Highlights
-
•
The host defence peptide LL-37 can reduce Human Rhinovirus 1B (HRV1B) replication in airway epithelial cells.
-
•
The antiviral activity of LL-37 is sequence specific.
-
•
LL-37 reduces the metabolic activity of cells infected with HRV1B without inducing substantial apoptotic or necrotic cell death.
-
•
The antiviral activity of cathelicidin peptides towards HRV1B is conserved in peptides from other mammalian species including pig and sheep.
Keywords: Cathelicidin, Rhinovirus, LL-37, Respiratory, Infection
Abstract
Human rhinoviruses (HRVs) are the most common cause of viral respiratory tract infections, and are associated with significant morbidity and mortality in immunocompromised individuals and patients with pre-existing pulmonary conditions. The therapeutic options available are extremely limited and therefore novel therapeutics for HRV infections are of significant interest. Cathelicidins have been shown to have potent antiviral activity against a range of pathogens and are known to be key immunomodulatory mediators during infection. We therefore assessed the antiviral potential of cathelicidins from humans and other mammalian species against HRV, together with the potential for the human cathelicidin to modulate apoptotic pathways and alter cell viability during HRV infection. We demonstrate that LL-37, the porcine cathelicidin Protegrin-1, and the ovine cathelicidin SMAP-29 display potent antiviral activity towards HRV and that this activity is visible when either the virus is exposed to the peptides prior to cell infection or after cells have been infected. We further demonstrate that, in contrast to established findings with bacterial infection models, LL-37 does not induce apoptosis or necrosis in HRV-infected lung epithelial cells at physiological or superphysiological concentrations, but does reduce the metabolic activity of infected cells compared to uninfected cells treated with similar peptide concentrations. Collectively, the findings from this study demonstrate that the mechanism of action of cathelicidins against rhinovirus is by directly affecting the virus and we propose that the delivery of exogenous cathelicidins, or novel synthetic analogues, represent an exciting and novel therapeutic strategy for rhinovirus infection.
1. Introduction
Human rhinoviruses (HRVs) are the most common cause of viral respiratory tract infections and are associated with significant morbidity and mortality in young children, in the elderly, in immunocompromised individuals, and in patients with pre-existing pulmonary conditions [1]. HRVs have historically been associated with relatively benign upper respiratory tract infections (i.e. the common cold). However, studies have recently provided clear evidence of the major role that rhinoviruses can play in triggering exacerbations of asthma and chronic obstructive pulmonary disease [2], [3], in causing severe bronchiolitis in infants and children [4], [5], as well as pneumonia [6]. There are no current specific effective treatments for HRV infection, and prevention by vaccination has thus far been shown to be almost impossible due to the fact that there have been more than 100 circulating serotypes of HRV identified [7]. The therapeutic options available are predominantly supportive and are only applicable for mild upper respiratory infections, however, for serious respiratory infections in immunocompromised individuals there are no efficient treatments available. The development of specific effective therapies for HRV infections is therefore an urgent requirement.
Cationic Host Defense Peptides (HDPs) constitute a major component of the innate immune system and can be found in a variety of life-forms, contributing to the first-line of defense against invading pathogens. The sole human cathelicidin, a cationic antimicrobial peptide of 18 kDa called hCAP-18, is encoded by the CAMP gene and is expressed in a variety of cell types including neutrophils, macrophages and epithelial cells. The precursor peptide hCAP-18 is cleaved in to its active form, LL-37, by extracellular proteinase-3 [8] and while concentrations of approximately 5 μg/ml have been detected in bronchoalveolar lavage (BAL) fluid in healthy infants [9], concentrations of LL-37 can rise to ∼30 μg/ml in the BAL fluid of patients with respiratory infection, or with cystic fibrosis (CF) [10]. Cathelicidins have been shown to modulate innate and adaptive immunity, predominantly mediated through their interaction with cells, such as monocytes, dendritic cells and T cells [11], [12], [13]. Cathelicidin knockout mouse-models show an increased susceptibility to bacterial infections of the skin, lung, urinary tract, and gastrointestinal tract which confirms the crucial role of cathelicidins in the immune response to bacterial infections [14], [15].
We have previously shown that LL-37 displays potent antiviral activity against the respiratory pathogen, influenza A virus (IAV) both in vitro and in vivo [16], and several other studies have revealed a role for LL-37 in defence against a variety of other viral pathogens including Respiratory Syncytial Virus (RSV), dengue virus, Human Immunodeficiency Virus-1 (HIV-1), Herpes Simplex Virus (HSV), vaccinia virus and Adenovirus [17], [18], [19], [20], [21], [22], [23], [24]. In addition, we and others have shown that cathelicidins from other mammalian sources such as murine (mCRAMP), porcine (Protegrin-1), ovine (SMAP-29) and bovine (BMAP-28, indolicidin) have direct antiviral activity against these human pathogens and other emerging pathogens, including Dengue virus [16], [20], [22], [25], [26], [27], [28] (Reviewed in detail in [29]). It has also recently been demonstrated that vitamin D-mediated upregulation of cathelicidin in human bronchial cells from CF patients resulted in reduced HRV load in vitro, and that treating cystic fibrosis bronchial epithelial cells with LL-37 (20 μg/ml) post-infection resulted in a significant reduction in viral replication, although the underlying mechanism remains unclear [30].
In addition to their direct antimicrobial and antiviral activity, cathelicidins have also been shown to have a potent capacity for altering inflammatory cytokine responses and apoptotic pathways in healthy and infected cells [31], [32], [33], [34], [35], at physiological, and inflammatory, concentrations. We have previously shown that LL-37 is capable of enhancing the apoptosis of infected airway epithelial cells, together with inducing rapid secondary necrosis in apoptotic neutrophils. Additionally, the porcine cathelicidin, Protegrin-1, has been shown to induce necrosis in U937 tumor cells, and Ca2+-dependent necrotic cell death in human K562 erythroleukemia cells [36], [37]. While the underlying mechanisms for the cathelicidin-mediated induction of necrotic cell death or apoptosis in a number of cell types remains to be fully established, it is a clear indicator of the immunomodulatory potential of cathelicidins, the role of which in viral infection remain to be understood.
Due to the potent antiviral activity of cathelicidins and their ability to modulate the immune system, along with the urgent need to develop specific effective antiviral treatments against rhinovirus, this study investigates the antiviral activity of the human cathelicidin, LL-37, together with cathelicidins from ovine (SMAP-29) and porcine (Protegrin-1) sources against HRV in in vitro models of infection. In addition, we also investigate the underlying antiviral and cell-based mechanisms of the anti-HRV activity displayed by LL-37 in order to more clearly understand the direct and indirect antiviral capacity of cathelicidins in HRV infection. We show that the human cathelicidin, LL-37, together with the porcine and ovine cathelicidins Protegrin-1 and SMAP-29, display potent antiviral activity against HRV, through a mechanism distinct from the induction of apoptosis.
2. Materials and methods
2.1. Reagents
Dulbecco’s modified Eagle’s medium (DMEM), Eagle’s minimal essential medium (E-MEM), l-glutamine, Foetal bovine serum (FBS), non-essential amino acids (NEAA), PBS, trypsin/EDTA, BSA, and Fibronectin were all purchased from Sigma-Aldrich, UK. Vectashield Hardset mounting medium with 49,6-diamidino- 2-phenylindole (DAPI) was supplied by Vector Laboratories, UK. CellTiter96 Aqueous Non-Radioactive Cell Proliferation Assay (MTS) and CytoTox 96 Non-Radioactive Cytotoxicity Assay (LDH) were supplied by Promega, UK. The Terminal Deoxynucleotidyl Transferase-Mediated Deoxyuridine Triphosphate Nick-End Labelling (TUNEL) Assay was supplied by Roche Applied Science. Reconstituted peptide masses were characterised by MALDI-TOF analysis (Proteomics Facility Moredun Research Institute, UK).
2.2. Peptide synthesis
The peptides were assembled using the Fmoc/tBu solid-phase peptide synthesis approach [38] using either model 433A (Applied Biosystems, CA, USA) or model Liberty (CEM Corporation, NC, USA) automated peptide synthesizers followed by cleavage in the trifluoroacetic acid (TFA)/phenol/thioanisole/ethanedithiol/water (10:0.75:0.5:0.25:0.5, w/w) mixture at 25 °C for 90 min followed by precipitation with cold diethyl ether. The crude peptides were purified by preparative reversed-phase high-pressure liquid chromatography (RP-HPLC). The peptide purity (>98%) was confirmed by analytical RP-HPLC, and the masses were confirmed by mass spectrometry. Following lyophilization, the purified peptides were obtained in the form of their TFA salts; namely: LL-37 (LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES), LL-37 analog having “scrambled” sequence (RSLEGTDRFPFVRLKNSRKLEFKDIKGIKREQFVKIL), termed sLL-37 (control peptide). Since porcine Protegrin-1 (PG-1; RGGRLCYCRRRFCVCVGR −amide) was obtained in its reduced form, its two disulfide bridges, connecting Cys-6 and Cys-15, and Cys-8 and Cys-13, were formed by air oxidation of the HPLC purified, all-reduced peptide. SMAP-29 was synthesized in the same way as described above for LL-37. All peptides were dissolved in endotoxin-free ultrapure water (Sigma-Aldrich, UK) and stored at −20 °C until use.
2.3. Respiratory epithelial cell culture
The WI-38 human fetal embryonic lung fibroblast cell line was purchased from the Public Health England (PHE) Culture Collection, Salisbury, UK, and cultured in Eagle’s minimum essential medium (E-MEM), supplemented with 1% l-glutamine (v/v), 1% non-essential amino acids (v/v), 1% streptomycin/penicillin (v/v) and 10% fetal bovine serum (v/v). The A549 adenocarcinoma human alveolar basal epithelial cells were provided by Edinburgh Napier University. Cells were cultured in DMEM, supplemented with 1% l-glutamine (v/v), 1% non-essential amino acids (v/v), 1% streptomycin/penicillin (v/v) and 10% fetal bovine serum (v/v). WI-38 cells and A549 were cultured at 37 °C in a humidified incubator with 5% CO2.
2.4. Virus propagation
Human Rhinovirus strain 1B (RV1B) was purchased from the National Collection of Pathogenic Viruses (NCPV) of Public Health England Virus Collection, Salisbury, UK. HRV was propagated in the Hela and WI-38 cell line by infecting cells in serum-free DMEM for 2 h at 33 °C. The inoculum was then removed and cells were incubated for 24–48 h in low serum media (5% FBS supplemented DMEM). WI-38 cells were then frozen at −20 °C for 3 h and virus was harvested by freezing and thawing the cells and subsequently centrifuging at 14,000 × g and recovering the supernatant, which was subsequently frozen and stored in aliquots at −80 °C until use.
2.5. Viral titer assessment (TCID50)
A TCID50 assay was used to determine the infectious titer of rhinovirus. In order to determine the virus titer of the virus stocks, WI-38 cells were seeded in a 96-well plate and cultured overnight. Ten-fold serial dilutions of the original virus stock were made using serum-free media and subsequently added to the cells which were then incubated for 1 h at 33 °C. Following incubation, the inoculum was removed and cells were cultured in 5% media (v/v) for a period of 5 days before analysis. TCID50 was subsequently determined using the Reed-Muench method [39]. The same procedure was repeated in A549 epithelial cells.
For experimental assessment of the antiviral effects of cathelicidins (human LL-37, scrambled LL-37, ovine SMAP-29, or porcine PG-1), WI-38 cells were seeded at 2 × 104 per well in a 96-well plate and cultured overnight. Rhinovirus (stock titer of 5.2 × 108 TCID50) was exposed to varying concentrations of cathelicidins for 2 h at 33 °C. After 2 h incubation, 10-fold serial dilutions of the HRV-peptide mixture were prepared. Cell media was aspirated and the serial dilutions of the peptide/virus mixture were used to infect WI-38 cells for 1 h. After 1 h the inoculum was removed and cells were cultured in low-serum (5% v/v) media and incubated for a period of 5 days at 33 °C before determining the virus titer by TCID50 assay.
2.6. Molecular quantitation of viral infectivity
To assess viral infectivity following cathelicidin exposure, an in vitro infection model was employed. Briefly, A549 cells were grown to confluency overnight in a 12 well plate incubated at 37 °C and were subsequently subjected to two different treatment approaches. For approach 1) HRV was exposed to peptide for 1 h prior to infection of confluent A549 cells at an MOI of 1 for 1 h. Cells were then washed with PBS, and re-immersed in low-serum (5% v/v) complete DMEM. For approach 2), cells were infected with serum-free media containing HRV at an MOI of 1 for 2 h, and subsequently washed with PBS, before re-immersion in DMEM containing 1% (v/v) Ultroser G with LL-37 (0–30 μg/ml) and incubated for 22 h at 33 °C. Media was aspirated and ice cold PBS was added to each well. Cells were scraped from the surface of the plate with a cell-scraper, and cell suspension was harvested and centrifuged at 230 × g for 5 min. Supernatant was aspirated and cell pellets were stored at −80 °C until extraction. Total RNA extraction was performed by resuspending cell pellets in situ in RLT buffer from RNeasy® mini kits (Qiagen, Crawley, UK) and collecting lysate into sterile DNase/RNase free tubes. Once samples were lysed, RNA was extracted from the lysates using the RNeasy® mini kit system following manufacturers protocols. RNA integrity was determined using an Agilent Bioanalyser (Agilent, UK), where RIN ≥ 8 was used as a quality filter for further downstream analyses. Viral RNA was quantified using a Genesig Human Rhinovirus (all subtypes) qPCR kit (Primer Design) according to manufacturer’s protocol.
2.7. Lactate dehydrogenase (LDH) quantification and MTS assay
The LDH assay was performed using the LDH cytotoxicity detection kit (Promega) according to the manufacturer’s instructions. Metabolic activity was assessed by use of the CellTiter96 Aqueous Non-Radioactive Cell Proliferation Assay (MTS assay) (Promega) according to the manufacturer’s instructions. Briefly, A549 cells were seeded at 2 × 104 per well in a 96-well plate and cultured overnight at 37 °C. Cells were infected with HRV at an MOI of 10 (with or without LL-37). Cells were also incubated with peptide alone. After incubation of cells with HRV and/or peptide at 33 °C, supernatant was aspirated and LDH activity was measured. Cells were resuspended in DMEM containing MTS reagent and metabolic conversion of MTS reagent was assessed after 2 h by measuring the optical density at 595 nm. Controls included untreated cells as negative control, and cells treated with Triton-X as positive control for reduction in metabolic activity.
2.8. Flow cytometric analysis
A549 cells were grown to confluency at 37 °C, and infected with serum-free media containing HRV at an MOI of 1 for 2 h, and subsequently washed with PBS, before re-immersion in DMEM containing 1% (v/v) Ultroser G with LL-37 (0–30 μg/ml) and incubated for 22 h at 33 °C. Media containing virus and peptide was aspirated and replaced with Trypsin/EDTA solution and incubated for 5 min to detach the cells from the surface of the plate. Trypsin activity was neutralised via the addition of DMEM containing 10% (v/v) FBS and cells were subsequently centrifuged at 230 × g for 5 min and supernatant was discarded. Cells were resuspended in 2mls of annexin V binding buffer and a wash step was repeated twice. Finally, cells were resuspended in 2mls of binding buffer containing FITC-labeled annexin V (BD Pharmigen, Oxford, UK) and incubated at room temperature in the dark for 20 min. Cells were once again centrifuged to remove FITC-annexin V, and subsequently resuspended in binding buffer before analysis by flow cytometry. Immediately prior to analysis, 5 μl of μg/ml propidium iodide was added to cells.
2.9. TUNEL assay
A549 cells (3 × 105) were grown in the apical compartment of Transwell® polycarbonate-permeable supports (pore size 0.4 μm, Corning Life Sciences) at 37 °C and 5% CO2. After cells had adhered to the membrane, the media in both compartments was aspirated and replaced with DMEM containing 1% (v/v) Ultroser G and LL-37 at varying concentrations (0–50 μg/ml) in the presence and absence of HRV (MOI 10). Cells were incubated for 6 h at 33 °C and 5% CO2. Cells were then fixed in 10% neutral buffered formalin (3.7% formaldehyde) for 10 min, then washed once in PBS. Cells were then permeabilised in ice cold 0.1% Triton X-100/0.1% sodium citrate for 3 min and washed twice with PBS. An in situ cell death detection kit (Roche Applied Science, UK) was used to label cells according to the manufacturer’s instructions. Transwell membranes were excised with a scalpel and mounted on a glass slide in 50 μl Vectashield Hardset (containing DAPI). At least four random fields of view were counted, each containing more than 80 cells. The total number of DAPI-positive nuclei was assessed for each treatment to evaluate total cell number and the number of TUNEL-positive cells is expressed as a percentage of the total number of DAPI-positive nuclei.
3. Results
3.1. LL-37 has direct antiviral effects against rhinovirus and reduces replication in airway epithelial cells
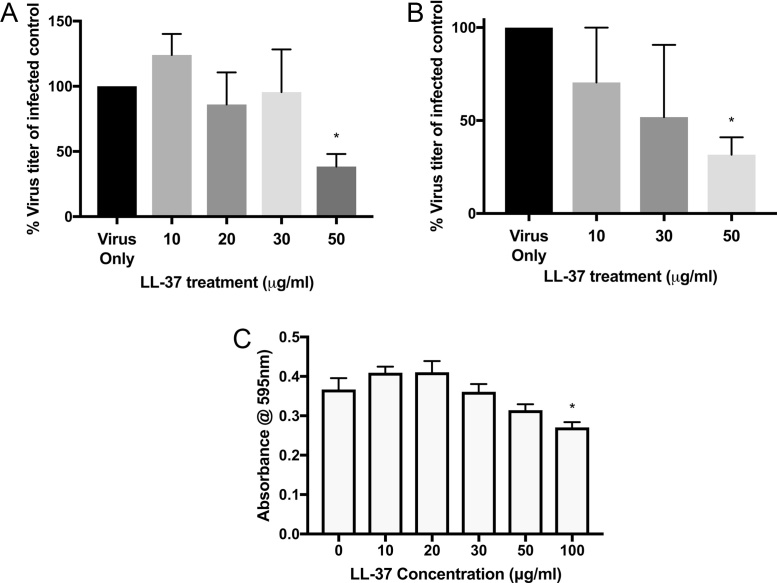
To determine if the human cathelicidin LL-37 has direct antiviral activity against HRV in an in vitro model of infection, two experimental approach were used to distinguish between the direct effects of LL-37 on the virus, or cell-mediated effects of the peptide on viral replication. Human rhinovirus (strain RV1B) was incubated with varying concentrations of exogenous LL-37 peptide (0–50 μg/ml) for 2 h prior to infection of human lung epithelial (WI-38) cells (Fig. 1A), or cells were exposed to LL-37 (0–50 μg/ml) during active HRV infection (Fig. 1B). For the virus pre-treatment regimen, following serial dilutions of the peptide/virus mixture, the virus titer in cell supernatants following peptide treatment was quantified by TCID50 assay 5 days post infection. It was found that, irrespective of treatment approach, LL-37 significantly reduced the HRV titer only at the highest concentration of peptide tested. We also assessed toxicity of the LL-37 peptide towards WI-38 in this context and found that no toxicity was observed at doses of LL-37 that were found to be antiviral (50 μg/ml) (Fig. 1C). We did, however, identify a statistically significant reduction in the metabolic activity of the cells at 100 μg/ml of LL-37.
Fig. 1.
The cathelicidin LL-37 displays direct antiviral activity against human rhinovirus. *Human rhinovirus was (A) pre-incubated with cathelicidin peptide (LL-37) for 2 h prior to infection of WI-38 cells, or (B) simultaneously exposed to LL-37 during infection of WI-38 cells. A TCID50 assay was subsequently performed to assess the virus titer in cells 4 days after infection. The virus used was RV1B. Figures are representative of at least four independent experiments and display% of virus remaining following peptide treatment compared to control (untreated) virus titer ± SEM. Statistical analysis was performed using a one-way ANOVA with Dunnett’s post-test to compare virus only (untreated) titer with virus + peptide treatments (***P ≤ 0.001). (C) WI-38 cell metabolic activity, indicative of viability, was assessed by MTS assay following exposure to LL-37 for 24 h. Figures are representative of three independent experiments and represent mean absorbance values ± SEM. Statistical analysis was performed using one-way ANOVA to compare LL-37 treated samples with negative control.
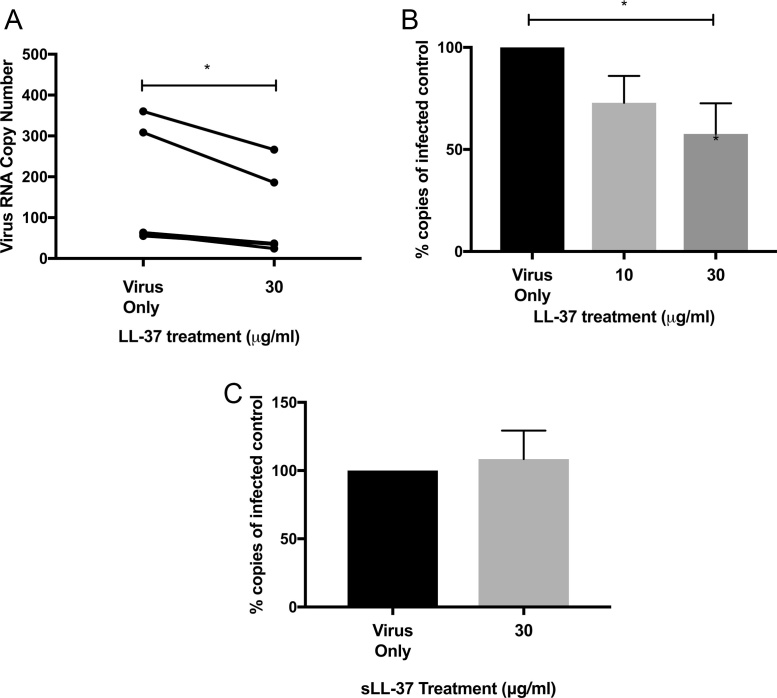
After determining that superphysiological concentrations of exogenous LL-37 were able to reduce the HRV-mediated cytopathic effects and thus total viable viral particles, we wanted to address whether LL-37 was able to reduce HRV RNA copy number. HRV was incubated with either a vehicle control (0 μg/ml), or an inflammatory concentration of LL-37 (30 μg/ml) prior to infection of A549 alveolar epithelial cells. Alternatively, HRV-infected cells were treated with physiologically normal or inflammatory, concentrations of LL-37 at 2 h post infection with virus. After a further 22 h, cells were harvested, RNA isolated and viral RNA was quantified using an HRV-specific qPCR kit. LL-37 pre-treatment (30 μg/ml) of virus resulted in a statistically significant reduction in viral copy number compared to the vehicle control (Fig. 2A). In addition, it was found that this concentration of LL-37, but not 10 μg/ml, elicited a statistically significant reduction in viral copies detected when a post infection treatment approach was employed (Fig. 2B). A control peptide with identical amino acid composition but scrambled sequence was also employed in order to determine the importance of sequence specificity in the antiviral effects observed. HRV was treated with 30 μg/ml of scrambled control peptide or vehicle control for 2 h prior to infection of cells. In contrast to the reduction in viral copy number observed when HRV was treated with 30 μg/ml LL-37 peptide (Fig. 2A), it was found that treatment with 30 μg/ml sLL-37 did not alter viral copy number detected after treatment (Fig. 2C).
Fig. 2.
LL-37 reduces rhinovirus replication in human airway epithelium. Human rhinovirus was (A) pre-incubated with cathelicidin peptide (LL-37) for 1 h prior to infection of A549 cells, (B) cells were infected for 2 h then washed and treated with peptide for 22 h or (C) pre-incubated with scrambled LL-37 (sLL-37) for 2 h prior to infection of A549 cells. The virus used was RV1B. Viral mRNA was isolated and quantified by RT-PCR. Figures are representative of at least four independent experiments and show total virus RNA copy number compared to control (untreated) virus (2A) or% of virus copy number compared to control. Statistical analysis was performed using a one way ANOVA to compare virus only (untreated) control with virus + peptide treatments (*P ≤ 0.05).
3.2. LL-37 reduces the metabolic activity of airway epithelial cells infected with rhinovirus but does not enhance host cell apoptosis or necrosis
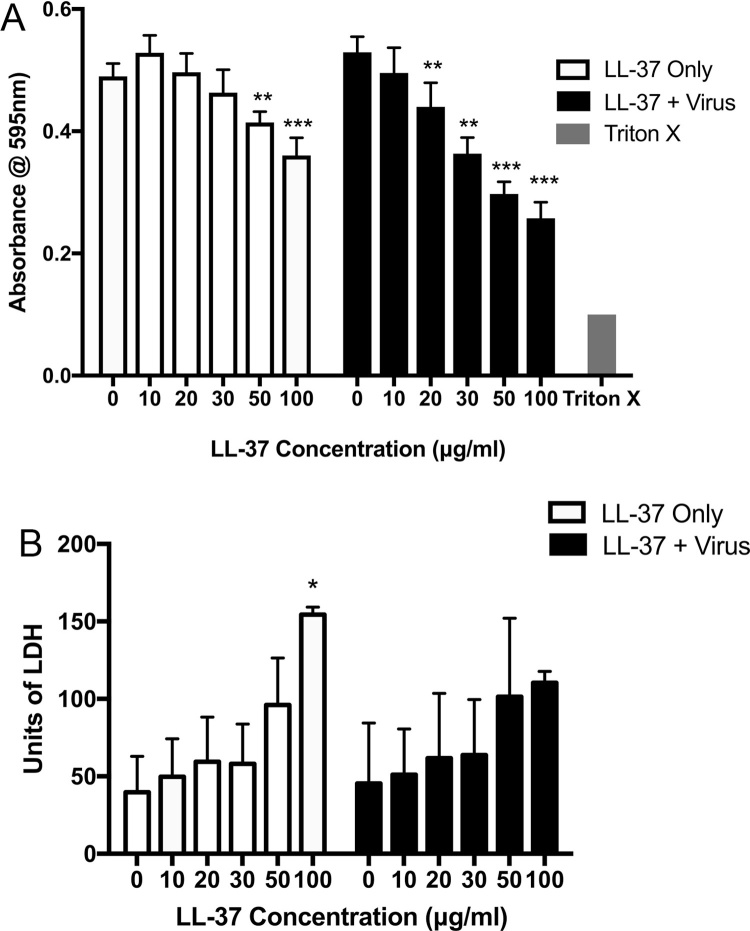
To evaluate the effect of LL-37 exposure on the viability of airway epithelial cells, A549 epithelial cells, highly permissive to Rhinovirus replication, were infected with HRV at an MOI of 10 for 2 h, prior to treatment with LL-37 (0–100 μg/ml) for 3 h. Peptide treatments were also conducted in cells in the absence of any viral infection as control. The mitochondrial metabolic potential of the cells was assessed by MTS assay, and cytosolic lactate dehydrogenase release, indicative of damage to the cell, was quantified. HRV infection did not result in any significant change to cell metabolic activity or LDH release, but LL-37 did induce significant reductions in A549 cell metabolic activity at superphysiological concentrations (50 and 100 μg/ml), and this effect was significantly more pronounced when cells were infected with HRV (at peptide concentrations ≥20 μg/ml) (Fig. 3A). However, there did not appear to be any major effects on LDH release by cells (Fig. 3B).
Fig. 3.
LL-37 reduces the metabolic activity of airway epithelial cells infected with rhinovirus but does not induce host cell necrosis. Human lung epithelial cells (A549) were infected with human rhinovirus (strain RV1B) for 2 h prior to treatment with the cathelicidin LL-37 (0–100 μg/ml) for 6 h. (A) Cell metabolic activity, indicative of viability, was assessed by MTS assay and (B) cytotoxic release of cytosolic lactate dehydrogenase (LDH) was assessed by LDH assay. Figures are representative of three independent experiments and represent mean absorbance values ± SEM. Statistical analysis was performed using one-way ANOVA to compare LL-37 treated samples with negative control (0) in the presence and absence of virus (** P ≤ 0.01, *** P ≤ 0.001).
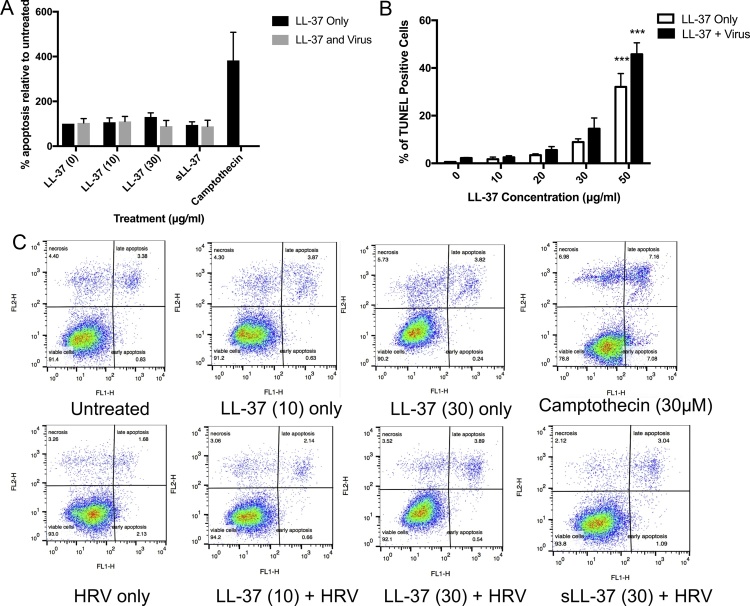
Apoptosis of airway epithelial cells induced by LL-37 in the presence of HRV infection was evaluated by FACS quantification of FITC-labelled annexin V and propidium iodide staining. A549 epithelial cells were infected with HRV at an MOI of 1 for 2 h prior to treatment with LL-37 (10 and 30 μg/ml), or camptothecin as a positive control (30 μM) for a further 22 h. Significant increases in apoptosis or necrosis were not observed in any of the treatment conditions utilised (Fig. 4A and B). This data was further confirmed by assessment of nuclear DNA fragmentation by TUNEL assay at 6 h post infection with an MOI of 1 in the presence and absence of LL-37 (Fig. 4C). Exposure of cells to the superphysiological concentration of 50 μg/ml LL-37 resulted in a statistically significant increase in cell death induced by LL-37 in both healthy and infected cells.
Fig. 4.
LL-37 does not modulate apoptotic cell death pathways in airway epithelial cells infected with human rhinovirus. Human lung epithelial cells (A549) were infected with Rhinovirus (strain RV1B) and treated with a range of LL-37 concentrations for (A)6 h or (B) 24 h. (A) Cells were fixed and apoptosis was assessed by TUNEL assay. Three random fields of view, each containing more than 100 cells, were counted for each sample, and the number of TUNEL-positive cells was expressed as a percentage of the number of DAPI-positive nuclei. (B) Cells were stained with Annexin V-Fluos and Propidium Iodide (PI) and the percentage of cell population that was either AV + PI- (early apoptosis) or AV + PI+ (late apoptosis) was categorized as apoptotic. Data represent mean values ± SEM, for n = 3 independent experiments for each condition (*** P ≤ 0.001 compared to control treatment).
3.3. Ovine and porcine cathelicidins display potent conserved antiviral activity against human rhinovirus
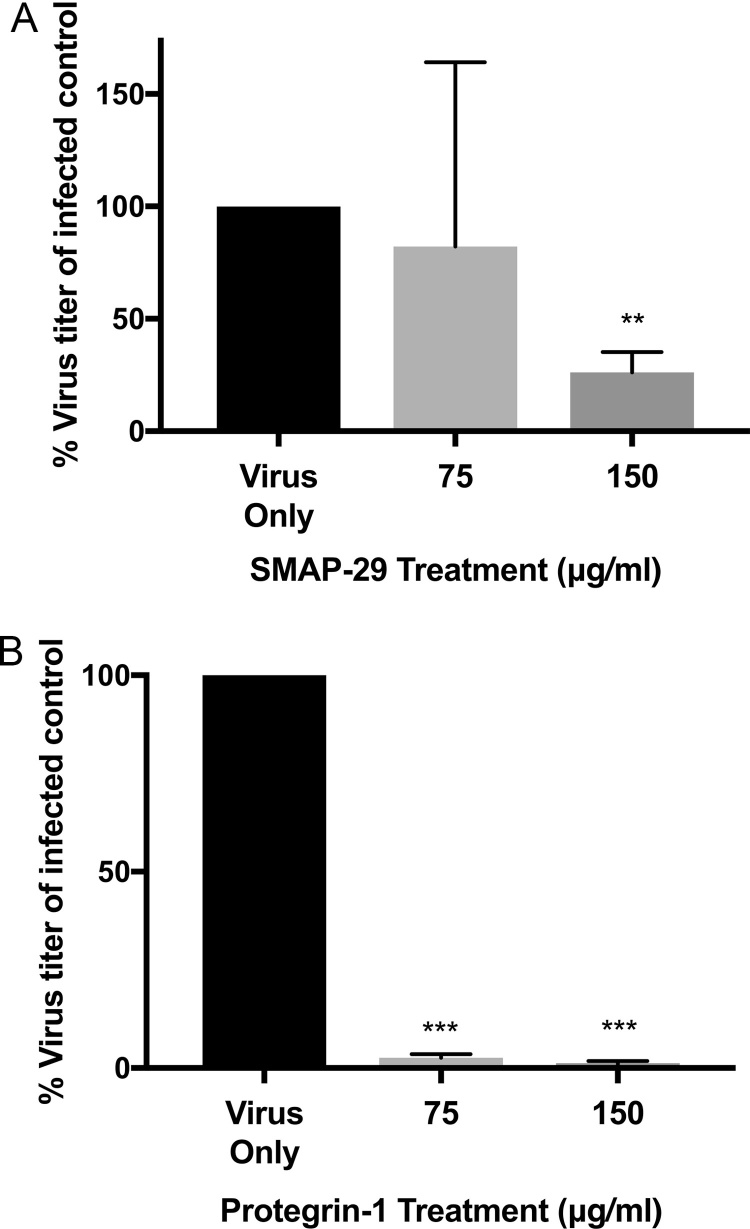
To assess whether the anti-rhinovirus activity of cathelicidins was a species-specific property, we synthesised cathelicidins from porcine (Protegrin-1) and ovine (SMAP-29) species. Human rhinovirus (strain RV1B) was then incubated with two concentrations of each peptide (75–150 μg/ml) for 2 h prior to infection of human lung epithelial (WI-38) cells. Consistent with human cathelicidin results, it was shown that SMAP-29 and Protegrin-1 exerted direct antiviral effects on the virus, although to a lesser extent than that exhibited by LL-37. The antiviral effect of SMAP-29 was demonstrated at 150 μg/ml, resulting in a 70% reduction in virus titer (Fig. 5A) although treatment with SMAP-29 at 75 μg/ml did not alter virus titer. The porcine cathelicidin Protegrin-1 exerted a direct antiviral effect at both concentrations tested, with approximately a 90% reduction in virus titer at the concentrations tested (Fig. 5B).
Fig. 5.
Cathelicidins display species specific antiviral activity against human rhinovirus. Rhinovirus (strain RV1B) was incubated with two concentrations of either (A) ovine cathelicidin peptide (SMAP-29) or (B) porcine cathelicidin peptide (Protegrin-1), or 500 μg/ml of scrambled LL-37 for 1 h. Virus concentration (TCID) was assessed by WI-38 lung epithelial cell lysis after four days. Figures are representative of four independent experiments and represent mean Log10 TCID values after treatment ± SEM. Statistical analysis was performed using a one way ANOVA with Dunnett’s post-test to compare virus only titer with virus + peptide treatments (** P ≤ 0.01, *** P ≤ 0.001 compared to control treatment).
4. Discussion
It is well established that cathelicidins possess broad-spectrum antimicrobial and antiviral activity against a wide range of bacterial and viral pathogens. The human cathelicidin, LL-37, has been shown to have antiviral activity against vaccinia virus, respiratory syncytial virus (RSV), influenza, HIV and adenovirus, among others [16], [17], [18], [19], [21], [22]. In addition, the murine cathelicidin, mCRAMP, the ovine cathelicidin SMAP-29 and the porcine cathelicidin, Protegrin-1, show specific antiviral potential against a number of viral pathogens including vaccinia virus, influenza, herpes simplex virus (HSV) and dengue [16], [22], [27], [40]. Additionally, vitamin D treatment of cells isolated from CF patients led to reduced HRV loads in vitro, and it was suggested that this effect was mediated by upregulation of hCAP-18. This was further supported by a reduction in virus observed after exposure of the CF cells to 20 μg/ml of recombinant LL-37 during the HRV infection process.
In this study, we demonstrate that the human cathelicidin displays direct antiviral activity against HRV in vitro, and that this effect was visible when the virus was exposed to peptide prior to infection, and when cells were treated with peptide post-infection. This is consistent with other studies investigating the antiviral effects of LL-37 against RSV which have revealed that LL-37 has the potential to act both directly on the virus and on the host cell, but in those instances, pre-incubation of peptide with virus yielded the most significant antiviral effect [18], [41]. Direct antiviral activity of LL-37 has also been characterised against Herpes Simplex Virus (HSV) type 1, a number of adenovirus serotypes, dengue virus, vaccinia virus and influenza virus [16], [21], [22], [24], [42]. The antiviral effect of the LL-37 peptide was demonstrated to be sequence specific as a scrambled LL-37 peptide with identical amino-acid composition but with a random sequence did not display any anti-HRV activity. This is in accord with our previous study examining the antiviral activity of LL-37 against influenza virus, which revealed that a scrambled LL-37 peptide did not show anti-influenza activity, in comparison to LL-37 which exhibited potent activity against influenza A in vitro and in vivo [16], together with other studies which have revealed a sequence specificity for LL-37 against RSV and dengue [18], [24].
In situ hybridisation studies have revealed that airway epithelial cells are the primary site of HRV infection and replication in the respiratory tract [43], therefore these cells are the preferred target for antiviral therapeutics aimed at combating established HRV infections. In order to establish if the reduction in HRV replication in airway epithelium induced by LL-37 that we observed could be attributed to induction of death in the host cell, we assessed mitochondrial metabolic activity and cytosolic lactate dehydrogenase release by healthy and HRV-infected host cells following peptide treatment. It was revealed that following HRV infection, there was a significant reduction in cell metabolic activity at lower concentrations of LL-37 (≤30 μg/ml) in infected cells, compared to healthy cells which did not show any alteration of metabolic activity until ≥50 μg/ml LL-37 was applied. Interestingly, there was no significant increase in LDH from the cytosol in HRV-infected cells at any LL-37 concentration tested, although 100 μg/ml of LL-37 in healthy cells did induce a significant increase in LDH released from cells, suggesting a cytotoxic effect only at the highest concentration. While decreased metabolic activity could be associated with increased cytotoxicity due to membrane damage, a lack of cytotoxicity at LL-37 concentrations <50 μg/ml in healthy cells is consistent with previous studies [21]. This data could indicate that the antiviral effects exhibited by LL-37 may not just be attributable to direct effects on the virus, but via a depression of the metabolic activity of the host cell. However, while our previous studies demonstrated a role for LL-37 in the rapid induction of necrosis in apoptotic neutrophils [32], this data suggests that increased susceptibility to necrosis induced by LL-37 is not exhibited in airway cells infected with HRV. Despite this, it is clear that mitochondrial specific cytotoxic effects of LL-37 resulting in reduced metabolic capacity in these cell types may be enhanced during infection, as we and others have indicated a role for LL-37 in inducing or enhancing mitochondrial-mediated apoptosis in healthy and infected cells [31], [44], [45].
Previously, it was shown that physiological concentrations of LL-37 could enhance the apoptotic cell death in airway epithelial cells infected with Pseudomonas aeruginosa [31], and thus the induction of apoptotic cell death by LL-37 in HRV-infected cells could represent a potential mechanism for peptide-mediated impairment of viral replication. In this context, we used Annexin V/P.I. staining, in tandem with TUNEL labelling for apoptosis specific DNA fragmentation and, in contrast to observations using bacterial infection, the data indicate that apoptosis was not induced by LL-37 in HRV-infected cells. Thus, induction of apoptosis did likely not represent the mechanism by which the viral loads were reduced by LL-37, and therefore the direct antiviral activity of the peptide was responsible. However, our previous studies have revealed that LL-37 does possess the capacity to alter inflammatory responses in murine models of influenza infection in vivo, and thus cathelicidin mediated alteration of inflammatory cytokine secretion in viral infection should be explored in future studies. This is particularly relevant in the context of HRV infection which has been demonstrated to induce upregulation of multiple proinflammatory cytokine/chemokine genes together with IFN-β secretion in the murine and human lung [2], [46], [47], leading to severe exacerbations of COPD and asthma. While the induction of inflammation may have a significant role to play in the host antiviral response against this infection, controlled attenuation of the inflammation by cathelicidins in the context of airway disease exacerbation may prove to be of important therapeutic value.
Cathelicidins from other mammalian species have been shown to display potent antimicrobial capacity against a range of human pathogens. The porcine β-sheet cathelicidin, Protegrin-1, has been shown to have antiviral activity against Dengue virus, lentivirus and HIV [27], [48], [49], [50], but lacks antiviral activity against influenza virus [16]. The ovine cathelicidin, SMAP-29, has previously been shown to have antibacterial activity against methicillin-resistant Staphylococcus aureus (MRSA), Escherichia coli and Pseudomonas aeruginosa among others, together with activity against Cryptococcus and Candida species [51], although its antiviral activity has yet to be established. To examine whether the antiviral activity of cathelicidins was species-specific, we synthesised both of these peptides and assessed their direct antiviral activity against HRV. Protegrin-1 displayed potent antiviral activity at both concentrations tested, with SMAP-29 displaying direct antiviral activity but to a lesser extent. This suggests that anti-HRV capacity is a conserved activity of cathelicidins from different sources, and that this family of peptides possesses direct antiviral capacity.
5. Conclusions
In summary, this study demonstrates that the human cathelicidin LL-37, together with ovine and porcine cathelicidins, display powerful and direct antiviral activity against HRV. Our data indicate that this is mediated by direct activity against the virion, rather than by induction of apoptotic or necrotic cell death in the host airway cells. However, the contribution of cathelicidins in the modulation of the host inflammatory response to this virus remains to be determined. Our data suggests that exogenous prophylactic or therapeutic synthetic peptide delivery, with design informed by cathelicidins from different mammalian species, could represent a key avenue for therapeutics designed to reduce the exacerbations of serious airway conditions as a result of infection with HRV.
Author contributions
FHS contributed to the experimental design, performed the experimental work, and contributed to the drafting of the manuscript. VC contributed to the experimental design, performed the experimental work and contributed to the drafting of the manuscript. FF contributed to the experimental design, performed the experimental work, and contributed to the drafting of the manuscript. CS contributed to the experimental design, and the drafting of the manuscript. PS performed the experimental work, and contributed to the drafting of the manuscript. JP performed the experimental work, and contributed to the drafting of the manuscript. LP contributed to the experimental design, and the drafting of the manuscript. PGB conceived the study, contributed to the experimental design, performed the experimental work, and contributed to the drafting of the manuscript.
Acknowledgments
This study was funded by the Chief Scientist Office (ETM/389) and Tenovus Scotland (E12/01).
References
- 1.Mackay I.M. Human rhinoviruses: the cold wars resume. J. Clin. Virol. 2008;42(4):297–320. doi: 10.1016/j.jcv.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hershenson M.B. Rhinovirus-induced exacerbations of asthma and COPD. Scientifica (Cairo) 2013;2013:405876. doi: 10.1155/2013/405876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gavala M.L., Bertics P.J., Gern J.E. Rhinoviruses, allergic inflammation, and asthma. Immunol. Rev. 2011;242(1):69–90. doi: 10.1111/j.1600-065X.2011.01031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Papadopoulos N.G., Moustaki M., Tsolia M., Bossios A., Astra E., Prezerakou A. Association of rhinovirus infection with increased disease severity in acute bronchiolitis. Am. J. Respir. Crit. Care Med. 2002;165(9):1285–1289. doi: 10.1164/rccm.200112-118BC. [DOI] [PubMed] [Google Scholar]
- 5.Pitrez P.M., Stein R.T., Stuermer L., Macedo I.S., Schmitt V.M., Jones M.H. Rhinovirus and acute bronchiolitis in young infants. J. Pediatr. (Rio J.) 2005;81(5):417–420. doi: 10.2223/JPED.1394. [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Garcia M.L., Calvo C., Pozo F., Villadangos P.A., Perez-Brena P., Casas I. Spectrum of respiratory viruses in children with community-acquired pneumonia. Pediatr. Infect. Dis. J. 2012;31(8):808–813. doi: 10.1097/INF.0b013e3182568c67. [DOI] [PubMed] [Google Scholar]
- 7.Arden K.E., Mackay I.M. Human rhinoviruses: coming in from the cold. Genome Med. 2009;1(4):44. doi: 10.1186/gm44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sorensen O.E., Follin P., Johnsen A.H., Calafat J., Tjabringa G.S., Hiemstra P.S. Human cathelicidin, hCAP-18, is processed to the antimicrobial peptide LL-37 by extracellular cleavage with proteinase 3. Blood. 2001;97(12):3951–3959. doi: 10.1182/blood.v97.12.3951. [DOI] [PubMed] [Google Scholar]
- 9.Schaller-Bals S., Schulze A., Bals R. Increased levels of antimicrobial peptides in tracheal aspirates of newborn infants during infection. Am. J. Respir. Crit. Care Med. 2002;165(7):992–995. doi: 10.1164/ajrccm.165.7.200110-020. [DOI] [PubMed] [Google Scholar]
- 10.Chen C.I., Schaller-Bals S., Paul K.P., Wahn U., Bals R. Beta-defensins and LL-37 in bronchoalveolar lavage fluid of patients with cystic fibrosis. J. Cyst. Fibros. 2004;3(1):45–50. doi: 10.1016/j.jcf.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 11.Agerberth B., Charo J., Werr J., Olsson B., Idali F., Lindbom L. The human antimicrobial and chemotactic peptides LL-37 and alpha-defensins are expressed by specific lymphocyte and monocyte populations. Blood. 2000;96(9):3086–3093. [PubMed] [Google Scholar]
- 12.Scott M.G., Davidson D.J., Gold M.R., Bowdish D., Hancock R.E. The human antimicrobial peptide LL-37 is a multifunctional modulator of innate immune responses. J. Immunol. 2002;169(7):3883–3891. doi: 10.4049/jimmunol.169.7.3883. [DOI] [PubMed] [Google Scholar]
- 13.Kin N.W., Chen Y., Stefanov E.K., Gallo R.L., Kearney J.F. Cathelin-related antimicrobial peptide differentially regulates T- and B-cell function. Eur. J. Immunol. 2011;41(10):3006–3016. doi: 10.1002/eji.201141606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chromek M., Slamova Z., Bergman P., Kovacs L., Podracka L., Ehren I. The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection. Nat. Med. 2006;12(6):636–641. doi: 10.1038/nm1407. [DOI] [PubMed] [Google Scholar]
- 15.Iimura M., Gallo R.L., Hase K., Miyamoto Y., Eckmann L., Kagnoff M.F. Cathelicidin mediates innate intestinal defense against colonization with epithelial adherent bacterial pathogens. J. Immunol. 2005;174(8):4901–4907. doi: 10.4049/jimmunol.174.8.4901. [DOI] [PubMed] [Google Scholar]
- 16.Barlow P.G., Svoboda P., Mackellar A., Nash A.A., York I.A., Pohl J. Antiviral activity and increased host defense against influenza infection elicited by the human cathelicidin LL-37. PLoS One. 2011;6(10):e25333. doi: 10.1371/journal.pone.0025333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bergman P., Walter-Jallow L., Broliden K., Agerberth B., Soderlund J. The antimicrobial peptide LL-37 inhibits HIV-1 replication. Curr. HIV Res. 2007;5(4):410–415. doi: 10.2174/157016207781023947. [DOI] [PubMed] [Google Scholar]
- 18.Currie S.M., Findlay E.G., McHugh B.J., Mackellar A., Man T., Macmillan D. The human cathelicidin LL-37 has antiviral activity against respiratory syncytial virus. PLoS One. 2013;8(8):e73659. doi: 10.1371/journal.pone.0073659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Currie S.M., Gwyer Findlay E., McFarlane A.J., Fitch P.M., Böttcher B., Colegrave N. Cathelicidins have direct antiviral activity against respiratory syncytial virus in vitro and protective function in vivo in mice and humans. J. Immunol. 2016:2699–2710. doi: 10.4049/jimmunol.1502478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yasin B., Pang M., Turner J.S., Cho Y., Dinh N.N., Waring A.J. Evaluation of the inactivation of infectious herpes simplex virus by host-defense peptides. Eur. J. Clin. Microbiol. Infect. Dis. 2000;19(3):187–194. doi: 10.1007/s100960050457. [DOI] [PubMed] [Google Scholar]
- 21.Gordon Y.J., Huang L.C., Romanowski E.G., Yates K.A., Proske R.J., McDermott A.M. Human cathelicidin (LL-37), a multifunctional peptide, is expressed by ocular surface epithelia and has potent antibacterial and antiviral activity. Curr. Eye Res. 2005;30(5):385–394. doi: 10.1080/02713680590934111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Howell M.D., Jones J.F., Kisich K.O., Streib J.E., Gallo R.L., Leung D.Y. Selective killing of vaccinia virus by LL-37: implications for eczema vaccinatum. J. Immunol. 2004;172(3):1763–1767. doi: 10.4049/jimmunol.172.3.1763. [DOI] [PubMed] [Google Scholar]
- 23.Dean R.E., O'Brien L.M., Thwaite J.E., Fox M.A., Atkins H., Ulaeto D.O. A carpet-based mechanism for direct antimicrobial peptide activity against vaccinia virus membranes. Peptides. 2010;31(11):1966–1972. doi: 10.1016/j.peptides.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 24.Alagarasu K., Patil P.S., Shil P., Seervi M., Kakade M.B., Tillu H. In-vitro effect of human cathelicidin antimicrobial peptide LL-37 on dengue virus type 2. Peptides. 2017;92:23–30. doi: 10.1016/j.peptides.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 25.Albiol Matanic V.C., Castilla V. Antiviral activity of antimicrobial cationic peptides against Junin virus and herpes simplex virus. Int. J. Antimicrob. Agents. 2004;23(4):382–389. doi: 10.1016/j.ijantimicag.2003.07.022. [DOI] [PubMed] [Google Scholar]
- 26.Benincasa M., Skerlavaj B., Gennaro R., Pellegrini A., Zanetti M. In vitro and in vivo antimicrobial activity of two alpha-helical cathelicidin peptides and of their synthetic analogs. Peptides. 2003;24(11):1723–1731. doi: 10.1016/j.peptides.2003.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rothan H.A., Abdulrahman A.Y., Sasikumer P.G., Othman S., Rahman N.A., Yusof R. Protegrin-1 inhibits dengue NS2B-NS3 serine protease and viral replication in MK2 cells. J. Biomed. Biotechnol. 2012;2012:251482. doi: 10.1155/2012/251482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pompilio A., Scocchi M., Pomponio S., Guida F., Di Primio A., Fiscarelli E. Antibacterial and anti-biofilm effects of cathelicidin peptides against pathogens isolated from cystic fibrosis patients. Peptides. 2011;32(9):1807–1814. doi: 10.1016/j.peptides.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Barlow P.G., Findlay E.G., Currie S.M., Davidson D.J. Antiviral potential of cathelicidins. Future Microbiol. 2014;9(1):55–73. doi: 10.2217/fmb.13.135. [DOI] [PubMed] [Google Scholar]
- 30.Schogler A., Muster R.J., Kieninger E., Casaulta C., Tapparel C., Jung A. Vitamin D represses rhinovirus replication in cystic fibrosis cells by inducing LL-37. Eur. Respir. J. 2015:520–530. doi: 10.1183/13993003.00665-2015. [DOI] [PubMed] [Google Scholar]
- 31.Barlow P.G., Beaumont P.E., Cosseau C., Mackellar A., Wilkinson T.S., Hancock R.E. The human cathelicidin LL-37 preferentially promotes apoptosis of infected airway epithelium. Am. J. Respir. Cell Mol. Biol. 2010;43(6):692–702. doi: 10.1165/rcmb.2009-0250OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li H.N., Barlow P.G., Bylund J., Mackellar A., Bjorstad A., Conlon J. Secondary necrosis of apoptotic neutrophils induced by the human cathelicidin LL-37 is not proinflammatory to phagocytosing macrophages. J. Leukoc. Biol. 2009;86(4):891–902. doi: 10.1189/jlb.0209050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barlow P.G., Li Y., Wilkinson T.S., Bowdish D.M., Lau Y.E., Cosseau C. The human cationic host defense peptide LL-37 mediates contrasting effects on apoptotic pathways in different primary cells of the innate immune system. J. Leukoc. Biol. 2006;80(3):509–520. doi: 10.1189/jlb.1005560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sall J., Carlsson M., Gidlof O., Holm A., Humlen J., Ohman J. The antimicrobial peptide LL-37 alters human osteoblast Ca2+ handling and induces Ca2+-independent apoptosis. J. Innate Immun. 2013;5(3):290–300. doi: 10.1159/000346587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bowdish D.M., Davidson D.J., Scott M.G., Hancock R.E. Immunomodulatory activities of small host defense peptides. Antimicrob. Agents Chemother. 2005;49(5):1727–1732. doi: 10.1128/AAC.49.5.1727-1732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paredes-Gamero E.J., Martins M.N., Cappabianco F.A., Ide J.S., Miranda A. Characterization of dual effects induced by antimicrobial peptides: regulated cell death or membrane disruption. Biochim. Biophys. Acta. 2012;1820(7):1062–1072. doi: 10.1016/j.bbagen.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 37.Koszalka P., Kamysz E., Wejda M., Kamysz W., Bigda J. Antitumor activity of antimicrobial peptides against U937 histiocytic cell line. Acta Biochim. Pol. 2011;58(1):111–117. [PubMed] [Google Scholar]
- 38.Zughaier S.M., Svoboda P., Pohl J., Stephens D.S., Shafer W.M. The human host defense peptide LL-37 interacts with Neisseria meningitidis capsular polysaccharides and inhibits inflammatory mediators release. PLoS One. 2010;5(10):e13627. doi: 10.1371/journal.pone.0013627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reed L.J., Muench H. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. 1938;27:493–497. [Google Scholar]
- 40.Howell M.D., Gallo R.L., Boguniewicz M., Jones J.F., Wong C., Streib J.E. Cytokine milieu of atopic dermatitis skin subverts the innate immune response to vaccinia virus. Immunity. 2006;24(3):341–348. doi: 10.1016/j.immuni.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 41.Harcourt J.L., McDonald M., Svoboda P., Pohl J., Tatti K., Haynes L.M. Human cathelicidin, LL-37, inhibits respiratory syncytial virus infection in polarized airway epithelial cells. BMC Res. Notes. 2016;9(1):11. doi: 10.1186/s13104-015-1836-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tripathi S., Tecle T., Verma A., Crouch E., White M., Hartshorn K.L. The human cathelicidin LL-37 inhibits influenza A viruses through a mechanism distinct from that of surfactant protein D or defensins. J. Gen. Virol. 2013;94(Pt. 1):40–49. doi: 10.1099/vir.0.045013-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arruda E., Boyle T.R., Winther B., Pevear D.C., Gwaltney J.M., Jr., Hayden F.G. Localization of human rhinovirus replication in the upper respiratory tract by in situ hybridization. J. Infect. Dis. 1995;171(5):1329–1333. doi: 10.1093/infdis/171.5.1329. [DOI] [PubMed] [Google Scholar]
- 44.Aarbiou J., Tjabringa G.S., Verhoosel R.M., Ninaber D.K., White S.R., Peltenburg L.T. Mechanisms of cell death induced by the neutrophil antimicrobial peptides alpha-defensins and LL-37. Inflamm. Res. 2006;55(3):119–127. doi: 10.1007/s00011-005-0062-9. [DOI] [PubMed] [Google Scholar]
- 45.Mader J.S., Mookherjee N., Hancock R.E., Bleackley R.C. The human host defense peptide LL-37 induces apoptosis in a calpain- and apoptosis-inducing factor-dependent manner involving Bax activity. Mol. Cancer Res. 2009;7(5):689–702. doi: 10.1158/1541-7786.MCR-08-0274. [DOI] [PubMed] [Google Scholar]
- 46.Wark P.A., Johnston S.L., Bucchieri F., Powell R., Puddicombe S., Laza-Stanca V. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J. Exp. Med. 2005;201(6):937–947. doi: 10.1084/jem.20041901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Proud D., Turner R.B., Winther B., Wiehler S., Tiesman J.P., Reichling T.D. Gene expression profiles during in vivo human rhinovirus infection: insights into the host response. Am. J. Respir. Crit. Care Med. 2008;178(9):962–968. doi: 10.1164/rccm.200805-670OC. [DOI] [PubMed] [Google Scholar]
- 48.Rothan H.A., Han H.C., Ramasamy T.S., Othman S., Rahman N.A., Yusof R. Inhibition of dengue NS2B-NS3 protease and viral replication in Vero cells by recombinant retrocyclin-1. BMC Infect. Dis. 2012;12:314. doi: 10.1186/1471-2334-12-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Steinstraesser L., Tippler B., Mertens J., Lamme E., Homann H.H., Lehnhardt M. Inhibition of early steps in the lentiviral replication cycle by cathelicidin host defense peptides. Retrovirology. 2005;2:2. doi: 10.1186/1742-4690-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tamamura H., Murakami T., Horiuchi S., Sugihara K., Otaka A., Takada W. Synthesis of protegrin-related peptides and their antibacterial and anti-human immunodeficiency virus activity. Chem. Pharm. Bull. (Tokyo) 1995;43(5):853–858. doi: 10.1248/cpb.43.853. [DOI] [PubMed] [Google Scholar]
- 51.Skerlavaj B., Benincasa M., Risso A., Zanetti M., Gennaro R. SMAP-29: a potent antibacterial and antifungal peptide from sheep leukocytes. FEBS Lett. 1999;463(1–2):58–62. doi: 10.1016/s0014-5793(99)01600-2. [DOI] [PubMed] [Google Scholar]