Abstract
Background
Ascomycete Cordyceps species have been using as valued traditional Chinese medicines. Particularly, the fruiting bodies of Cordyceps cicadae (syn. Isaria cicadae) have long been utilized for the treatment of chronic kidney disease. However, the genetics and bioactive chemicals in this fungus have been largely unexplored.
Results
In this study, we performed comprehensive omics analyses of C. cicadae, and found that, in contrast to other Cordyceps fungi, C. cicadae produces asexual fruiting bodies with the production of conidial spores instead of the meiotic ascospores. Genome sequencing and comparative genomic analysis indicate that the protein families encoded by C. cicadae are typical of entomopathogenic fungi, including the expansion of proteases and chitinases for targeting insect hosts. Interestingly, we found that the MAT1-2 mating-type locus of the sequenced strain contains an abnormally truncated MAT1-1-1 gene. Gene deletions revealed that asexual fruiting of C. cicadae is independent of the MAT locus control. RNA-seq transcriptome data also indicate that, compared to growth in a liquid culture, the putative genes involved in mating and meiosis processes were not up-regulated during fungal fruiting, further supporting asexual reproduction in this fungus. The genome of C. cicadae encodes an array of conservative and divergent gene clusters for secondary metabolisms. Based on our analysis, the production of known carcinogenic metabolites by this fungus could be potentially precluded. However, the confirmed production of oosporein raises health concerns about the frequent consumption of fungal fruiting bodies.
Conclusions
The results of this study expand our knowledge of fungal genetics that asexual fruiting can occur independent of the MAT locus control. The obtained genomic and metabolomic data will benefit future investigations of this fungus for medicinal uses.
Electronic supplementary material
The online version of this article (doi:10.1186/s12864-017-4060-4) contains supplementary material, which is available to authorized users.
Keywords: Cordyceps cicadae, Genomics, Mating type, Asexual fruiting, Secondary metabolism, Bioactive metabolites
Background
Ascomycete fungi belonging to Cordyceps sensu lato account to more than 500 known species that are classified into the three families, Cordycipitaceae, Ophiocordycipitaceae, and Clavicipitaceae [1, 2]. The family Cordycipitaceae includes the genera Cordyceps, Isaria, Beauveria, and Lecanicillium. The sexual stages of several species belonging to the genera Beauveria and Lecanicillium have been clarified as the Cordyceps spp. [1, 3]. In the family Ophiocordycipitaceae, some species such as Ophiocordyceps sinensis (better known as the caterpillar fungus C. sinensis) and O. unilateralis are highly host-specific and the infections by these fungi can alter insect host behaviors [4, 5]. The mechanisms of entomopathogenicity have been well studied in a number of species including Beauveria spp., and Metarhizium spp. belonging to the family Clavicipitaceae [2], which have been frequently using as insect biocontrol agents [6, 7]. The fruiting bodies produced by C. militaris, O. sinensis, and C. cicadae have long been used as valued traditional Chinese medicines (TCMs) for anticancer, immunomodulation, anti-fatigue, and anti-impotence [8, 9]. C. cicadae has once been called as C. sobolifera, C. sinclairii, Paecilomyces cicadae, and Isaria cicadae. The last name has become the recognized synonym of this fungus [10]. In contrast to C. militaris and O. sinensis with clear sexual lifecycles [11], the sexuality of C. cicadae is still enigmatic.
In Cordyceps, sexual reproduction is either heterothallic or homothallic that is controlled by the mating-type (MAT) loci [11]. The MAT1-1 locus in general contains two-three genes whereas the MAT1-2 locus has only one MAT1-2-1 gene [11, 12]. For example, the MAT1-1 loci in Metarhizium species contain three genes (i.e., MAT1-1-1, MAT1-1-2 and MAT1-1-3) whereas the MAT1-1 loci in B. bassiana and C. militaris have two genes [11, 13]. Interestingly, recent genome sequencing of different B. bassiana strains identified an additional MAT1-2-8 gene in the MAT1-2 type isolates [14]. Loss-of-function studies of MAT genes in C. militaris have indicated that both MAT1-1 and MAT1-2 loci are required for fungal fruiting and fertility but the three genes MAT1-1-1, MAT1-1-2, and MAT1-2-1 play diverse roles in regulating fertility and the formation of perithecia, asci, or ascospore [15]. In the homothallic fungus Fusarium graminearum, all MAT genes are not required for the formation of perithecia [16]. Until this study, the structure of MAT loci in C. cicadae is unclear, and their precise functions remain unknown.
In Asia, the fruiting bodies of C. cicadae together with the mycosed cicada cadaver are used as medicinal herbs for the treatment of chronic kidney disease [17, 18]. One study reported that the anti-fibrotic activity of ergosterol peroxide isolated from C. cicadae was responsible for its renoprotective effect [18]. Other studies exploring bioactive metabolites identified different adenosine analogs and cyclodepsipeptides such as cordycecin A and beauvericins in C. cicadae [19, 20]. Reports on whether or not C. cicadae can produce the anticancer compound cordycepin (i.e., 3′-deoxyadenosine) are inconsistent [21, 22]. The potent immunosuppressant drug Fingolimod was developed from the atypical amino acid myrocin produced by Isaria sinclairii [23]. Moreover, safety concerns of consuming Cordyceps have also been frequently raised due to uncertainty on fungal production of human-toxic mycotoxins [9, 24].
In this study, we performed fruiting-body induction, de novo genome sequencing and comparative genomic analysis of C. cicadae to help understand the genetic nature of fungal developmental controls. Fungal development-associated metabolomic analyses, transcriptomics, and deletion of MAT genes were also performed to better understand the secondary metabolisms and genetics of this fungus.
Results
Asexual fruiting and insect pathogenicity of C. cicadae
In nature, the fruiting bodies of C. cicadae form uniquely on pupated cicadae (Platylomia spp.) (Fig. 1a) and are referred to as “cicada flower” in China [25]. Due to the difficulty in rearing cicadae in the laboratory, we used the pupae of Chinese tussah silkworm (Antheraea pernyi) for inoculation with the conidia of C. cicadae. We found that the silkworm pupae could be killed and mycosed by the fungus 8 days post inoculation (dpi) (Fig. 1b). Fungal primordia formed 13 dpi (Fig. 1c), and the mature stroma/fruiting bodies were produced 22 dpi (Fig. 1d). The stroma of C. cicadae formed on artificial rice medium ca. 3 weeks post inoculation (Fig. 1e). Microscopic examinations indicated that the asexual conidial spores but not the sexual ascospores were produced on field-collected or lab-induced fruiting bodies (Fig. 1f). Thus, in contrast to other Cordyceps species that produce sexual fruiting bodies in the fields [1], C. cicadae forms synnema-like asexual structures.
Fig. 1.

Phenotypes and development of asexual fruiting in C. cicadae. a Field collected fruiting bodies of C. cicadae developed on the cadaver of cicada Platylomia sp. b-d Fruiting body production of C. cicadae on the pupae of Chinese tussah silkworm (Antheraea pernyi) after inoculation for different times (labeled in each panel). Dpi, days post inoculation. e Fruiting bodies of C. cicadae produced on the rice medium 23 days post inoculation. f Asexual conidial spores produced on the fruiting bodies (arrows in the panels A, D and E). CO, conidium; Bar, 5 μm
To examine the ability of C. cicadae to infect non-cicada insect hosts, we induced formation of the infection structure appressorium on the hind wings of the mealworm Tenebrio molitor. The results revealed that unlike C. militaris, C. cicadae could produce appressoria on beetle wings similar to M. robertsii, B. bassiana, and I. fumosorosea (Additional file 1: Fig. S1A). Insect bioassays against the mealworm larvae confirmed that C. cicadae could kill insects like B. bassiana and M. robertsii within 4 to 5 days post topical infection whereas C. militaris could not infect beetles (Additional file 1: Fig. S1B). In addition, we found that both C. cicadae and I. fumosorosea but not M. robertsii and B. bassiana could form synnema-like structures on insect cadavers (Additional file 1: Fig. S1C).
Genome sequencing and protein families involved in fungal entomopathogenicity
To better understand the physiology of C. cicadae, de novo genome sequencing was performed to obtain a 98.8% completeness of the genome. The genome size (33.9 Mb) and gene-coding capacity (9701 genes) of the fungus are equivalent to four other ascomycete insect pathogenic fungi C. militaris, I. fumosorosea, B. bassiana, and M. robertsii (Table 1). Protein family analyses indicated that C. cicadae has fewer numbers of total conserved protein families, and putative proteins involved in pathogen-host interaction (PHI) when compared to other pathogens. However, C. cicadae encodes higher numbers of small secreted cysteine-rich proteins (SSCPs), proteases, G-protein coupled receptors (GPCRs), lipases, glycoside hydrolases (GHs), and core genes involved in secondary metabolisms when compared to C. militaris or other fungi (Table 1). For example, C. cicadae encodes more lipases (35) than C. militaris (23), I. fumosorosea (21), and B. bassiana (28). In addition, more bacterial-like protein toxins are encoded by C. cicadae (16) than by C. militaris (6) and I. fumosorosea (12) but fewer than by B. bassiana (26) (Table 1). Overall, C. cicadae has the conventional genome features of entomopathogenic fungi [2, 26], including the genome expansion of serine proteases and chitinases (GH18) that are used by the fungus to degrade the protein- and chitin-rich cuticles of insect hosts (Additional file 1: Table S1-S4).
Table 1.
Comparison of the sequencing and genome features of C. cicadae with other entomopathogenic fungi
| Featuresa | C. cicadae | I. fumosorosea | C. militaris | B. bassiana | M. robertsii |
|---|---|---|---|---|---|
| Size (Mb) | 33.9 | 33.5 | 32.2 | 33.7 | 39 |
| Coverage fold | 80× | 86.99× | 147× | 76.6 × | 100× |
| Scaffold no. (>1 kb) | 599 | 430 | 13 | 242 | 176 |
| Scaffold N50 (Mb) | 0.21 | 0.87 | 4.55 | 0.73 | 1.96 |
| % G + C content | 53.0 | 53.6 | 51.4 | 51.5 | 51.5 |
| % Repeat rate | 3.19 | 3.84 | 3.04 | 2.03 | 0.98 |
| Protein-coding genes | 9701 | 10,060 | 9684 | 10,366 | 10,582 |
| Gene density (per Mb) | 286 | 300 | 301 | 308 | 271 |
| Exons per gene | 2.6 | 2.6 | 3 | 2.7 | 2.8 |
| Protein families | 2592 | 2876 | 2736 | 3002 | 2797 |
| Putative PHI genes | 1490 | 1604 | 1547 | 2121 | 1828 |
| SSCPs | 268 | 287 | 207 | 305 | 283 |
| Secreted proteins | 1031 | 1390 | 1133 | 1378 | 1333 |
| Proteases | 388 | 395 | 371 | 386 | 390 |
| Secreted proteases | 127 | 158 | 120 | 142 | 1660 |
| GPCRs | 38 | 39 | 29 | 32 | 64 |
| Lipases | 29 | 21 | 23 | 28 | 35 |
| Secondary metabolisms | 34 | 36 | 29 | 42 | 62 |
| Glycoside hydrolases | 135 | 143 | 134 | 144 | 149 |
| Cytochrome P450s | 67 | 76 | 65 | 89 | 133 |
| Bacterial-like toxins | 16 | 12 | 6 | 26 | 16 |
a,Abbreviations: Mb mega base, PHI pathogen-host interaction, SSCP small secreted cysteine-rich protein, GPCR G-protein coupled receptor
Phylogenetic and syntenic relationships
To infer the phylogeny of C. cicadae, a maximum likelihood phylogenomic tree was generated by including other insect pathogens, plant pathogens, and mycoparasites using 47 single-copy and conserved orthologous protein sequences. Consistent with a previous analysis [27], we observed that the three families of Cordyceps species could be well separated from each other and confirmed that C. cicadae belongs to the Cordycipitaceae family. In addition, the phylogeny revealed that C. cicadae is more closely related to the Isaria genus than to the Cordyceps lineage (Additional file 1: Fig. S2). The whole genome Blast score ratio analysis also indicated that the proteins encoded by C. cicadae have higher similarities to those in I. fumosorosea than to those in C. militaris (Fig. 2a). In addition, the pairwise comparison analysis based on oriented scaffolds demonstrated that the genome structure of C. cicadae is highly syntenic with that of I. fumosorosea whereas the fragmented, transverse, and/or reverse-oriented relationships are observed between the genomes of C. cicadae and C. militaris (Fig. 2b and c). C. cicadae and I. fumosorosea are therefore closely related to each other.
Fig. 2.
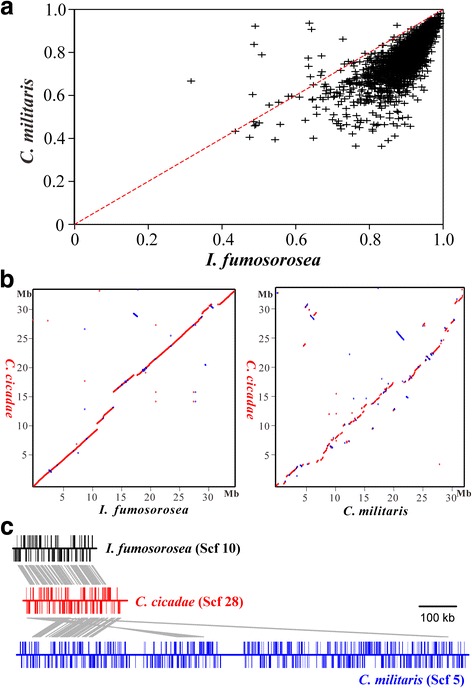
Genome structure comparison between C. cicadae and other closely-related fungi. a Scatter plots of Blast score ratio analysis of C. cicadae showing that the fungus is more closely related to I. fumosorosea than to C. militaris. b Dot blot analysis of C. cicadae, I. fumosorosea and C. militaris using ordered scaffold data. The blue spots show reverse-oriented relationships between the genomes. c Syntenic analysis of the representative scaffold structures between the three fungi. ISF, I. fumosorosea; CCAD, C. cicadae; CCM, C. militaris
Divergent structure and function of mating-type loci
Fungal sexual lifecycle is controlled by the MAT loci that are also called as the mini sex-chromosomes [11, 28]. Except for the presence of two opposite MAT loci in the haploid genome of O. sinensis (i.e., being homothallically sexual), a single MAT locus is observed in the haploid genomes of most Cordyceps species, i.e., being sexually heterothallic [11, 12]. To determine the potential mechanism of asexual fruiting in C. cicadae, the structure of MAT locus was examined. We identified a MAT1-2 type in the sequenced strain of C. cicadae (Fig. 3a). However, an additional gene (CCAD_01633, encoding a protein of 226 aa) was also identified in the MAT1-2 locus that shows similarities to the MAT1-1-1 (CCM_06523, 456 aa; 52% identity) of C. militaris and MAT1-1-1 (BBA_07733, 456 aa; 51% identity) of B. bassiana. Further analysis indicated the lack of the conserved α-domain of HMG (high mobility group) box in CCAD_01633 (Fig. 3b). Thus, a truncated type of MAT1-1-1 is present in the MAT1-2 locus of C. cicadae.
Fig. 3.
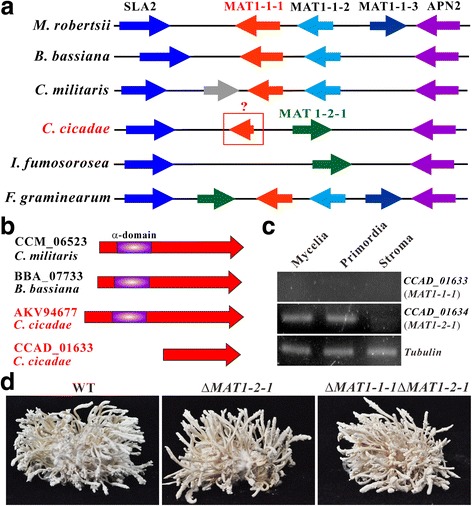
Structure, gene expression and loss-of-function analyses of the mating-type loci. a Structure comparison of the idiomorphic region between different fungi. In contrast to the structures of heterothallic or homothallic fungi, the MAT1-2 type of C. cicadae contains a truncated MAT1-1-1 gene. b Structure comparison of the MAT1-1-1 protein between different fungi. The truncated MAT1-1-1 encoded in the MAT1-2 type of C. cicadae has lost the N-terminus α-box domain. The MAT1-1-1 (AKV94677) from a MAT1-1 type of C. cicadae has a structure similar to those of C. militaris and B. bassiana. c RT-PCR analysis of MAT genes expressed by C. cicadae at different developmental stages. The mycelia harvested from the SDB (3 dpi), and primordia (13 dpi) and stroma (23 dpi) produced on silkworm pupae were used for RNA extraction and gene expression analysis. d Fruiting body production by the WT and mutant strains. Conidia of the WT and mutant strains were injected in the tussah silkworm pupae for 25 days
To examine the function of MAT1-2-1 and truncated MAT1-1-1 in controlling asexual fruiting in C. cicadae, we first examined the gene expression profiles during fungal developments. Consistent with the transcription profile of MAT1-2-1 in C. militaris [15], MAT1-2-1 of C. cicadae was transcribed during fungal growth in a liquid culture and early fruiting-body development (i.e., the primordium formation stage) but down-regulated during the fruiting-body maturation stage (i.e., stromata formation stage). Nevertheless, the truncated MAT1-1-1-like gene was not expressed by the fungus under the examined growth conditions (Fig. 3c). To further determine the MAT genes’ effect on fungal fruiting, single and joint gene deletions were performed by homologous replacement. We found that deletion of either single MAT1-2-1 or both MAT1-2-1/MAT1-1-1 had no effect on the fruiting-body formation of C. cicadae on caterpillar pupae (Fig. 3d). Thus, asexual fruiting of C. cicadae is not regulated by the MAT locus.
Conservation and divergence of the gene clusters involved in secondary metabolisms
With the obtained genome information of C. cicadae, we identified the gene clusters putatively involved in secondary metabolisms, including the 34 core enzymes of non-ribosomal peptide synthetase (NRPS), polyketide synthase (PKS), NRPS-PKS hybrid, and terpene synthase. We found that while C. cicadae encodes more gene clusters than C. militaris (29) it encodes fewer gene clusters than I. fumosorosea (36) and other selected fungi (Fig. 4a). For example, similar to C. militaris, fewer NRPS clusters (8 in total) are encoded in C. cicadae than those (average 14) in other fungi. Conservation analysis of these gene clusters revealed the presence of 18 clusters being conserved in four closely-related fungal species while six clusters are species-specific to C. cicadae (Fig. 4b). Consistent with their conserved genome structures (Fig. 2), C. cicadae shares more conserved gene clusters with I. fumosorosea than with other fungi. RNA-seq analysis indicated that these core enzyme genes were differentially transcribed by the fungus under different growth conditions or developmental stages. Overall, relative to the growth in Sabouraud dextrose broth (SDB), more genes were up-regulated when the fungus was grown on the silkworm pupae, especially during the maturation of fruiting bodies (Fig. 4c).
Fig. 4.
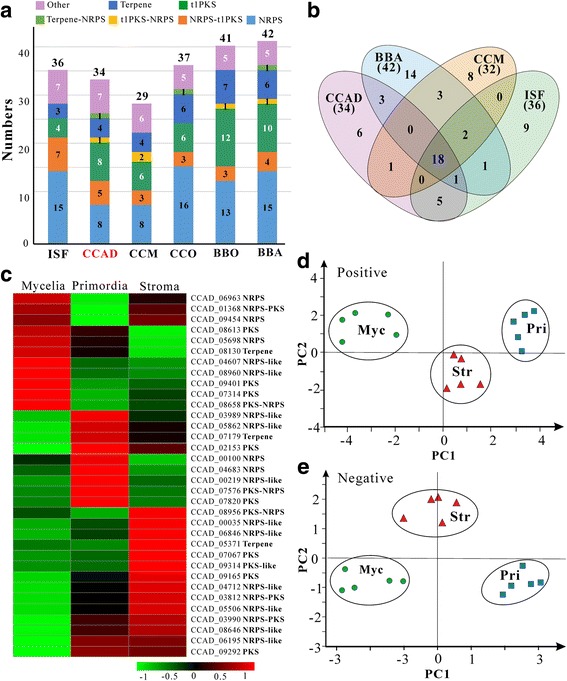
Fungal secondary metabolisms. a Comparison of the core genes involved in secondary metabolisms between C. cicadae (CCAD) and other closely-related fungi. ISF, I. fumosorosea; CCM, C. militaris; CCO, C. confragosa (anamorph: Lecanicillium lecanii); BBO, B. brongniartii; BBA, C. bassiana. b Venn diagram analysis of the secondary metabolic gene clusters between C. cicadae and other fungi. c Differential expression of the core secondary metabolic genes in C. cicadae at different developmental stages. PCA plotting based on metabolomic data obtained from the positive (d) and negative ion mode (e) of LC-MS analysis. There were five independent repeats for each sample. Myc, mycelia grown in SDB for seven days; Pri, primordia harvested from the mycosed tussah silkworm pupae 13 days post injection; Str, stroma harvested from the mycosed silkworm pupae 22 days post injection
Considering that C. cicadae is being consumed as a medicinal/healthy fungus, we performed phylogenetic and enzyme modulation analysis of PKSs and NRPSs with the counterparts that produce human toxicogenic/carcinogenic toxins in other fungi. Phylogeny analysis based on the ketoacyl synthase domain of PKSs indicated the groupings of some PKSs encoded by C. cicadae with those involved in human mycotoxin-producing enzymes. However, comparative modulation analysis revealed the divergent nature of the corresponding PKSs (Additional file 1: Fig. S3). For example, CCAD_09292 clusters with the fumonisin-producing PKS but the structures of these two enzymes are different from each other. Similar analysis was performed for the NRPSs of C. cicadae by using the retrieved adenylation domain sequences. The results confirmed the absence of HC-toxin, gliotoxin, and enniatin-producing gene clusters in the genome of C. cicadae (Additional file 1: Fig. S4). On the other hand, we found that a PKS gene cluster of C. cicadae is highly conserved with the cluster involved in the biosynthesis of bibenzoquonine oosporein in B. bassiana [29] (Fig. 5a). Based on our previous protocols [29], we performed oosporein extraction and high-performance liquid chromatography (HPLC) analysis of C. cicadae for verification. The results confirmed that, similar to B. bassiana, C. cicadae could produce oosporein in both the liquid culture and fruiting bodies (Fig. 5b). Despite the close relationship between C. cicadae and I. fumosorosea (Additional file 1: Fig. S2), the latter does not have an oosporein-biosynthetic gene cluster. A conserved NRPS cluster is also highly conserved in C. cicadae, I. fumosorosea, B. bassiana, and the plant pathogen Fusarium oxysporum (Fig. 5c). This NRPS cluster of B. bassiana is responsible for the production of the insecticidal cyclopeptide beauvericins [30]. HPLC analysis also verified that, similar to B. bassiana, both C. cicadae and I. fumosorosea could produce beauvericin (Fig. 5d). Thus, both the conserved and divergent features of secondary metabolic gene clusters are observed between C. cicadae and other fungi.
Fig. 5.
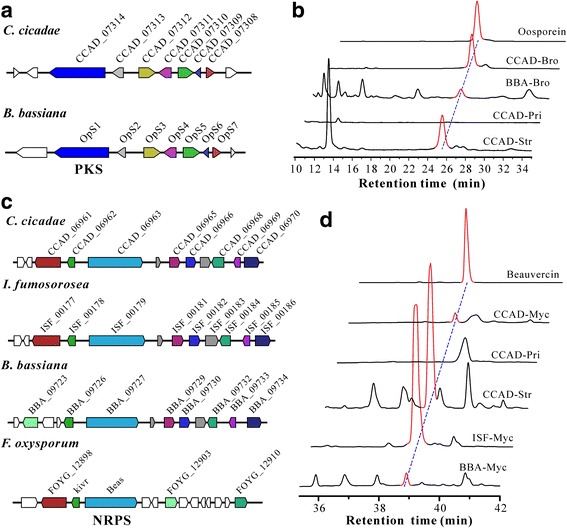
Conservation and metabolite production analysis of the gene clusters involved in biosynthesis of oosporein and beauvericin in C. cicadae. a Schematic map of the oosporein biosynthetic gene cluster in C. cicadae and B. bassiana. b HPLC verification of oosporein (red peak) production in C. cicadae and B. bassiana. c Schematic map of the beauvericin biosynthetic gene cluster in different fungi. d HPLC analysis of beauvericin (red peak) production in different fungi. CCAD, C. cicadae; BBA, B. bassiana; ISF, I. fumosorosea. Myc, mycelia harvested from SDB broth; Pri, primordia; Str, stroma
Metabolomic profiles
To corroborate the differential expression of secondary metabolic genes (Fig. 4c), high throughput LC-mass spectrometry (MS) metabolomic analysis was conducted by using five independent extracts isolated from liquid mycelia, primordia and stroma formed on insect pupae. Interestingly, principal component analysis based on the LC-MS data obtained from both the positive and negative ion modes could well separate the samples into three groups in association with fungal growth conditions or developmental stages (Fig. 4d). The results indicate that fungal metabolisms (including secondary metabolisms) are tightly linked with fungal developments. Consistent with the above analysis, both oosporein and beauvericins were detected in the metabolome data (Additional file 2). In addition, the adenosine analog, N 6-(2-hydroxyethyl)-adenosine, a Ca2+ antagonist in mammalian cells and possessing sedative activity [31], was also identified in the LC-MS analysis of C. cicadae. Relative to the growth in SDB, different species of phospholipids, amino acids (e.g., arginine, threonine, glutamine and saccharopine), and organic acids (e.g., oxalic acid, fumaric acid, gluconic acid and linoleic acid) were found to be accumulated in the primordial and stromata samples (Additional file 2; Additional file 1: Fig. S5A and S5B). The metabolites previously unreported from C. cicadae were also identified in our metabolomic analysis (Additional file 2; Additional file 1: Fig. S5C). For example, cyclodipeptide cordysinin A, first identified in the caterpillar fungus C. sinensis [32], was detected in our metabolomic data. Additional compounds first identified in non-insect pathogens were also detected, including the antibiotic fumimycin first reported in Aspergillus fumisynnematus [33], lichenicolin A reported in a lichenicolous fungus [34], alkaloid sinensine B from the basidiomycete Ganoderma sinense [35], and cycloheximide acid A from a bacterium [36].
Transcriptional profiles associated with fungal developments
We also performed high throughput RNA-seq transcriptomic analyses of C. cicadae grown in SDB or on silkworm pupae. Some stage-specifically expressed genes were observed but most genes (7851) were transcribed at several developmental stages (Additional file 1: Fig. S6A). At least, six transcriptional patterns associated with fungal development were evident (Additional file 1: Fig. S6B). For example, relative to the growth in artificial medium, ca. 3000 genes were up-regulated and 2500 genes were down-regulated when the fungus was grown on silkworm pupae. Otherwise, 1377 genes were specifically up-regulated, and 806 genes down-regulated in the fungus during the maturation of fruiting bodies (Additional file 1: Fig. S6B). Then, we performed gene ontology (GO) enrichment analysis of the genes that were up-regulated >2-fold with individual P value of t-test less than 0.05 between samples [37]. Different GO terms were identified with a P value <0.05 and FDR (false discovery rate) < 0.05 (Additional file 3). For example, relative to the mycelial sample obtained from SDB, the GO term “secondary metabolic process” was enriched 2.5-fold in primordia and 3.3-fold in stroma, which is consistent with the up-regulations of the core secondary metabolic genes (Fig. 4c). Consistent with the metabolomic data of oxalic acid (OS) accumulation in fruiting bodies (Additional file 1: Fig. S5A), the core genes involved in OS biosynthesis were found to be highly activated during fungal fruiting. For example, relative to the growth in SDB, the putative oxalate decarboxylase (CCAD_04067) [38] was up-regulated 7- and 6-fold in primordia and stroma, respectively. In addition, we found that the putative oxaloacetate acetylhydrolase (CCAD_00875) was up-regulated 137-fold in primordia and 147-fold in stroma. Considering that OS is a risk factor for the formation of calcium-oxalate kidney stones [39], OS quantification analysis was performed. Consistently, relative to the SDB mycelial samples (1.66 ± 1.04 mg/g), OS content was significantly (P = 0.01) increased in the fruiting-bodies (5.89 ± 0.33 mg/g) of C. cicadae.
We established that conidial spores instead of ascospores are produced by the asexual fruiting bodies of C. cicadae (Fig. 1A). The expression of the genes putatively involved in mating and meiosis was examined, and the data revealed that most of these genes were not up-regulated in C. cicadae during asexual fruiting (Additional file 4). Consistent with the reverse transcription PCR (RT-PCR) analysis (Fig. 3C), the truncated MAT1-1-1 was not transcribed in the fungus whereas MAT1-2-1 was not up-regulated during fungal fruiting. We also found that a putative alpha-factor pheromone gene (CCAD_03382), which is similar to the homolog CPP1 (50% identity) identified in C. militaris [15], was not transcribed in C. cicadae. A yeast IME1-like positive regulator of meiosis (CCAD_07081, 41% identity) [40] was expressed at similar levels by the fungus during different growth stages. The protein CCAD_04741, which is similar to the yeast SPO11 (22% identity) required for the formation of double-strand breaks and the initiation of meiotic recombination [41], was not transcribed by the fungus during the maturation of stroma. On the other hand, relative to the growth in SDB, most genes involved in conidiation were up-regulated during fungal fruiting. For example, the Aspergillus-like conidiophore development regulator AbaA (AN0422 vs. CCAD_06931, 40% identity) and the conidium wall factor hydrophobin RodA (AN8803 vs. CCAD_03400, 38% identity) were up-regulated 15- and 736-fold in stroma, respectively (Additional file 4). Overall, the transcriptome data support the non-mating and non-meiosis but conidiation processes of C. cicadae asexual fruiting.
Discussion
Like other organisms, fungi reproduce sexually or asexually to transmit genes to the next generations [42]. We previously found that the haploid isolate of C. militaris could produce fruiting bodies in the laboratory with few conidia formed on the mature stroma [43], and this capacity could be lost during successive maintenance of the cultures [44]. In this study, we established that the fruiting bodies of C. cicadae are asexually formed in the field or could be induced in the laboratory. Unlike the same sex mating or mating-type switch in yeasts [42], the sexual structures such as perithecia, asci, and ascospores are not produced on the fruiting bodies of C. cicadae but the production of conidial spores. These structures are somehow similar to the synnemata produced by C. bassiana [3] or I. japonica [45]. Asexual fruiting (also called as monokaryotic fruiting) has also been observed in the basidiomycete mushrooms such as Schizophylloum commune [46] and Agrocybe aegerita [47]. However, in contrast to C. cicadae, the sexual structure basidia could still be formed but beard only two instead of four basidiospores during the asexual cycle of A. aegerita. The evolution and developmental control of asexual fruiting in C. cicadae remain elusive. It cannot be precluded that sexual reproduction of C. cicadae does not occur at all in the fields. The nymphs and pupae of cicadae are soil dwelling. Thus, similar to the “summit disease syndrome” of insects killed by some fungal species [48], the production of conidia on the protruded fruiting bodies of C. cicadae may have been selected to benefit the dispersal of fungal spores to initiate the next infection cycle.
The teleomorph of some Isaria species has been verified to be Cordyceps spp., for example, C. takaomontana for I. tenuipes and C. memorabilis for I. farinosa [1]. Comparative analysis of the MAT loci among different fungi indicated that C. cicadae MAT1-2 locus contains a truncated type of MAT1-1-1 without the conserved α-box domain. Genome survey of the C. cicadae BA-001 strain sequenced by another group (GenBank accession: AEIW00000000) confirmed the similar presence of the truncated MAT1-1-1 gene in the MAT1-2 locus, implying that this gene is not a pseudogene. The HMG-box domain of MAT1-1-1 mediates DNA binding and functions as a transcription factor to activate the mating-type specific transcription of pheromone and pheromone receptor genes [49]. The loss of α-box domain suggests that this truncated MAT1-1-1 could be non-functional for gene activations. The deletion of α-box domain in the truncated MAT1-1-1 has also been observed in the conifer pathogens Grosmannia spp. [50] and the beetle-associated fungi Leptographium procerum and L. profanum [51], suggesting a general pattern of MAT gene truncation. In contrast to our observation (Fig. 3C), the expression of the truncated MAT1-1-1 was detected in G. clavigera during fungal vegetative growth on a solid medium [50]. The biological function(s) of the truncated MAT1-1-1 in fungal sexuality and/or developmental regulation remains to be determined.
Comparative genome structure analysis revealed a high level of non-syntenic relationships between C. cicadae and C. militaris. Since C. militaris can readily perform sexual reproduction to increase genetic recombination [11], this finding further supports that the lifecycle of C. cicadae is largely asexual and clonal in nature. An additional support to the asexual lifecycle of C. cicadae comes from gene deletion studies that the MAT locus is not required for fruiting (Fig. 3D). Moreover, our transcriptomic data indicate that most of the genes putatively involved in mating and meiosis are not up-regulated during fruiting-body formation (Additional file 4). The mechanisms of asexual fruiting in C. cicadae remain to be further investigated.
Insect bioassays confirmed that, in contrast to the caterpillar-specific fungus C. militaris, C. cicadae could infect and kill non-cicada insect hosts. Comparative genomic analysis revealed that the protein families encoded in C. cicadae have the typical features of entomopathogenic fungi, including the expansion of serine proteases and chitinases to effectively target the protein- and chitin-rich insect hosts [2, 26]. Consistent with the trajectory of protein family expansions in Metarhizium species to diverge from a specialist ancestor to generalist species [13], C. cicadae encodes more GPCRs, effector-like SSCPs, and secondary metabolic gene clusters than C. militaris that may contribute to the former for recognition, immune evasion, and infection of a wider range of insect hosts. Appressorium induction assays indicated that the specialist C. militaris did not form appressoria on the hind wings of Tenebrio and failed to kill beetle larvae (Additional file 1: Fig. S1). However, the homolog of a benzoquinone oxidoreductase (BBA_01593), characterized in B. bassiana to detoxify benzoquinone-containing defensive secretions in the tenebrionid beetles [52], is present in C. militaris (CCM_06005, 85% identity) and as well as in C. cicadae (CCAD_01949, 86% identity), I. fumosorosea (ISF_00350, 79% identity), and other insect pathogens. Thus, this enzyme may not particularly contribute to the detoxification of insect protective benzoquinone secretions. Similar to B. bassiana, C. cicadae but not C. militaris can produce beauvericins and oosporein. Both types of metabolites have non-selective insecticidal activities [29, 30], which may additionally contribute to the adaptation of C. cicadae to a wide host range.
Like other TCM herbs, the safety of consuming Cordyceps is still concerned [24]. In Asian countries, C. cicadae is one of the widely used Cordyceps species with renopretective activity [17]. However, potential adverse side effects of consuming this fungus are poorly understood. Our genome data reveal an array of secondary metabolic genes clusters encoded in C. cicadae, and comparative analysis suggests that no carcinogenic mycotoxins are likely to be produced by the fungus. However, the confirmation of oosporein production in C. cicadae indicates that this fungus could potentially cause avian gout or mortality in chickens and birds [53, 54]. Previous in vivo and in vitro assays also indicated that oosporein could cause cytotoxicity in the canine kidney and lead to tissue damages in mice in a dose-dependent manner by inducing oxidative stress [55]. In addition, our transcriptomic and metabolomic data indicated that the oxalic acid (OS) biosynthetic pathway was up-regulated during fungal fruiting, and OS was more highly accumulated in the fruiting-body samples when compared to the liquid cultures. OS has a strong metal-chelating property [56], and the frequent dietary intake of OS/oxalate may pose a risk for kidney stone disease [39]. Frequent consumption of C. cicadae fruiting bodies should therefore be limited to reduce the potential risk of calcium-oxalate kidney stone disease. Biologically, OS has been elucidated as a virulence factor of plant pathogenic fungi [38, 57], and B. bassiana against invertebrate hosts [58]. OS production could also increase fungal tolerance to toxic metals or ability to mobilize nutrients from minerals [56, 59]. Thus, OS accumulation in C. cicadae may benefit fungal colonization of insect hosts and beyond. From both the safety and biological implication points of view, the physiology and metabolism of OS in C. cicadae require further investigation.
Consistent with previous metabolomic analyses of B. bassiana [60] and Metarhizium species [61], metabolomic investigation of C. cicadae not only revealed fungal development-associated metabolic patterns but also identified the bioactive molecules that have not been reported before in C. cicadae (Additional file 1: Fig. S5C). In addition to the identification of oosporein for the first time, we also found the production of cordysinin A, fumimycin and lichenicolin A, which have not been reported before in C. cicadae. Fumimycin is a peptide deformylase inhibitor and has antibacterial activity [33]. Lichenicolin A shows antibiotic activity against gram-positive bacteria [34]. The identification of these compounds can benefit the medicinal uses of C. cicadae. On the other hand, some metabolites previously reported in C. cicadae were not detected in this study. For example, cordycecin A [20] and myriocin [62] were not identified in our metabolomic data. Consistent with a previous report [22], we also found that C. cicadae does not produce the adenosine analog cordycepin but N 6-(2-hydroxyethyl)-adenosine. Overall, future investigations are required to fully elucidate the chemical constituents of C. cicadae.
Conclusions
In conclusion, we performed comprehensive omics analyses of the medicinal fungus C. cicadae in this study. The combined data reveal the uncommon biological features and genetic nature of asexual fruiting in this fungus. Genome mining and comparative analysis of core enzymes for secondary metabolisms and metabolomic inspections support to some extent the safe consumption of C. cicadae fruiting bodies. Future efforts are still required to fully dissect the biosynthetic potentials of this fungus for pharmaceutical explorations and safety concerns as well.
Methods
Fungal strain and maintenance
The C. cicadae strain CCAD02 was isolated from the fruiting body of a mycosed cicada sample collected from Zhejiang Province, China. Cultures of C. cicadae were maintained either in SDB (Difco) or on potato dextrose agar (PDA, Difco). For fruiting body induction, conidial spore suspension (2 × 107 conidia/ml) was injected into the pupae (20 μl each) of Chinese Tussah silkworm (Antheraea pernyi) or inoculated on the artificial rice medium as described [63]. The strains of M. robertsii ARSEF 23, B. bassiana ARSEF 2860, C. militaris Cm 01 and I. fumosorosea ARSEF 2679 [64] were also used for comparative insect bioassays.
Appressorium induction and insect bioassays
Conidial spores of each species harvested from the two-week old complete medium (CM) [65] were subjected to appressorium induction on the hind wings of the mealworm beetle Tenebrio molitor for 24 h. The samples were then examined under a Nikon microscopy (Eclipse Ni-U). To determine the virulence of different entomopathogenic fungi, insect bioassays were conducted against the last instar larvae of T. molitor. Thus, 20 insects per group were sprayed with 0.8 ml of the aqueous suspension (1 × 107 conidia/ml). Each sample had three repeats and the experiments were repeated three times. Mortality was recorded every 12 h and the median lethal time (LT50) was calculated by Kaplan-Meier analysis [66].
Genome sequencing, assembly and annotation
The genome of CCAD02 strain was sequenced with the Illumina HiSeq2500 system at the National Center for Gene Research, Chinese Academy of Sciences (Shanghai, China). Three DNA libraries with the fragment sizes of 200-300 bp, 700-800 bp and 5 kb were constructed for sequencing, and the obtained data were assembled with SOAPdenovo2 (version 2.23) using a k-mer value of 51. After filling the gaps with the program GapCloser (version 1.12), the scaffolds were then assembled with the contigs to construct the draft genome. The gene structures of C. cicadae were predicted with a combination of different algorithms as we described previously [67, 68]. A mapping analysis for core eukaryotic genes was performed to assess the completeness of the genome [69]. The whole project has been deposited at GenBank under the accession no. MWMN00000000.
Phylogenomic and syntenic analysis
A set of 47 conserved and single-copy proteins used for generating robust fungal phylogeny [64, 70] was selected from C. cicadae and 25 other fungal species. The orthologous proteins were aligned with the program MUSCLE (ver. 3.8.31) [71], and the concatenated amino acid sequences were used to generate a maximum likelihood tree using the program TREE-PUZZLE [72]. For pairwise syntenic analysis of genome structures, the scaffolds of the paired genomes of C. cicadae, C. militaris and I. fumosorosea were oriented by MEGABLAST and Argo Genome Browser for dot plotting analysis [67]. Blast score ratio (BSR) analysis was conducted to compare the difference between the paired genomes [73]. The normalized pairs of BSR indices were visualized with the R program (version 3.1.3).
Protein family classifications
All predicted gene models of C. cicadae were subjected to InterProScan analysis for identifying conserved protein families. To identify potential pathogenicity genes, whole genome blast analysis was conducted against the protein sequences catalogued at the Pathogen-Host Interaction database [74] and the top hits were selected with a cut-off E value of 1e-5. The protease families were classified by Blastp analysis against the MEROPS peptidase database (E < 1e-50). G-protein coupled receptors and GH families were classified by protein blast analyses against the GPCRDB (http://gpcrdb.org/), and CAZy databases (http://www.cazy.org/), respectively. To identify fungal secondary metabolite gene clusters, the genome data was analyzed with the program AntiSMASH (version 3.0.4) [75]. For phylogenetic analysis, the adenylation domain sequences of NRPS enzymes or the ketoacyl synthase-domain sequences of PKS proteins were extracted and aligned with Clustal X 2.0. The phylogenetic trees were generated with the program MEGA7 [76] with a Dayhoff model, 1000 bootstrap replications and pair-wise deletions for gaps or missing data.
Mating-type gene express assays and gene deletions
Genome analysis indicated that the sequenced strain of C. cicadae is a MAT1-2 type but containing a truncated MAT1-1-1 in the MAT locus. To determine the function of MAT genes in controlling C. cicadae fruiting, RT-PCR analysis of MAT1-2-1 (CCAD_01634) and truncated MAT1-1-1 (CCAD_01633) was performed using different primer pairs (Additional file 1: Table S5). A β-tubulin gene (CCAD_032660) was used as a reference. Null mutants of MAT1-2-1, and MAT1-1-1/MAT1-2-1 (i.e. double deletions) were generated by homologous replacement as we described previously [15]. In brief, the 5′- and 3′-flanking sequences of the target gene were amplified with the corresponding primers (Additional file 1: Table S5) using the genomic DNA as a template and the PhataTM Super Fidelity DNA Polymerase (Vazyme, Piscataway, NJ, USA). The amplified products were cloned into the corresponding enzyme-restriction sites of the binary vector pDHt-Bar (conferring resistance against ammonium glufosinate) for Agrobacterium-mediated transformation of the WT strain. Drug-resistant transformants were selected and verified by PCR analysis. Fruiting-body induction assays were conducted using the silkworm pupae as described above.
Metabolomic analysis
To determine the chemical constituents of C. cicadae grown at different developmental stages, the conidia harvested from two-week’s old PDA plates were inoculated into SDB and incubated in a rotatory shaker at 220 rpm for 7 days at 25 °C. The mycelia were harvested and washed twice with sterile water. The nascent (primordia) and mature (stroma) fruiting bodies induced on silkworm pupae were harvested. Each sample had five independent replicates. The mycelia and fruiting bodies were lyophilized at −80 °C using a freeze-dryer (Labconco Corporation), and the homogenized samples (30 mg each) were extracted with 1 ml of methanol (with the addition of 2 mg/l Fmoc-Glycine as an internal standard) by ultrasonication for 0.5 h and kept at 4 °C for 12 h. The samples were filtered through a 0.22 μm syringe filter before analysis. LC-MS analysis was performed using an Agilent 6210 TOF/Q-TOF LC-Mass Spectrometer System equipped with an electrospray ionization (ESI) source. The samples were analyzed in ESI positive and negative ion modes [77]. Aliquots of 5 μl sample were injected for analysis, and the MS data were collected between m/z 50 and 1000 Da. The raw data were subjected to MassHunter software for molecular feature extraction and then processed with the program MetaboAnalyst (ver. 3.0) for normalization [78]. PCA analysis was performed to determine the optimal separation of samples. Heatmap analysis of the identified compounds was performed using the program MultiExperiment Viewer (ver. 4.9.0).
HPLC verification of oosporein and beauvericin productions
To verify the productions of oosporein and beauvericin, C. cicadae and B. bassiana and or I. fumosorosea were used for parallel incubations, metabolite extractions and HPLC analysis. To determine oosporein production, the spores of C. cicadae (strain CCAD02) and B. bassiana (strain ARSEF 2860) were inoculated in SDB and incubated in a rotatory shaker at 220 rpm for 7 days at 25 °C. The mycelia were harvested for oosporein extraction and HPLC analysis using a LC-20 AD HPLC system (Shimadzu Scientific Instruments) equipped with a Athena C18 reverse phase column (4.6 × 250 mm, 5 μm) as we previously described [29]. For beauvericin detection, the cultures of C. cicadae, B. bassiana and I. fumosorosea were extracted with methanol and analyzed with the same HPLC system. The mobile phase consisted of phase A (water) and phase B (acetonitrile). The gradient program was 0-40 min, 20-100% B; 40-55 min, 100% B; 55-56 min, 100%-20% B; 56-60 min 20% B. The flow rate was set at 1 ml/min and the elution was monitored at 210 nm [30].
RNA-seq transcriptome analysis
To corroborate the metabolomic analysis and determine fungal development-associated transcriptional profiles, high throughput RNA-seq transcriptomic analysis was performed. Thus, the samples obtained in parallel for metabolomic analysis, i.e., the SDB mycelia, primordia and stroma harvested from insect pupae, were used for RNA extractions with the QIAGEN RNeasy Plant Mini Kit. Three independent replicates were prepared for each sample. cDNA libraries were constructed and sequenced with the Illumina HiSeq2500 system. The reads with only one copy detected or those could be mapped to different transcripts were excluded in further analysis. Other tags were mapped to the genome or annotated genes if they possessed no more than one nucleotide mismatch. The read data have been deposited at NCBI with an accession no. SRP100593. The level of gene transcription was converted to fragment per kb of exon model per million mapped reads (FPKM) for expressional comparison between samples. Differentially expressed genes with more than 2-fold change and a cut-off P value of less than 0.05 were subjected to GO enrichment analysis with the program DAVID (ver. 6.7) [37]. The GO terms were considered to be significantly enriched with the cutoff statistics of P < 0.05 and FDR < 0.05.
Quantification of oxalic acid
The content of oxalic acid was quantitatively determined by displacement reaction with tribromoarsenazo and zirconium adjusted from the previous protocol [79]. In brief, 30 mg (dry weight) of the mycelial and fruiting body samples obtained above were homogenized and extracted with 2 ml of water by ultrasonication at 40 kHz for 30 min. The supernatants were collected by centrifugation and the pigments were removed using activated carbon. The supernatants were then individually added with 0.5 ml of 5 mM tribromoarsenazo, 0.55 ml of 0.5 g/l zirconium and 1 M HCl to a total reaction volume of 4 ml for 30 min. The optical density of each repeat was measured at 650 nm. The content of oxalic acid in each sample was determined by reference to the standard curve generated with the anhydrous oxalic acid (99%, Aladdin).
Additional files
Contains supplemental results and figure legends to the supplemental Figs. S1 to S6 as well as the supplemental Tables S1. to Table S5. Figure S1, Spore differential induction and insect bioassays. Figure S2, Phylogenetic relationship of C. cicadae. Figure S3. and S4. Phylogenetic and modular analysis of PKSs (Fig. S3) and NRPSs (Fig. S4) encoded in C. cicadae in comparison with those involved in the production of human mycotoxins. Figure S5, Metabolomic analysis of C. cicadae. Figure S6, Transcriptomic profiling of C. cicadae at different developmental stages. Table S1, Comparative analysis of putative protease genes between C. cicadae and other insect pathogens. Table S2, Comparative analysis of carbohydrate-degrading enzymes between C. cicadae and other insect pathogens. Table S3, Comparative analysis of putative lipase genes between C. cicadae and other insect pathogens. Table S4, Comparative analysis of putative bacterial-like protein toxins between C. cicadae and other insect pathogens. Table S5, Primers used in this study. (DOCX 2852 kb)
Relative quantification of the metabolites produced by C. cicadae at different developmental stages. (XLSX 31 kb)
GO analysis of the genes up-regulated between different samples of C. cicadae. (XLSX 51 kb)
Expression of putative mating and meiosis process-related genes by C. cicadae at different growth stages. (XLSX 13 kb)
Acknowledgements
We thank Qi Feng and Qiang Zhao at the National Center for Gene Research, Chinese Academy of Sciences for their helps for de novo genome sequencing and RNA-seq analyses.
Funding
This work was supported by the National Natural Science Foundation of China (31530001) and the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB11030100).
Availability of data and materials
The genome assembly of Cordyceps cicadae CCAD02 has been deposited at NCBI with an accession no. MWMN00000000. RNA-seq data have been deposited at NCBI with an accession no. SRP100593.
Abbreviations
- GH
Glycoside hydrolase
- GPCR
G-protein coupled receptor
- HPLC
High-performance liquid chromatography
- MAT
Mating type
- NRPS
Non-ribosomal peptide synthetase
- OS
Oxalic acid
- PKS
Polyketide synthase
- SSCPs
Small secreted cysteine-rich proteins
- TCMs
Traditional Chinese medicines
Authors’ contributions
Conceived and designed the experiments: CW. Performed the experiments: YL, FL. Analyzed the data: CW, YL, KC, SZ, FL, GX, YY. Contributed reagents/materials/analysis tools: ZL, CL, HZ. Manuscript preparation: CW, YL. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Field permission is not required for the obtaining of fungal samples in this study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1186/s12864-017-4060-4) contains supplementary material, which is available to authorized users.
Contributor Information
Huizhan Zhang, Email: huizhzh@ecust.edu.cn.
Chengshu Wang, Phone: 86-21-5492 4157, Email: cswang@sibs.ac.cn.
References
- 1.Sung GH, Hywel-Jones NL, Sung JM, Luangsa-Ard JJ, Shrestha B, Spatafora JW. Phylogenetic classification of Cordyceps and the clavicipitaceous fungi. Stud Mycol. 2007;57(57):5–59. doi: 10.3114/sim.2007.57.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang CS, Wang SB. Insect pathogenic fungi: genomics, molecular interactions, and genetic improvements. Annu Rev Entomol. 2017;62:73–90. doi: 10.1146/annurev-ento-031616-035509. [DOI] [PubMed] [Google Scholar]
- 3.Lee JO, Shrestha B, Kim TW, Sung GH, Sung JM. Stable formation of fruiting body in Cordyceps bassiana. Mycobiology. 2007;35(4):230–234. doi: 10.4489/MYCO.2007.35.4.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shang Y, Feng P, Wang C. Fungi that infect insects: altering host behavior and beyond. PLoS Pathog. 2015;11(8):e1005037. doi: 10.1371/journal.ppat.1005037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hughes DP, Andersen SB, Hywel-Jones NL, Himaman W, Billen J, Boomsma JJ. Behavioral mechanisms and morphological symptoms of zombie ants dying from fungal infection. BMC Ecol. 2011;11:13. doi: 10.1186/1472-6785-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ortiz-Urquiza A, Luo ZB, Keyhani NO. Improving mycoinsecticides for insect biological control. Appl Microbiol Biotechnol. 2015;99(3):1057–1068. doi: 10.1007/s00253-014-6270-x. [DOI] [PubMed] [Google Scholar]
- 7.Wang CS, Feng MG. Advances in fundamental and applied studies in China of fungal biocontrol agents for use against arthropod pests. Biol Control. 2014;68:129–135. doi: 10.1016/j.biocontrol.2013.06.017. [DOI] [Google Scholar]
- 8.Yue K, Ye M, Zhou Z, Sun W, Lin X. The genus Cordyceps: a chemical and pharmacological review. J Pharm Pharmacol. 2013;65(4):474–493. doi: 10.1111/j.2042-7158.2012.01601.x. [DOI] [PubMed] [Google Scholar]
- 9.Paterson RR. Cordyceps: a traditional Chinese medicine and another fungal therapeutic biofactory? Phytochemistry. 2008;69(7):1469–1495. doi: 10.1016/j.phytochem.2008.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luangsa-ard JJ, Hywel-Jones NL, Manoch L, Samson RA. On the relationships of Paecilomyces sect. Isarioidea species Mycol Res. 2005;109(Pt 5):581–589. doi: 10.1017/S0953756205002741. [DOI] [PubMed] [Google Scholar]
- 11.Zheng P, Xia YL, Zhang SW, Wang CS. Genetics of Cordyceps and related fungi. Appl Microbiol Biotechnol. 2013;97(7):2797–2804. doi: 10.1007/s00253-013-4771-7. [DOI] [PubMed] [Google Scholar]
- 12.Hu X, Zhang YJ, Xiao GH, Zheng P, Xia YL, Zhang XY, St Leger RJ, Liu XZ, Wang CS. Genome survey uncovers the secrets of sex and lifestyle in caterpillar fungus. Chin Sci Bull. 2013;58(23):2846–2854. doi: 10.1007/s11434-013-5929-5. [DOI] [Google Scholar]
- 13.Hu X, Xiao G, Zheng P, Shang Y, Su Y, Zhang X, Liu X, Zhan S, St Leger RJ, Wang C. Trajectory and genomic determinants of fungal-pathogen speciation and host adaptation. Proc Natl Acad Sci U S A. 2014;111(47):16796–16801. doi: 10.1073/pnas.1412662111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valero-Jimenez CA, Faino L, Spring In't, Veld D, Smit S, Zwaan BJ, van Kan JA. Comparative genomics of Beauveria bassiana: uncovering signatures of virulence against mosquitoes. BMC Genomics 2016;17(1):986. [DOI] [PMC free article] [PubMed]
- 15.Lu Y, Xia Y, Luo F, Dong C, Wang C. Functional convergence and divergence of mating-type genes fulfilling in Cordyceps militaris. Fungal Genet Biol. 2016;88:35–43. doi: 10.1016/j.fgb.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 16.Zheng Q, Hou R, Juanyu Z, Ma J, Wu Z, Wang G, Wang C, Xu JR. The MAT locus genes play different roles in sexual reproduction and pathogenesis in Fusarium graminearum. PLoS One. 2013;8(6):e66980. doi: 10.1371/journal.pone.0066980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu R, Chen YP, Deng YY, Zheng R, Zhong YF, Wang L, Du LP. Cordyceps cicadae extracts ameliorate renal malfunction in a remnant kidney model. J Zhejiang Univ Sci B. 2011;12(12):1024–1033. doi: 10.1631/jzus.B1100034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu R, Zheng R, Deng Y, Chen Y, Zhang S. Ergosterol peroxide from Cordyceps cicadae ameliorates TGF-β1-induced activation of kidney fibroblasts. Phytomedicine. 2014;21(3):372–378. doi: 10.1016/j.phymed.2013.08.022. [DOI] [PubMed] [Google Scholar]
- 19.Chu ZB, Chang J, Zhu Y, Sun X. Chemical constituents of Cordyceps cicadae. Nat Prod Commun. 2015;10(12):2145–2146. [PubMed] [Google Scholar]
- 20.Wang J, Zhang DM, Jia JF, Peng QL, Tian HY, Wang L, Ye WC. Cyclodepsipeptides from the ascocarps and insect-body portions of fungus Cordyceps cicadae. Fitoterapia. 2014;97:23–27. doi: 10.1016/j.fitote.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Guo Y, Zhang L, Wu J. Characterizations of a new Cordyceps cicadae isolate and production of adenosine and cordycepin. Braz J Microbiol. 2012;43(2):449–455. doi: 10.1590/S1517-83822012000200004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeng W-B, Yu H, Ge F, Yang J-Y, Chen Z-H, Wang Y-B, Dai Y-D, Adams A. Distribution of nucleosides in populations of Cordyceps cicadae. Molecules. 2014;19(5):6123–6141. doi: 10.3390/molecules19056123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strader CR, Pearce CJ, Oberlies NH. Fingolimod (FTY720): a recently approved multiple sclerosis drug based on a fungal secondary metabolite. J Nat Prod. 2011;74(4):900–907. doi: 10.1021/np2000528. [DOI] [PubMed] [Google Scholar]
- 24.Sum SS, Ziegler J. Lingzhi and Cordyceps: two commonly used Chinese medicinal herbs, safe or not? Nutr Clin Pract. 2016;31(5):695–697. doi: 10.1177/0884533616662990. [DOI] [PubMed] [Google Scholar]
- 25.Dong C, Guo S, Wang W, Liu X. Cordyceps industry in China. Mycology. 2015;6(2):121–129. doi: 10.1080/21501203.2015.1043967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang JB, St Leger RJ, Wang CS. Advances in genomics of entomopathogenic fungi. Adv Genet. 2016;94:67–105. doi: 10.1016/bs.adgen.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 27.Sung GH, Poinar GO, Jr, Spatafora JW. The oldest fossil evidence of animal parasitism by fungi supports a cretaceous diversification of fungal-arthropod symbioses. Mol Phylogenet Evol. 2008;49(2):495–502. doi: 10.1016/j.ympev.2008.08.028. [DOI] [PubMed] [Google Scholar]
- 28.Ni M, Feretzaki M, Sun S, Wang XY, Heitman J. Sex in Fungi. Annual Review Genetics, Vol 45 2011;45:405-30. [DOI] [PMC free article] [PubMed]
- 29.Feng P, Shang Y, Cen K, Wang C. Fungal biosynthesis of the bibenzoquinone oosporein to evade insect immunity. Proc Natl Acad Sci U S A. 2015;112(36):11365–11370. doi: 10.1073/pnas.1503200112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu Y, Orozco R, Wijeratne EM, Gunatilaka AA, Stock SP, Molnar I. Biosynthesis of the cyclooligomer depsipeptide beauvericin, a virulence factor of the entomopathogenic fungus Beauveria bassiana. Chem Biol. 2008;15(9):898–907. doi: 10.1016/j.chembiol.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 31.Lu MY, Chen CC, Lee LY, Lin TW, Kuo CF. N6-(2-hydroxyethyl) adenosine in the medicinal mushroom Cordyceps cicadae attenuates lipopolysaccharide-stimulated pro-inflammatory responses by suppressing TLR4-mediated NF-kappaB signaling pathways. J Nat Prod. 2015;78(10):2452–2460. doi: 10.1021/acs.jnatprod.5b00573. [DOI] [PubMed] [Google Scholar]
- 32.Yang ML, Kuo PC, Hwang TL, Wu TS. Anti-inflammatory principles from Cordyceps sinensis. J Nat Prod. 2011;74(9):1996–2000. doi: 10.1021/np100902f. [DOI] [PubMed] [Google Scholar]
- 33.Kwon YJ, Sohn MJ, Zheng CJ, Kim WG. Fumimycin: a peptide deformylase inhibitor with an unusual skeleton produced by Aspergillus fumisynnematus. Org Lett. 2007;9(13):2449–2451. doi: 10.1021/ol0703231. [DOI] [PubMed] [Google Scholar]
- 34.He H, Bigelis R, Yang HY, Chang LP, Singh MP. Lichenicolins a and B, new bisnaphthopyrones from an unidentified lichenicolous fungus, strain LL-RB0668. J Antibiot (Tokyo) 2005;58(11):731–736. doi: 10.1038/ja.2005.99. [DOI] [PubMed] [Google Scholar]
- 35.Liu J-Q, Wang C-F, Peng X-R, Qiu M-H. New alkaloids from the fruiting bodies of Ganoderma sinense. Nat Prod Bioprospect. 2011;1(2):93–96. doi: 10.1007/s13659-011-0026-4. [DOI] [Google Scholar]
- 36.Xu XL, Yin LY, Wang SY, Liu HH, Gao JH, Zhao SJ. Cycloheximide acid a, a new cycloheximide derivative from marine derived Streptomyces sp from east China sea. Rec Nat Prod. 2013;7(4):292–295. [Google Scholar]
- 37.Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 38.Williams B, Kabbage M, Kim HJ, Britt R, Dickman MB. Tipping the balance: Sclerotinia sclerotiorum secreted oxalic acid suppresses host defenses by manipulating the host redox environment. PLoS Pathog. 2011;7(6):e1002107. doi: 10.1371/journal.ppat.1002107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robertson WG. Dietary recommendations and treatment of patients with recurrent idiopathic calcium stone disease. Urolithiasis. 2016;44(1):9–26. doi: 10.1007/s00240-015-0849-2. [DOI] [PubMed] [Google Scholar]
- 40.Kassir Y, Granot D, Simchen G. IME1, a positive regulator gene of meiosis in S. cerevisiae. Cell. 1988;52(6):853–862. doi: 10.1016/0092-8674(88)90427-8. [DOI] [PubMed] [Google Scholar]
- 41.Martini E, Keeney S. Sex and the single (double-strand) break. Mol Cell. 2002;9(4):700–702. doi: 10.1016/S1097-2765(02)00512-9. [DOI] [PubMed] [Google Scholar]
- 42.Lee SC, Ni M, Li W, Shertz C, Heitman J. The evolution of sex: a perspective from the fungal kingdom. Microbiol Mol Biol Rev. 2010;74(2):298–340. doi: 10.1128/MMBR.00005-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zheng P, Xia Y, Xiao G, Xiong C, Hu X, Zhang S, Zheng H, Huang Y, Zhou Y, Wang S, et al. Genome sequence of the insect pathogenic fungus Cordyceps militaris, a valued traditional Chinese medicine. Genome Biol. 2011;12(11):R116. doi: 10.1186/gb-2011-12-11-r116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiong CH, Xia YL, Zheng P, Wang CS. Increasing oxidative stress tolerance and subculturing stability of Cordyceps militaris by overexpression of a glutathione peroxidase gene. Appl Microbiol Biotechnol. 2013;97(5):2009–2015. doi: 10.1007/s00253-012-4286-7. [DOI] [PubMed] [Google Scholar]
- 45.Yamanaka K, Inatomi S. XV. Cultivation of Isaria japonica fruit bodies on mixed plant/insect media. Food Rev Int. 1997;13(3):455–460. doi: 10.1080/87559129709541132. [DOI] [Google Scholar]
- 46.Esser K, Saleh F, Meinhardt F. Genetics of fruit body production in higher basidiomycetes II. Monokaryotic and dikaryotic fruiting in Schizophyllum commune. Curr Genet. 1979;1(1):85–88. doi: 10.1007/BF00413309. [DOI] [PubMed] [Google Scholar]
- 47.Esser K, Meinhardt F. A common genetic control of dikaryotic and monokaryotic fruiting in the basidiomycete Agrocybe aegerita. Mol Gen Genet MGG. 1977;155(1):113–115. doi: 10.1007/BF00268568. [DOI] [Google Scholar]
- 48.Roy HE, Steinkraus DC, Eilenberg J, Hajek AE, Pell JK. Bizarre interactions and endgames: Entomopathogenic fungi and their arthropod hosts. Annu Rev Entomol. 2006;51:331–357. doi: 10.1146/annurev.ento.51.110104.150941. [DOI] [PubMed] [Google Scholar]
- 49.Martin T, Lu SW, van Tilbeurgh H, Ripoll DR, Dixelius C, Turgeon BG, Debuchy R. Tracing the origin of the fungal alpha1 domain places its ancestor in the HMG-box superfamily: implication for fungal mating-type evolution. PLoS One. 2010;5(12):e15199. doi: 10.1371/journal.pone.0015199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsui CK, DiGuistini S, Wang Y, Feau N, Dhillon B, Bohlmann J, Hamelin RC. Unequal recombination and evolution of the mating-type (MAT) loci in the pathogenic fungus Grosmannia clavigera and relatives. G3. 2013;3(3):465–480. doi: 10.1534/g3.112.004986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Duong TA, de Beer ZW, Wingfield BD, Wingfield MJ. Characterization of the mating-type genes in Leptographium procerum and Leptographium profanum. Fungal Biol. 2013;117(6):411–421. doi: 10.1016/j.funbio.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 52.Pedrini N, Ortiz-Urquiza A, Huarte-Bonnet C, Fan Y, Juarez MP, Keyhani NO. Tenebrionid secretions and a fungal benzoquinone oxidoreductase form competing components of an arms race between a host and pathogen. Proc Natl Acad Sci U S A. 2015;112(28):E3651–E3660. doi: 10.1073/pnas.1504552112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pegram RA, Wyatt RD. Avian gout caused by oosporein, a mycotoxin produced by Caetomium trilaterale. Poult Sci. 1981;60(11):2429–2440. doi: 10.3382/ps.0602429. [DOI] [PubMed] [Google Scholar]
- 54.Manning RO, Wyatt RD. Comparative toxicity of Chaetomium contaminated corn and various chemical forms of oosporein in broiler chicks. Poult Sci. 1984;63(2):251–259. doi: 10.3382/ps.0630251. [DOI] [PubMed] [Google Scholar]
- 55.Ramesha A, Venkataramana M, Nirmaladevi D, Gupta VK, Chandranayaka S, Srinivas C. Cytotoxic effects of oosporein isolated from endophytic fungus Cochliobolus kusanoi. Front Microbiol. 2015;6:870. doi: 10.3389/fmicb.2015.00870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fomina M, Hillier S, Charnock JM, Melville K, Alexander IJ, Gadd GM. Role of oxalic acid overexcretion in transformations of toxic metal minerals by Beauveria caledonica. Appl Environ Microbiol. 2005;71(1):371–381. doi: 10.1128/AEM.71.1.371-381.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cessna SG, Sears VE, Dickman MB, Low PS. Oxalic acid, a pathogenicity factor for Sclerotinia sclerotiorum, suppresses the oxidative burst of the host plant. Plant Cell. 2000;12(11):2191–2200. doi: 10.1105/tpc.12.11.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kirkland BH, Eisa A, Keyhani NO. Oxalic acid as a fungal acaracidal virulence factor. J Med Entomol. 2005;42(3):346–351. doi: 10.1093/jmedent/42.3.346. [DOI] [PubMed] [Google Scholar]
- 59.Landeweert R, Hoffland E, Finlay RD, Kuyper TW, van Breemen N. Linking plants to rocks: ectomycorrhizal fungi mobilize nutrients from minerals. Trends Ecol Evol. 2001;16(5):248–254. doi: 10.1016/S0169-5347(01)02122-X. [DOI] [PubMed] [Google Scholar]
- 60.Luo F, Wang Q, Yin C, Ge Y, Hu F, Huang B, Zhou H, Bao G, Wang B, Lu R, et al. Differential metabolic responses of Beauveria bassiana cultured in pupae extracts, root exudates and its interactions with insect and plant. J Invertebr Pathol. 2015;130:154–164. doi: 10.1016/j.jip.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 61.Xu Y-J, Luo F, Li B, Shang Y, Wang C. Metabolic conservation and diversification of Metarhizium species correlate with fungal host-specificity. Front Microbiol. 2016;7:2020. doi: 10.3389/fmicb.2016.02020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fujita T, Inoue K, Yamamoto S, Ikumoto T, Sasaki S, Toyama R, Chiba K, Hoshino Y, Okumoto T. Fungal metabolites. Part 11. A potent immunosuppressive activity found in Isaria sinclairii metabolite. J Antibiot (Tokyo) 1994;47(2):208–215. doi: 10.7164/antibiotics.47.208. [DOI] [PubMed] [Google Scholar]
- 63.Xiong C, Xia Y, Zheng P, Shi S, Wang C. Developmental stage-specific gene expression profiling for a medicinal fungus Cordyceps militaris. Mycology. 2010;1(1):25–66. doi: 10.1080/21501201003674581. [DOI] [Google Scholar]
- 64.Shang Y, Xiao G, Zheng P, Cen K, Zhan S, Wang C. Divergent and convergent evolution of fungal pathogenicity. Genome Biol Evol. 2016;8(5):1374–1387. doi: 10.1093/gbe/evw082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Duan ZB, Chen YX, Huang W, Shang YF, Chen PL, Wang CS. Linkage of autophagy to fungal development, lipid storage and virulence in Metarhizium robertsii. Autophagy. 2013;9(4):538–549. doi: 10.4161/auto.23575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gao Q, Lu Y, Yao H, Xu YJ, Huang W, Wang C. Phospholipid homeostasis maintains cell polarity, development and virulence in metarhizium robertsii. Environ Microbiol. 2016;18(11):3976–3990. doi: 10.1111/1462-2920.13408. [DOI] [PubMed] [Google Scholar]
- 67.Gao Q, Jin K, Ying SH, Zhang Y, Xiao G, Shang Y, Duan Z, Hu X, Xie XQ, Zhou G, et al. Genome sequencing and comparative transcriptomics of the model entomopathogenic fungi Metarhizium anisopliae and M. acridum. PLoS Genet. 2011;7(1):e1001264. doi: 10.1371/journal.pgen.1001264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xiao G, Ying SH, Zheng P, Wang ZL, Zhang S, Xie XQ, Shang Y, St Leger RJ, Zhao GP, Wang C, et al. Genomic perspectives on the evolution of fungal entomopathogenicity in Beauveria bassiana. Sci Rep. 2012;2:483. doi: 10.1038/srep00483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Parra G, Bradnam K, Korf I. CEGMA: a pipeline to accurately annotate core genes in eukaryotic genomes. Bioinformatics. 2007;23(9):1061–1067. doi: 10.1093/bioinformatics/btm071. [DOI] [PubMed] [Google Scholar]
- 70.Floudas D, Binder M, Riley R, Barry K, Blanchette RA, Henrissat B, Martinez AT, Otillar R, Spatafora JW, Yadav JS, et al. The Paleozoic origin of enzymatic lignin decomposition reconstructed from 31 fungal genomes. Science. 2012;336(6089):1715–1719. doi: 10.1126/science.1221748. [DOI] [PubMed] [Google Scholar]
- 71.Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32(5):1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schmidt HA, Petzold E, Vingron M, von Haeseler A. Molecular phylogenetics: parallelized parameter estimation and quartet puzzling. J Parallel Distr Com. 2003;63(7–8):719–727. doi: 10.1016/S0743-7315(03)00129-1. [DOI] [Google Scholar]
- 73.Rasko DA, Myers GS, Ravel J. Visualization of comparative genomic analyses by BLAST score ratio. BMC Bioinformatics. 2005;6:2. doi: 10.1186/1471-2105-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Urban M, Cuzick A, Rutherford K, Irvine A, Pedro H, Pant R, Sadanadan V, Khamari L, Billal S, Mohanty S, et al. PHI-base: a new interface and further additions for the multi-species pathogen-host interactions database. Nucleic Acids Res. 2017;45(D1):D604–Dd10. doi: 10.1093/nar/gkw1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Weber T, Blin K, Duddela S, Krug D, Kim HU, Bruccoleri R, Lee SY, Fischbach MA, Muller R, Wohlleben W, et al. antiSMASH 3.0-a comprehensive resource for the genome mining of biosynthetic gene clusters. Nucleic Acids Res. 2015;43(W1):W237–W243. doi: 10.1093/nar/gkv437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33(7):1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xu Y-J, Luo F, Gao Q, Shang Y, Wang C. Metabolomics reveals insect metabolic responses associated with fungal infection. Anal Bioanal Chem. 2015;407(16):4815–4821. doi: 10.1007/s00216-015-8648-8. [DOI] [PubMed] [Google Scholar]
- 78.Xia JG, Sinelnikov IV, Han B, Wishart DS. MetaboAnalyst 3.0-making metabolomics more meaningful. Nucleic Acids Res. 2015;43(W1):W251–W2W7. doi: 10.1093/nar/gkv380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Savvin S. Analytical use of arsenazo III: determination of thorium, zirconium, uranium and rare earth elements. Talanta. 1961;8(9):673–685. doi: 10.1016/0039-9140(61)80164-1. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Contains supplemental results and figure legends to the supplemental Figs. S1 to S6 as well as the supplemental Tables S1. to Table S5. Figure S1, Spore differential induction and insect bioassays. Figure S2, Phylogenetic relationship of C. cicadae. Figure S3. and S4. Phylogenetic and modular analysis of PKSs (Fig. S3) and NRPSs (Fig. S4) encoded in C. cicadae in comparison with those involved in the production of human mycotoxins. Figure S5, Metabolomic analysis of C. cicadae. Figure S6, Transcriptomic profiling of C. cicadae at different developmental stages. Table S1, Comparative analysis of putative protease genes between C. cicadae and other insect pathogens. Table S2, Comparative analysis of carbohydrate-degrading enzymes between C. cicadae and other insect pathogens. Table S3, Comparative analysis of putative lipase genes between C. cicadae and other insect pathogens. Table S4, Comparative analysis of putative bacterial-like protein toxins between C. cicadae and other insect pathogens. Table S5, Primers used in this study. (DOCX 2852 kb)
Relative quantification of the metabolites produced by C. cicadae at different developmental stages. (XLSX 31 kb)
GO analysis of the genes up-regulated between different samples of C. cicadae. (XLSX 51 kb)
Expression of putative mating and meiosis process-related genes by C. cicadae at different growth stages. (XLSX 13 kb)
Data Availability Statement
The genome assembly of Cordyceps cicadae CCAD02 has been deposited at NCBI with an accession no. MWMN00000000. RNA-seq data have been deposited at NCBI with an accession no. SRP100593.


