stress relationships as previously described [15].
2.5. Immunohistochemistry
Fluorescent and bright-field immunohistochemistry were used to quantify protein expression in the different components of the aorta in different groups as previously described [10]. Briefly, a 2 mm segment of thoracic aorta was fixed in 3% paraformaldehyde, dehydrated in ethanol series, paraffin embedded, and transversely sectioned in 5μm. To evaluate the formation of aortic 3-nitrotyrosine (3-NT) as a representative of nitrosylated oxidation products, sections were incubated with 1:200 rabbit polyclonal anti-3-NT overnight at room temperature and appropriate secondary antibody, and the signals were visualized by diaminobenzidine (DAB) chromogen system (Dako).
2.6. Biochemical assays
Blood samples were collected from a subset of fasting mice in each treatment group, and plasma was stored at −80°C for plasma uric acid determination, glucose and insulin assay and homeostatic model assessment of insulin resistance as previously described [13]. Plasma cholesterol and triglyceride assay was performed automated clinical chemistry analyzer (AU680, Beckman-Coulter, Inc., Brea, CA). Aortic XO activity was determined in tissue protein supernatant using a XO assay kit (Abcam, Cambridge, MA) [13].
Urine samples were collected into chilled 4°C tubes within one week prior to sacrifice from mice placed in metabolic cages for 24 hours were subsequently stored at −80 C for further analysis. Total protein and creatinine content in the urine were determined using a colorimetric assay developed by Beckman Coulter to be run on an automated clinical chemistry analyzer (AU680, Beckman-Coulter, Inc., Brea, CA) platform. Urine sodium was measured by the same Beckman-Coulter analyzer employing ion-specific electrodes.
Fibronectin protein levels in renal cortical tissue was evaluated by western immunoblot employing the primary antibody at a 1:1000 dilution (Abcam) [13].
2.7. Gene expression
Total RNA was isolated using the TRIzol reagent (Sigma) method as previously described [13]. The yield of RNA was quantified using a Nanodrop spectrophotometer (Thermo Scientific, Wilmington, DE). First-strand cDNA synthesis was done using 1 μg total RNA with oligo dT (1 μg), 5× reaction buffer, MgCl2, dNTP mix, RNAse inhibitor, and Improm II reverse transcriptase as per Improm II reverse transcription kit (Promega, Madison, WI). After the first strand synthesis, real-time PCR (qRT PCR) was done using 8 μl cDNA, 10 μl SYBR green PCR master mix (Bio-Rad Laboratories) and forward and reverse primers (10 pM/μl) (Integrated DNA Technologies, San Diego, CA) using a real-time PCR system (CFX96; Bio-Rad Laboratories). The specificity of the primers was analyzed by running a melting curve. The PCR cycling conditions used were 5 minutes at 95°C for initial denaturation, 40 cycles of 30 seconds at 95°C, 30 seconds at 58°C and 30 seconds at 72°C. Each real-time PCR was carried out using three individual samples in triplicates, and the threshold cycle values were averaged. Calculations of relative normalized gene expression were done using the Bio-Rad CFX manager software. The results were normalized against housekeeping gene GAPDH. For the expression of Toll-like receptor 4 (TLR4) the results were normalized against 18S rRNA. PCR cycling conditions were 5 minutes at 95°C for initial denaturation, 35 cycles of 30 seconds at 95°C, 1 minute at 60 °C and 1 minute at 72°C. The primers used for PCR are listed in Supplemental Table 1.
2.9. Statistical analysis
All data are presented as mean ± standard error (SE). Independent t-test and one- or two-way ANOVA was used to evaluate the effects of allopurinol on all dependent variables. For all statistical tests, differences were considered significant when P<0.05.
3. Results
3.1. XO inhibition and biochemical parameters
Compared to control diet (CD), consumption of a WD increased plasma uric acid levels and this increase was abrogated by allopurinol (Supplemental Table 2). Body weight, total body fat and reproductive fat were increased in WD-fed mice compared to CD-fed mice (P<0.05). XO inhibition had no effect on these parameters (Supplemental Table 2). In addition, plasma cholesterol levels were increased in WD-fed mice and were not affected by XO inhibition (Supplemental Table 2). WD-induced increase in insulin resistance as evaluated by HOMA-IR was not affected by allopurinol administration (Supplemental Table 2).
3.2. XO inhibition prevents WD-induced increases in aortic EC cortical and vascular smooth muscle cell (VSMC)/extracellular matrix (ECM) stiffness
WD-fed mice exhibited a 4-fold increase in EC cortical stiffness, assessed by AFM, compared to mice fed a CD (P<0.05), and this was prevented by allopurinol (Fig. 1A). The endothelium has been shown to modulate VSMC/ECM stiffness, in part, through changes in bioavailable NO [10, 16]. Therefore, we examined the stiffness of the underlying VSMC and ECM in endothelium denuded aortae. VSMC/ECM stiffness was significantly increased 3.5-fold (P<0.05) by WD feeding (Fig. 1B). Further, the WD-induced increase in VSMC/ECM stiffness was substantially decreased by treatment with the XO inhibitor (P<0.05). Recently, we and others have shown an important role for the EnNaC in regulation of endothelial and vascular stiffness [11]. To evaluate EnNaC expression, we immuno-stained aorta sections and quantified fluorescence signal in the endothelial layer. Compared to the CD-fed mice, EnNaC immunofluorescence was increased in the aortic endothelium of WD-fed mice and this was prevented with XO inhibition (Fig. 1C).
Figure 1. XO inhibition prevents WD-induced increases in aortic EC and vascular smooth muscle/extracellular matrix stiffness.
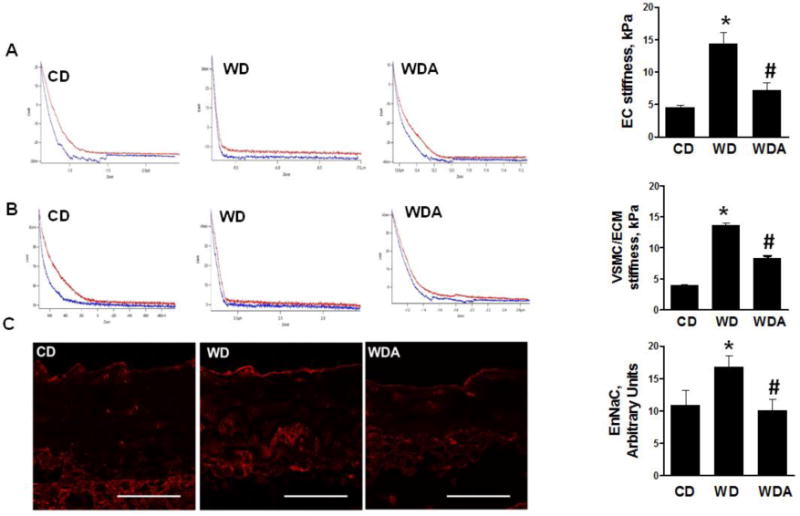
A. Representative force curve (red trace) from CD, WD, WDA group showing increased EC stiffness. The steeper the slope in the deflection trace, the stiffer the tissue. Blue curve represents retraction force that varies depending on the underlying region but not accounted for force curve generation. The bar graph summarizes the increased EC stiffness in WD-fed mice and its suppression by XO inhibitor in ex vivo aortic explants. B. Representative force curve from CD, WD, WDA group for VSMC/ECM stiffness and bar graph representing VSMC/ECM or sub-endothelial stiffness was obtained after denudation of endothelium of aortic explants. C. Representative immune-staining for the expression of EnNaC from CD, WD and WDA group. N = 3 to 5 per group. CD - control diet, WD - Western Diet, WDA - Western diet plus allopurinol. Data are expressed as means ± SE. * P<0.05 vs CD; # p<0.05 vs WD.
3.3. WD-induced oxidative stress is prevented by XO inhibition
Accumulation of vascular peroxynitrite, as a proxy for ROS, was evaluated by determination of aortic 3-NT staining in EC/intimal, smooth muscle and adventitial layers of the aorta (Fig. 2A–D). 3-NT staining was significantly increased in EC/intimal layer compared to other layers of the aortic wall. XO inhibition prevented accumulation of 3-NT in all layers of the aortic wall in WD-fed mice (P<0.05). We further examined the changes in XO activity in aortic tissue (Fig. 2E). Aortic XO activity was increased 2-fold by WD compared to CD (P<0.05), and this increase was prevented with XO inhibition (P<0.05). Collectively, these data suggest that WD increases in oxidative stress are driven, in part, by increased XO activity in vascular tissue.
Figure 2. XO inhibition reduces the levels of ROS and XO activity and decreases plasma uric acid levels induced by WD.
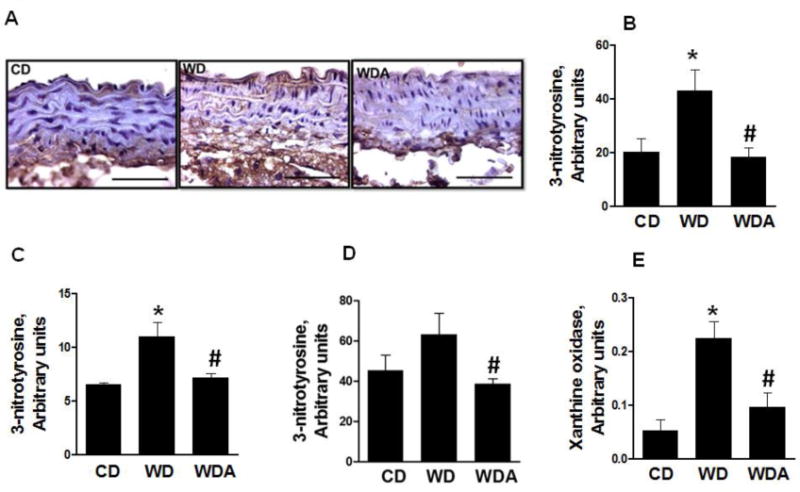
A. Representative images for quantification of 3-nitrotyrosine by immunostaining. B–D. The bar graph showing increased oxidative stress in EC (B), VSMC (C) and adventitial layer (D) of aorta and its prevention by XO inhibition. E. XO activity in aortic tissue was significantly increased in WD group but not in WD plus XO inhibitor group. N = 3 to 6 per group. CD - control diet, WD - Western Diet, WDA - Western diet plus allopurinol. Data are expressed as means ± SE. * P<0.05 vs. CD; # p<0.05 vs WD.
3.4. XO inhibition suppresses WD-induced maladaptive immune inflammatory responses involving macrophage recruitment and M1 polarization
To evaluate the potential contributions of macrophage recruitment and polarization of T cells to vascular inflammation caused by WD feeding, we next ascertained the expression of monocyte chemoattractant protein (MCP-1) in aortic tissue as a factor that drives monocytes to EC adhesion and trans-endothelial macrophage migration (Fig. 3A). The expression of MCP-1 was significantly increased in aorta by WD and this increase was significantly inhibited by XO inhibition (P<0.05). We further examined the accumulation of total macrophages and the M1 macrophage pro-inflammatory phenotype. The gene expression of CD11b, representing accumulation of total macrophages, and mRNA expression of CD86 and interleukin (IL)-6, representing mainly M1 macrophage polarization, were increased in WD-fed mice (P<0.05) (Fig. 3B–D). In contrast, XO inhibition attenuated the WD increases in expression of CD11b, CD 86 and IL-6 (Fig. 3B–D). Taken together, these data suggest the possibility that increased WD-induced XO activity and oxidative stress are involved in the maladaptive immune responses associated with consumption of a WD.
Figure 3. XO inhibition suppresses WD-induced inflammatory gene expression in aorta.
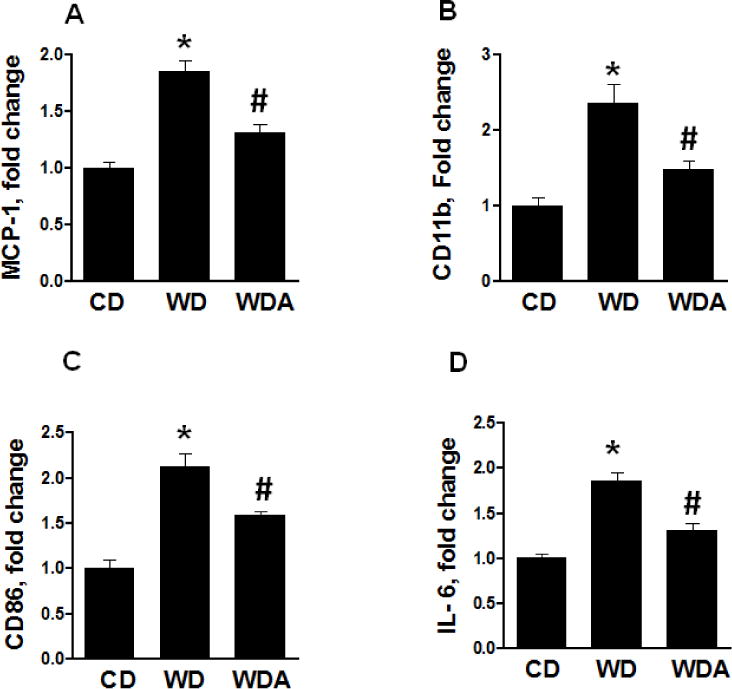
A. mRNA expression of MCP-1, a marker of EC activation, is increased in WD-fed mice which was prevented by XO inhibition B. CD11b as indicator of macrophage recruitment is increased in WD-fed group but not in WD plus XO inhibitor group. C. CD 86 expression is increased in WD-fed mice indicating M1 polarization and this response was blunted by XO inhibition. D. IL-6 expression is a marker of M1 polarization and enhanced pro-inflammatory response to WD feeding. This response is prevented by XO inhibitor treatment in WD-fed mice. N = 3 per group. CD - control diet, WD - Western Diet, WDA - Western diet plus allopurinol. Data are expressed as means ± SE. * P<0.05 vs CD; # p<0.05 vs WD.
3.5. XO inhibition decreases WD-induced microvessel stiffness, kidney fibrosis and proteinuria
Mesenteric resistance arteries serve as a surrogate for microvascular response in many tissue beds including the kidney microcirculation [15]. We have recently reported increased stiffness of mesenteric arteries in male mice fed a WD as shown by changes in circumferential strain and stress measured in pressurized arteries and increases in incremental modulus of elasticity [15]. In the current study, allopurinol significantly improved WD-induced changes in strain and stress response (P<0.05, Fig. 4A) and decreased the incremental modulus of elasticity (P<0.05, Fig. 4B). These data suggest XO inhibition reduces microvascular stiffness promoted by consumption of WD.
Figure 4. Effect of allopurinol treatment on WD-induced mesenteric artery stiffness.
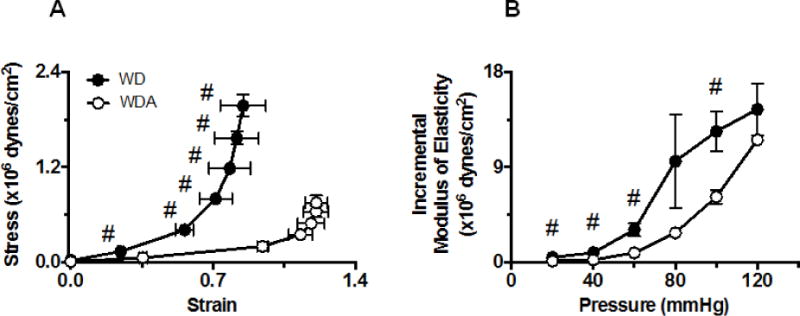
A. Comparison of strain-stress relationship curves between arteries isolated from WD and WDA-treated mice. B. Comparison of the incremental moduli of elasticity between the same arteries as in A. Data are expressed as means ± SE. # Data points were significantly different between WD and WDA groups at p<0.05. N=3 to 4 number of animals (vessels) per treatment group. WD -Western Diet, WDA - Western diet plus allopurinol.
EC stiffness and inflammatory responses are implicated in the development of glomerular endothelial injury, fibrosis and proteinuria in obesity [8, 16]. We therefore evaluated protein excretion in mice consuming a WD with and without XO inhibition. Protein excretion was significantly increased in WD-fed mice compared to CD-fed mice (Fig. 5A). Allopurinol administration resulted in less urine protein excretion in mice consuming a WD (P<0.05). To examine if there was any impact of XO inhibition on conventional renal epithelial sodium channels, we determined urine sodium excretion. XO inhibition had no significant effect on the level of urine sodium (Supplemental Table 2). Uric acid increases kidney fibrosis and inflammation, in part, through upregulation of fibronectin and TLR4 expression [17]. Therefore, we examined expression of fibronectin and TLR4 in kidney tissue. Fibronectin expression was increased in WD-fed mice and XO inhibition abrogated this increase in expression of this pro-fibrotic molecule (P<0.05, Fig. 4B). As shown in Fig. 5C, WD also induced an increase in expression of TLR-4 (P<0.05) and this was prevented by allopurinol.
Figure 5. XO inhibition suppresses WD-induced kidney fibrosis and proteinuria.
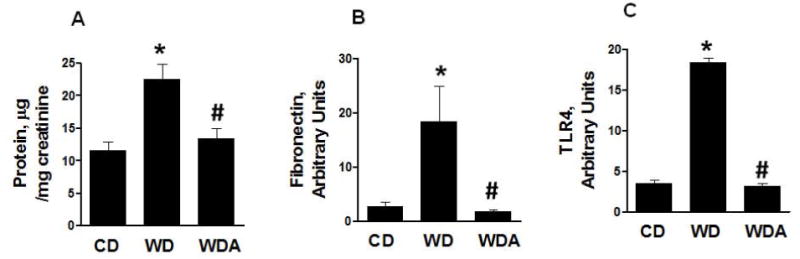
A. The bar graph shows increased excretion of protein in WD-fed mice and its prevention of treatment with XO inhibitor. B. WD tended increased fibronectin expression that is decreased by XO inhibition. C. TLR4 receptor expression is increased in WD-fed mice and tended to decrease in XO inhibitor treated group. N =4 to 8 per group. CD - control diet, WD - Western Diet, WDA - Western diet plus allopurinol. Data are expressed as means ± SE. * P<0.05 vs CD; # p<0.05 vs WD.
4. Discussion
This investigation demonstrates for the first time that consumption of a WD, enriched in fat and fructose not only increases uric acid levels, but also XO activity and oxidative stress in the aorta. Further, these changes were accompanied by increased expression of EnNaC and enhanced EC cortical and sub-endothelial vascular stiffness. The observed increased plasma uric acid and enhanced vascular XO activity with WD consumption was associated with an increase in vascular oxidative stress, maladaptive immune inflammatory responses and increased kidney fibronectin and proteinuria. Importantly, administration of allopurinol to WD-fed mice mitigated the increase in vascular XO activity, endothelial and sub-endothelial stiffness, oxidative stress, as well as, the maladaptive immune inflammatory responses and proteinuria. These collective findings support an undescribed mechanism to date on the contribution that uric acid has on endothelial stiffness, endothelial dysfunction and proteinuria through XO-induced oxidative stress and inflammatory responses.
Hypertension is an important risk factor for the development of vascular remodeling and increased vascular and arterial stiffness; however, stiffness is often increased in normotensive people [12]. Moreover, rodent studies also show that arterial stiffness precedes hypertension in mice fed high fat/high refined carbohydrates and removal of a high fat/high fructose diet results in a decrease in arterial stiffness [12]. In this regard, we have previously reported that blood pressure was not increased by WD-feeding in male mice and that allopurinol had no impact on blood pressure [13]. Therefore, our observation that allopurinol attenuated the WD-induced endothelial and VSMC stiffness in this study without lowering BP suggests this occurred through a blood pressure-independent effect. Our additional observation that allopurinol improved vascular stiffness without a significant impact on body weight or systemic insulin resistance as determined by HOMA-IR, further supports potential direct effect of XO inhibition on the vasculature.
Increased plasma levels of uric acid have been associated with enhanced oxidative stress in tissues and cultured ECs[16]. Further, impaired EC function in the setting of hyperuricemia can be mitigated by reducing XO in the vasculature [4]. In the present study, inhibition of aortic tissue XO activity in concert with reductions in oxidative stress suggest that XO is an important enzyme contributing to generation of reactive oxygen species (ROS) in the vasculature. Increases in ROS increases destruction of NO which plays a significant role in EC stiffness as well as aortic stiffness [13]. Oxidative stress also induces recruitment of immune cells to the vasculature and this event occurs early during the development of vascular dysfunction and arterial stiffness [18]. Immune cell recruitment is a sequential, multistep event resulting from interaction of leukocytes, monocytes and endothelial adhesion molecules [19]. Although this depends on adhesion molecule expression on immune cells and modulation of their expression by vascular cells, endothelial dependent mechanisms appear to be critical in the recruitment of immune cells including macrophages. MCP-1 is a molecule that is involved in attraction and adhesion of monocytes to EC [20]. Both decreased availability of NO and increased accumulation of ROS increase MCP-1 expression in EC [21]. Endothelial cortical stiffness additionally contributes to macrophage recruitment through its effect of decreasing bioavailable NO, enhancement of monocyte adhesion enhanced endothelial permeability and trans-endothelial migration of monocytes [16, 22]. These data are consistent with our finding that WD-induced increases in 3–NT content along with MCP-1 expression. Our observation then that XO inhibition reduced MCP-1 along with 3-NT and vascular stiffness measures suggest that tissue XO generation of ROS is an important mechanism by which a WD and excess uric acid promote endothelial dysfunction and vascular stiffness through immune inflammatory responses.
The vasculature pro-inflammatory immune response is constituted by both increases in macrophage infiltration and increases in the pro-inflammatory M1 macrophage phenotype [23]. M1 macrophage polarization which is a prototypical pro-inflammatory phenotypic alteration of macrophages, is associated with increased production of ROS and reactive nitrogen species (RNS), and increased expression of pro-inflammatory cytokines [24]. In the current study consumption of a WD increased CD11b, a total macrophage cell marker as well as M1 polarization markers CD86 and IL6 which were prevented by allopurinol. Increased M1 polarized macrophages produce more pro-inflammatory IL-6, and increased IL-6 levels are present in obese individuals [25]. A recent study demonstrated cholesterol-induced increases in macrophage XO and pro-inflammatory responses that were effectively prevented by XO inhibition [26]. However, while WD consumption induced an increase in plasma cholesterol, XO inhibition had no effect on plasma cholesterol in the current study. Collectively, data in this study suggest that direct tissue activation of XO activity with consumption of a WD promotes oxidative stress and associated pro-inflammatory immune responses leading to vascular fibrosis and stiffness.
We report the novel observation that increased vascular stiffness with consumption of a WD occurs in association with increased expression of vascular EnNaC, and that this process is suppressed by XO inhibition. Classically, ENaC is considered a regulator of Na+ transport in kidney epithelial cells, but ENaC is also expressed in non-epithelial cells, including endothelial cells thereby the terminology EnNaC [27, 28]. Uric acid has been shown to increase the expression of ENaC in renal epithelial cells [29]. However, this is the first report showing that diet induced obesity is associated with enhanced EnNaC expression, and that this is prevented XO inhibition. The activation of EnNaC results in polymerization of G- to F-actin [30]. This results in decreased flow-mediated NO production due to disruption of the endothelial glycocalyx (i.e., surface layer comprising a complex of glycoproteins located on the luminal surface of EC) and its interaction with endothelial nitric oxide synthase (eNOS) during flow-mediated vasodilation [31]. Decreased bioavailable NO is one of the major factors contributing to endothelial dysfunction and vascular stiffness in obesity [32]. Accordingly, our findings that XO inhibition attenuated the diet induced increases in EnNaC suggest a potential novel mechanism by which WD induced increases in uric acid production promotes stiffness in the vasculature.
The presence of albuminuria in obesity is thought to be associated with glomerular hyperfiltration. Importantly, a recent study showed a positive correlation between microvessel stiffness, increased renal resistance index, and albuminuria [33]. Thus, current data would suggest that WD-induced aortic stiffness and alterations in resistance artery (ie. mesenteric artery) function may be an important contributor to proteinuria associated with diet induced obesity. In this context, our findings that WD-induced increases in mesenteric artery stiffness suggest that a WD increases the arterial wave velocity to the glomerulus and thus contributes to hyperfiltration. Resistance mesenteric arteries are often used as a surrogate for other vessel beds such as the kidney microcirculation [15]. Thereby, our observation that XO inhibition reduced proteinuria along with mesenteric resistance artery elasticity s would be consistent with this notion.
In addition to indirect vascular effects, the direct kidney effects of XO inhibition may also account for prevention of WD induced kidney fibrosis and proteinuria. Although uric acid has been implicated in mediating glomerular injury in several preclinical studies, including ours in WD-fed male mice [6, 34], the role of XO activation in ECs has also been implicated in endothelial cortical stiffness through glycocalyx polymerization and shedding [35–37]. Indeed, glycocalyx shedding is one of the mechanisms by which glomerular filtration barrier is compromised resulting in proteinuria [38, 39]. We also observed that consumption of a WD was associated with increased renal tissue TLR4, and that this process was mitigated by allopurinol administration. Importantly, TLR4 is implicated in the kidney inflammatory responses and uric acid increases the expression of TLR4 in kidney [17]. Signaling through toll-like receptors activates dendritic cells and inflammatory cytokines to promote inflammation and fibrosis. Moreover, soluble uric acid causes increased expression of fibronectin which is considered to be one of the potential mediators of kidney fibrosis [40]. In this regard, we have also observed increased renal expression of TLR4 and an important fibrosis protein, fibronectin in conjunction with increased proteinuria in WD-fed mice. The attenuation of WD increases in both renal tissue TLR4 and fibronectin with allopurinol administration uncovers potential mechanisms by which dietary elevations in uric acid may promote kidney tissue fibrosis and proteinuria.
In conclusion, consumption of a WD leads to elevations in plasma uric acid and increased vascular XO activity, vascular stiffness and proteinuria all of which are attenuated with allopurinol administration. We have previously reported that allopurinol reduced WD-induced increases in cardiac XO activity and attenuated cardiac fibrosis [15]. The improvement of vascular stiffness and proteinuria with allopurinol was associated with decreases in vascular XO activity suggesting that both increased plasma uric acid and tissue XO activity with WD consumption contribute to cardiovascular and renal injury.
Supplementary Material
Acknowledgments
This research was supported by the National Institutes of Health (R01-HL73101 and R01-HL1079100) and Veterans Affairs Merit System (0018) to James R Sowers. The authors wish to thank Brenda Hunter for her assistance in editing the manuscript.
Abbreviations
- CVD
cardiovascular disease
- CKD
chronic kidney disease
- EnNaC
endothelial sodium channel
- CD
control diet
- WD
western diet
- 3-NT
3-nitrotyrosine
- TLR4
Toll -like receptor 4
- XO
xanthine oxidase
- VSMC
vascular smooth muscle cell
- ECM
extracellular matrix
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
Author contributions
All authors contributed to the design and conduct of the study data collection and analysis, data interpretation and manuscript writing.
References
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. Jama. 2010;303(3):235–41. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Sowers JR, Whaley-Connell A, Hayden MR. The Role of Overweight and Obesity in the Cardiorenal Syndrome. Cardiorenal medicine. 2011;1(1):5–12. doi: 10.1159/000322822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agirbasli M, Tanrikulu AM, Berenson GS. Metabolic Syndrome: Bridging the Gap from Childhood to Adulthood. Cardiovascular therapeutics. 2016;34(1):30–6. doi: 10.1111/1755-5922.12165. [DOI] [PubMed] [Google Scholar]
- 4.Borghi C, Rosei EA, Bardin T, Dawson J, Dominiczak A, Kielstein JT, et al. Serum uric acid and the risk of cardiovascular and renal disease. Journal of hypertension. 2015;33(9):1729–41. doi: 10.1097/HJH.0000000000000701. discussion 41. [DOI] [PubMed] [Google Scholar]
- 5.MacIsaac RL, Salatzki J, Higgins P, Walters MR, Padmanabhan S, Dominiczak AF, et al. Allopurinol and Cardiovascular Outcomes in Adults With Hypertension. Hypertension. 2016;67(3):535–40. doi: 10.1161/HYPERTENSIONAHA.115.06344. [DOI] [PubMed] [Google Scholar]
- 6.Chaudhary K, Malhotra K, Sowers J, Aroor A. Uric Acid - key ingredient in the recipe for cardiorenal metabolic syndrome. Cardiorenal medicine. 2013;3(3):208–20. doi: 10.1159/000355405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jia G, Aroor AR, Sowers JR. Arterial Stiffness: A Nexus between Cardiac and Renal Disease. Cardiorenal medicine. 2014;4(1):60–71. doi: 10.1159/000360867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jia G, Aroor AR, Whaley-Connell AT, Sowers JR. Fructose and uric acid: is there a role in endothelial function? Current hypertension reports. 2014;16(6):434. doi: 10.1007/s11906-014-0434-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.George J, Carr E, Davies J, Belch JJ, Struthers A. High-dose allopurinol improves endothelial function by profoundly reducing vascular oxidative stress and not by lowering uric acid. Circulation. 2006;114(23):2508–16. doi: 10.1161/CIRCULATIONAHA.106.651117. [DOI] [PubMed] [Google Scholar]
- 10.DeMarco VG, Habibi J, Jia G, Aroor AR, Ramirez-Perez FI, Martinez-Lemus LA, et al. Low-Dose Mineralocorticoid Receptor Blockade Prevents Western Diet-Induced Arterial Stiffening in Female Mice. Hypertension. 2015;66(1):99–107. doi: 10.1161/HYPERTENSIONAHA.115.05674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jia G, Habibi J, Aroor AR, Martinez-Lemus LA, DeMarco VG, Ramirez-Perez FI, et al. Endothelial Mineralocorticoid Receptor Mediates Diet-Induced Aortic Stiffness in Females. Circulation research. 2016;118(6):935–43. doi: 10.1161/CIRCRESAHA.115.308269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weisbrod RM, Shiang T, Al Sayah L, Fry JL, Bajpai S, Reinhart-King CA, et al. Arterial stiffening precedes systolic hypertension in diet-induced obesity. Hypertension. 2013;62(6):1105–10. doi: 10.1161/HYPERTENSIONAHA.113.01744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jia G, Habibi J, Bostick BP, Ma L, DeMarco VG, Aroor AR, et al. Uric acid promotes left ventricular diastolic dysfunction in mice fed a Western diet. Hypertension. 2015;65(3):531–9. doi: 10.1161/HYPERTENSIONAHA.114.04737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu X, Sun Z, Meininger GA, Muthuchamy M. Application of atomic force microscopy measurements on cardiovascular cells. Methods in molecular biology (Clifton, NJ) 2012;843:229–44. doi: 10.1007/978-1-61779-523-7_22. [DOI] [PubMed] [Google Scholar]
- 15.Foote CA, Castorena-Gonzalez JA, Ramirez-Perez FI, Jia G, Hill MA, Reyes-Aldasoro CC, et al. Arterial Stiffening in Western Diet-Fed Mice Is Associated with Increased Vascular Elastin, Transforming Growth Factor-beta, and Plasma Neuraminidase. Frontiers in physiology. 2016;7:285. doi: 10.3389/fphys.2016.00285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aroor AR, Demarco VG, Jia G, Sun Z, Nistala R, Meininger GA, et al. The role of tissue Renin-Angiotensin-aldosterone system in the development of endothelial dysfunction and arterial stiffness. Frontiers in endocrinology. 2013;4:161. doi: 10.3389/fendo.2013.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao J, Zhang XL, Fu C, Han R, Chen W, Lu Y, et al. Soluble uric acid increases NALP3 inflammasome and interleukin-1beta expression in human primary renal proximal tubule epithelial cells through the Toll-like receptor 4-mediated pathway. International journal of molecular medicine. 2015;35(5):1347–54. doi: 10.3892/ijmm.2015.2148. [DOI] [PubMed] [Google Scholar]
- 18.Aroor AR, McKarns S, Demarco VG, Jia G, Sowers JR. Maladaptive immune and inflammatory pathways lead to cardiovascular insulin resistance. Metabolism: clinical and experimental. 2013;62(11):1543–52. doi: 10.1016/j.metabol.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schaefer A, Hordijk PL. Cell-stiffness-induced mechanosignaling - a key driver of leukocyte transendothelial migration. Journal of cell science. 2015;128(13):2221–30. doi: 10.1242/jcs.163055. [DOI] [PubMed] [Google Scholar]
- 20.Melgarejo E, Medina MA, Sanchez-Jimenez F, Urdiales JL. Monocyte chemoattractant protein-1: a key mediator in inflammatory processes. The international journal of biochemistry & cell biology. 2009;41(5):998–1001. doi: 10.1016/j.biocel.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 21.Magenta A, Greco S, Capogrossi MC, Gaetano C, Martelli F. Nitric oxide, oxidative stress, and p66Shc interplay in diabetic endothelial dysfunction. BioMed research international. 2014;2014:193095. doi: 10.1155/2014/193095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jia G, Aroor AR, DeMarco VG, Martinez-Lemus LA, Meininger GA, Sowers JR. Vascular stiffness in insulin resistance and obesity. Frontiers in physiology. 2015;6:231. doi: 10.3389/fphys.2015.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schiffrin EL. Immune mechanisms in hypertension and vascular injury. Clinical science. 2014;126(4):267–74. doi: 10.1042/CS20130407. [DOI] [PubMed] [Google Scholar]
- 24.Kalupahana NS, Moustaid-Moussa N, Claycombe KJ. Immunity as a link between obesity and insulin resistance. Molecular aspects of medicine. 2012;33(1):26–34. doi: 10.1016/j.mam.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 25.Fuster JJ, Walsh K. The good, the bad, and the ugly of interleukin-6 signaling. The EMBO journal. 2014;33(13):1425–7. doi: 10.15252/embj.201488856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nomura J, Busso N, Ives A, Matsui C, Tsujimoto S, Shirakura T, et al. Xanthine oxidase inhibition by febuxostat attenuates experimental atherosclerosis in mice. Scientific reports. 2014;4:4554. doi: 10.1038/srep04554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown NJ. Contribution of aldosterone to cardiovascular and renal inflammation and fibrosis. Nature reviews Nephrology. 2013;9(8):459–69. doi: 10.1038/nrneph.2013.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Castorena-Gonzalez JA, Staiculescu MC, Foote CA, Polo-Parada L, Martinez-Lemus LA. The obligatory role of the actin cytoskeleton on inward remodeling induced by dithiothreitol activation of endogenous transglutaminase in isolated arterioles. American journal of physiology Heart and circulatory physiology. 2014;306(4):H485–95. doi: 10.1152/ajpheart.00557.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu W, Huang Y, Li L, Sun Z, Shen Y, Xing J, et al. Hyperuricemia induces hypertension through activation of renal epithelial sodium channel (ENaC) Metabolism: clinical and experimental. 2016;65(3):73–83. doi: 10.1016/j.metabol.2015.10.026. [DOI] [PubMed] [Google Scholar]
- 30.Foote CA, Castorena–Gonzalez JA, Staiculescu MC, Clifford PS, Hill MA, Meininger GA, et al. Brief serotonin exposure initiates arteriolar inward remodeling processes in vivo that involve transglutaminase activation and actin cytoskeleton reorganization. American journal of physiology Heart and circulatory physiology. 2016;310(2):H188–98. doi: 10.1152/ajpheart.00666.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fels J, Jeggle P, Liashkovich I, Peters W, Oberleithner H. Nanomechanics of vascular endothelium. Cell and tissue research. 2014;355(3):727–37. doi: 10.1007/s00441-014-1853-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DeMarco VG, Aroor AR, Sowers JR. The pathophysiology of hypertension in patients with obesity. Nature reviews Endocrinology. 2014;10(6):364–76. doi: 10.1038/nrendo.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Calabia J, Torguet P, Garcia I, Martin N, Mate G, Marin A, et al. The relationship between renal resistive index, arterial stiffness, and atherosclerotic burden: the link between macrocirculation and microcirculation. Journal of clinical hypertension. 2014;16(3):186–91. doi: 10.1111/jch.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nistala R, Habibi J, Lastra G, Manrique C, Aroor AR, Hayden MR, et al. Prevention of obesity-induced renal injury in male mice by DPP4 inhibition. Endocrinology. 2014;155(6):2266–76. doi: 10.1210/en.2013-1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Battelli MG, Bolognesi A, Polito L. Pathophysiology of circulating xanthine oxidoreductase: new emerging roles for a multi-tasking enzyme. Biochimica et biophysica acta. 2014;1842(9):1502–17. doi: 10.1016/j.bbadis.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 36.Battelli MG, Polito L, Bolognesi A. Xanthine oxidoreductase in atherosclerosis pathogenesis: not only oxidative stress. Atherosclerosis. 2014;237(2):562–7. doi: 10.1016/j.atherosclerosis.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 37.Borghi C, Desideri G. Urate-Lowering Drugs and Prevention of Cardiovascular Disease: The Emerging Role of Xanthine Oxidase Inhibition. Hypertension. 2016;67(3):496–8. doi: 10.1161/HYPERTENSIONAHA.115.06531. [DOI] [PubMed] [Google Scholar]
- 38.Lipowsky HH, Lescanic A. The effect of doxycycline on shedding of the glycocalyx due to reactive oxygen species. Microvascular research. 2013;90:80–5. doi: 10.1016/j.mvr.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salmon AH, Satchell SC. Endothelial glycocalyx dysfunction in disease: albuminuria and increased microvascular permeability. The Journal of pathology. 2012;226(4):562–74. doi: 10.1002/path.3964. [DOI] [PubMed] [Google Scholar]
- 40.Yang Z, Xiaohua W, Lei J, Ruoyun T, Mingxia X, Weichun H, et al. Uric acid increases fibronectin synthesis through upregulation of lysyl oxidase expression in rat renal tubular epithelial cells. American journal of physiology Renal physiology. 2010;299(2):F336–46. doi: 10.1152/ajprenal.00053.2010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


