Abstract
Objectives
To evaluate hospital-specific health economic implications of different protocols using high-sensitivity troponin I for the assessment of patients with chest pain.
Design
A cost prediction model and an economic microsimulation were developed using a cohort from a single centre recruited as part of the (ADAPT) trial, a prospective observational trial conducted from 2008 to 2011. The model was populated with 40 000 bootstrapped samples in five high-sensitivity troponin I-enabled algorithms versus standard care.
Setting
Adult emergency department (ED) of a tertiary referral hospital.
Participants
Data were available for 938 patients who presented to the ED with at least 5 min of symptoms suggestive of acute coronary syndrome. The analyses included 719 patients with complete data.
Main outcome(s)/measure(s)
This study examined direct hospital costs, number of false-negative and false-positive cases in the assessment of acute coronary syndrome.
Results
High-sensitivity troponin I-supported algorithms increased diagnostic accuracy from 90.0% to 94.0% with an average cost reduction per patient compared with standard care of $490. The inclusion of additional criteria for accelerated rule-out (limit of detection and the modified 2-hour ADAPT trial rules) avoided 7.5% of short-stay unit admissions or 25% of admissions to a cardiac ward. Protocols using high-sensitivity troponin I alone or high-sensitivity troponin I within accelerated diagnostic algorithms reduced length of stay by 6.2 and 13.6 hours, respectively. Overnight stays decreased up to 43%. Results were seen for patients with non-acute coronary syndrome; no difference was found for patients with acute coronary syndrome.
Conclusions
High-sensitivity troponin I algorithms are likely to be cost-effective on a hospital level compared with sensitive troponin protocols. The positive effect is conferred by patients not diagnosed with acute coronary syndrome. Implementation could improve referral accuracy or facilitate safe discharge. It would decrease costs and provide significant hospital benefits.
Trial registration
The original ADAPT trial was registered with the Australia-New Zealand Clinical trials Registry, ACTRN12611001069943.
Keywords: health economics, public health
Strengths and limitations of this study.
This study was based on an individual-level modelling design to allow for more realistic comparisons of different settings, assessment strategies or risk stratification rules.
As opposed to previous evaluations, costs and all management assumptions were based on actual patient information that was prospectively collected. In addition, we considered realistic management rules. For example, if patients were not discharged before 6:30 pm, they required an overnight stay.
Model results were based on a sampling strategy that created a large cohort with a wide spectrum of individual information, thus reflecting population heterogeneity and common variation in clinical practice.
Troponin results must be interpreted in concert with clinical presentation, ECG changes and other available information. Diagnostic accuracy used in this study refers to results of the complete pathway consisting of troponin results, ECG and cardiac work-up. All hospital costs accrued from assessment, management and events during 30 days of follow-up were considered in the analysis.
Cost data were based on information from an administrative database. The cost prediction was limited to activities during the assessment period. Information about inpatient treatment other than time was not available.
Economic implications from breaching specific emergency department targets or access blocks were not taken into account but may have a significant impact; it appears likely that considering such aspects would strengthen the results in favour of accelerated protocols.
Generalisability might be hindered by the variety of assessment processes. Exploiting the value of highly sensitive cardiac troponin I relies on the appropriateness of testing and the implementation of adequate protocols.
Introduction
Chest pain is a leading presenting complaint for adults seeking emergency department (ED) care.1 The most common serious underlying causes are acute coronary syndrome (ACS), including acute myocardial infarction and unstable angina. After detailed assessment, most patients are diagnosed with a non-cardiac cause (eg, musculoskeletal pain or gastrointestinal causes) for their symptoms. In Australia, over 500 000 persons per year present with chest pain, but fewer than 20% were diagnosed with ACS.2 3 The identification of the majority of chest pain presentations at low risk for ACS remains an organisational challenge for ED.
Accelerated assessment strategies for the rule-in and rule-out of acute myocardial infarction have recently been reported.3–12 Such strategies use clinical decision rules and/or troponin testing to identify a sizeable proportion of patients as low risk. Some protocols also accurately identify patients as high risk for acute myocardial infarction.3 4 The use of high-sensitivity troponin on presentation or within 2 hours is a key feature of several accelerated assessment strategies.6–10 For example, the modified ADAPT accelerated diagnostic protocol (ADP) uses highly sensitive troponin assays to support the identification of 40% of patients as low risk.4
While research into novel accelerated strategies has usually reported clinical outcomes, few studies have assessed the health economic implications of such protocols, or made comparisons to define optimum strategies. The incorporation of high-sensitivity troponin I (hsTnI) assays into clinical practice may have additional health economic benefits on the hospital level; however, this aspect has not been explored to date. The aim of this study was to evaluate the hospital-specific costs of different protocols using hsTnI for assessment of ED patients with chest pain, compared with standard care. The hypothesis was that hsTnI-enabled algorithms would streamline ED processes with equal or better diagnostic accuracy, thus leading to savings in direct hospital costs when compared with standard care.
Methods
Study design and setting
This study used data from a prospective, single centre observational study in Brisbane, Australia. Participants were recruited as part of the ADAPT trial,3 and included if they were aged 18 years or older, presented to the ED with at least 5 min worth of chest pain suggestive of ACS, and were being evaluated for ACS. Pain suggestive of ACS was defined in accordance with American Heart Association case definitions.13 Recruitment was performed by research staff in collaboration with the senior treating clinician. Patients were excluded if there was a clear non-ACS cause for their symptoms (eg, findings of pneumonia), they were unwilling or unable to provide informed consent, staff considered that recruitment was inappropriate (eg, patients undergoing palliative treatment), they were transferred from another hospital, were pregnant, were previously recruited to the study within the past 45 days or were unable or unwilling to be contacted after discharge. Recruitment included consecutive eligible cases during working hours at each site. Enrolment occurred between January 2008 and November 2010. All patients were managed according to standard care, which included ECG and troponin testing on presentation and at greater than 6 hours after presentation to the ED. Patients were classified into risk groups according to the Heart Foundation of Australia/Cardiac Society of Australia and New Zealand guidelines.14 The clinical assay in use as the reference troponin assay was the Beckman Coulter second-generation AccuTnI (Beckman Coulter, Chaska, Minnesota, USA). A value above the 99th percentile of greater than 40 ng/L was considered abnormal.
Original data were collected prospectively, using standardised case report forms.15 Research nursing staff collected demographic and clinical data from patient interviews. Telephone follow-up and medical record review was conducted 30 days after initial attendance for the diagnosis of ACS. Information was obtained from the patient and from hospital databases about all additional cardiac events, investigations or contact with any healthcare providers during the 30-day period. Follow-up information was verified through contact with the healthcare provider, and original copies of medical records and investigations were obtained. Ethical approval of the research project HREC/14/QRBW/320 was obtained from the Royal Brisbane and Women’s Hospital Human Research Ethics Committee (EC 00172) on 11August 2014. All patients provided written informed consent for data collection and the ethics committee waived the requirement for consent for this analysis.
Each patient was assigned one or more endpoints to explain the reason for their index presentation, or any events occurring within 30 days of admission. There were 15 possible endpoints, including both cardiovascular and non-cardiovascular endpoints. Patients were considered to meet the definition for ACS if they were assigned any of the following endpoints: cardiovascular death, cardiac arrest, revascularisation procedure, cardiogenic shock, acute myocardial infarction or unstable angina pectoris. One cardiologist from a group of three potential cardiologists adjudicated the outcome independently. Cardiologists had knowledge of the clinical record, ECG and troponin results from standard care and used such information to determine whether the patient met the predefined criteria for the cardiovascular endpoints.15 Patients not meeting such endpoints were classed as having a non-cardiovascular problem. A second cardiologist from the group conducted a blind review of all ACS cases and 10% of non-ACS cases. In cases of disagreement, endpoints were agreed on by consensus by the two cardiologists involved in endpoint adjudication and one emergency physician. This was achieved for all endpoints.
In addition to sampling for routine clinical care, blood was drawn on presentation and 2 hours later. Samples were later tested with the ARCHITECT High Sensitive STAT Troponin-I assay (Abbott Laboratories, Abbott Park, Illinois, USA). Laboratory technicians were blinded to patient data. The hsTnI assay has a 99th percentile concentration of 26.2 ng/L with a corresponding co-efficient of variation of <5% and a limit of detection (LoD) of 1.2 ng/L.16
Cost prediction model
As described previously,17 individual cost data were extracted from hospital administration records and adjusted for inflation to 2011 A$. To use a consistent cost matrix across all strategies, a prediction model was developed in four steps. First, we analysed the data and predefined exclusion criteria (see online supplementary table S1). Patients who received coronary bypass surgery were excluded because they were transferred to another hospital for surgery with no available outcome data and unknown accuracy of cost information. Cases with inconsistent or missing costs were excluded. Patients with a hospital length of stay (LOS) greater than 12 days were excluded to reduce bias from non-cardiac stays. Second, we considered key activities for evaluating an ACS in a generalised Box-Cox transformed model. Third, we dropped non-significant variables (second troponin, p=0.9; stress echocardiography, p=0.6) from the predictor variables, checked for relevant multicollinearity between variables and excluded cases that showed extreme discrepancies to the predicted results (n=4; see online supplementary table S1). Fourth, we run the final analysis that led to the cost prediction model and the 95% CIs for each predictor. The final model was based on data from 891 individuals. The following predictors were used: ED time, inpatient time, performed activities (exercise stress test, myocardial perfusion scan, CT coronary angiography, echocardiography and angiography), admission to short-stay unit (SSU) or admission to an inpatient ward. More information is given in the supplement (online supplementary eMethods).
bmjopen-2016-013653supp001.pdf (846.3KB, pdf)
Health economic model
We developed a microsimulation cost-effectiveness model that compared six assessment strategies (table 1). The standard of care was based on a protocol using cardiac troponin I (cTnI) at baseline and 6 hours after arrival (strategy 1). All other strategies used hsTnI at presentation and 2 hours. Strategy 2 (termed hsTnI) was the same as standard care except that a 2-hour high-sensitivity troponin was used rather than a 6-hour sensitive troponin. Strategy 3 (hsTnI+LoD) also used a 2-hour hsTnI, but allowed a patient to be directly ruled out on admission with no further work-up if their baseline hsTnI was below the assay’s LoD. Strategy 4 (hsTnI+ADP) used baseline and 2-hour hsTnI but enabled patients to be directly ruled out with no further work-up using the modified ADAPT accelerated diagnostic protocol (ADP). That is, patients could be ruled out if their Thrombolysis In Myocardial Infarction (TIMI) risk score was ≤1, their baseline and 2-hour troponin were below the diagnostic cut-off and their presentation ECG was non-ischaemic. Strategy 5 (hsTnI+LoD+ADP) was a combination of strategies 3 and 4 in that patients could be ruled out if their baseline hsTnI was below the LoD or if they met the criteria according to the modified ADAPT ADP. Finally, strategy 6 (hsTnI+LoD+ADP+direct rule-in) employed the same rule-out criteria as strategy 5, but also enabled patients with hsTnI at presentation >52 ng/L to be directly ruled in and admitted for ACS management (strategy 6).18
Table 1.
Assessment strategies evaluated in the model
| No | Strategy | Troponin assay | Protocol (hours) | Diagnostic cut-off* | Dynamic cut-off† | Direct rule-in‡ | Direct rule-out§ | Accelerated rule out¶ | Reference |
| 1 | Standard | cTnI | 0/6 | >40.0 | delta<10 | No | No | No | Standard care |
| 2 | hsTnI | hsTnI | 0/2 | >26.2 | delta<2 | No | No | No | 9 11 |
| 3 | hsTnI+LoD | hsTnI | 0/2 | >26.2 | delta<2 | No | Yes | No | 9 12 |
| 4 | hsTnI+ADP | hsTnI | 0/2 | >26.2 | delta<2 | No | No | Yes | 4 9 |
| 5 | hsTnI+LoD+ADP | hsTnI | 0/2 | >26.2 | delta<2 | No | Yes | Yes | 4 9 12 |
| 6 | hsTnI+LoD+ADP +direct rule-in |
hsTnI | 0/2 | >26.2 | delta<2 | Yes | Yes | Yes | 4 9 12 18 |
A troponin value greater than the diagnostic cut-off was considered as elevated.
†A delta between troponin values at different time points of <10 ng/L (cTnI) or 2 ng/L (hsTnI) was used to distinguish and rule-out a rise and/or fall in troponin associated with acute cardiac conditions.
‡Direct rule-in of individuals with a hsTnI value at baseline above 52 ng/L.
§Direct rule-out of individuals with a hsTnI value at baseline below the LoD of 1.2 ng/L.
¶Referring to the modified ADAPT accelerated diagnostic protocol (ADP). Accelerated rule-out applied to individuals with hsTnI values at 0 and 2 hours below the diagnostic cut-off and a TIMI risk score ≤1.
All values in ng/L.
ADAPT, Accelerated Diagnostic protocol to Assess Patients with chest pain symptoms using contemporary Troponin as the only biomarker; cTnI, sensitive cardiac troponin I; hsTnI, high-sensitivity cardiac troponin I; LoD, limit of detection; TIMI, Thrombolysis In Myocardial Infarction.
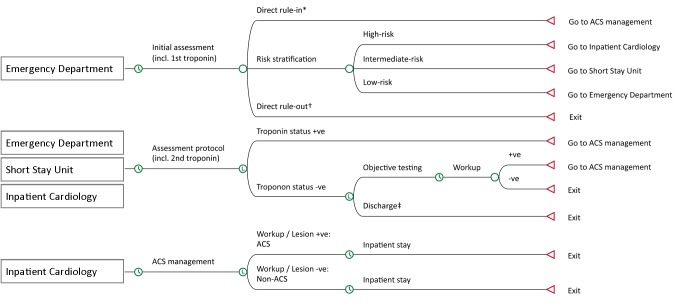
The model structure and the evaluation pathway are described in figure 1 and online supplementary figure S1, respectively. Individuals entering the model were stratified in the ED based on individual characteristics, first ECG and baseline troponin. Patients classified as high risk were admitted to inpatient cardiology. Low-risk patients were kept in the ED to await final assessment. Intermediate-risk patients were referred to the SSU for further cardiac work-up. Patients referred to the SSU or inpatient ward were counted as admitted.
Figure 1.
Basic model structure. Troponin statuses according to online supplementary table S2. *In strategy 6: if hsTnI at baseline ≥52 ng/L.†In strategies 3, 5 and 6: if hsTnI at baseline ≤1.2 ng/L (limit of detection). ‡In strategies 4, 5 and 6: if hsTnI values at baseline and 2 hours are below the diagnostic cut-off of 26.2 ng/L, and TIMI risk score ≤1, according to the modified ADAPT accelerated diagnostic protocol. hsTnI, high-sensitivity cardiac troponin I.
If the final troponin was performed later than 6.30 pm, patients stayed overnight. Total LOS comprised ED LOS, SSU LOS and inpatient stay. The maximum LOS was limited to 12 days to avoid bias in the effects from prolonged stays in patients with non-cardiac diagnoses. A 30-day follow-up event was assumed for individuals who were ruled out by the respective strategy, and who had a reported 30-day clinical outcome of ACS.
A minimum required dataset was defined for the cohort used in the model (see online supplementary table S3), and 219 patients with missing troponin values were excluded. Work-up, work-up duration and LOS were analysed from the model cohort and transformed into statistical distributions (see online supplementary table S4A–D). Patient attributes (age, sex, clinical characteristics, adjudicated diagnosis, ECG status and troponin values) were individually sampled from the model cohort by bootstrapping. This created a hypothetical cohort of 40 000 patients who followed the model for each of the strategies. Work-up and times for each patient were randomly sampled from distributions. Costs were estimated by considering attributes, work-up activities, work-up duration and LOS in the cost prediction model with coefficients individually sampled from the 95% CI of the respective predictor. The model followed a 30-day hospital perspective. Costs for the index event and follow-up were estimated from the cost prediction model.
Differences between strategies were expressed in terms of total hospital costs per patient and diagnostic accuracy. Diagnostic accuracy was defined as the percentage of correctly diagnosed patients compared with the final adjudicated diagnosis. In addition, LOS, referral rates, admission rates and overnight stays were evaluated. We conducted one-way and probabilistic sensitivity analyses to test the robustness of the microsimulation results. Model structure, parameters and assumptions are described in detail in the supplement.
Patient involvement
No patients were involved in setting the research question or the outcome measures, nor were they involved in developing plans for design or implementation of the study. No patients were asked to advise on interpretation or writing up of results. Patients were asked whether they wished to receive a summary of these results. These individuals were posted a lay summary of the results.
Results
Cost prediction and model validation
Characteristics of 719 patients meeting the minimum required dataset for the model and of the generated cohort of 40 000 patients are described in the supplement (see online supplementary table S5). The cost prediction model showed excellent regression quality (R-square 88.3%; see online supplementary table S6). The model was validated for the standard strategy against actual statistics with good prediction accuracy for all patients (p value vs actual costs: 0.723) as well as for low, intermediate and high-risk patients (p=0.946, 0.256, 0.761, respectively; table 2, see online supplementary figure S2A-B).
Table 2.
Comparison of cost data and model validation.
| Total costs, $ | Item | Cullen 20157 | Model cohort* | Model prediction† | Prediction versus cohort (p value) |
| All | n (%) | 926 (100%) | 719 (100%) | 719 (100%) | |
| Mean cost (95% CI) | 5272 (4835 to 5708) | 5303 (4796 to 5810) | 5437 (4897 to 5977) | 0.72 | |
| Median cost (25th to 75th percentile) | 2433 (1458 to 6778) | 2497 (1449 to 6663) | 2169 (1747 to 6384) | ||
| Low risk | n (%) | 9 (1.0%) | 9 (1.3%) | 9 (1.3%) | |
| Mean cost (95% CI) | 2040 (1306 to 2774) | 2040 (1125 to 2955) | 2010 (1559 to 2460) | 0.95 | |
| Median cost (25th to 75th percentile) | 1530 (1298 to 3050) | 1530 (1080 to 3359) | 1907 (1569 to 2438) | ||
| Intermediate risk | n (%) | 580 (62.6%) | 468 (65.1%) | 468 (65.1%) | |
| Mean cost (95% CI) | 3304 (2963 to 3644) | 3413 (3050 to 3775) | 3755 (3288 to 4223) | 0.26 | |
| Median cost (25th to 75th percentile) | 1849 (1376 to 3570) | 1925 (1389 to 3628) | 1946 (1668 to 3270) | ||
| High risk | n (%) | 329 (35.5%) | 242 (33.7%) | 242 (33.7%) | |
| Mean cost (95% CI) | 8919 (7971 to 9867) | 9081 (7878 to 10 284) | 8816 (7593 to 10 040) | 0.76 | |
| Median cost (25th to 75th percentile) | 6452 (2650 to 11 829) | 6405 (2752 to 11 309) | 5566 (2355 to 11 130) |
All costs referred to inflated costs in A$.
*Excluded individuals not meeting the minimum required dataset for the model
†Excluded individuals with cost outliers, missing and inconsistent data.
Patient referral and management
During initial assessment, 1.3% of patients were classified as low risk and managed in the ED (see online supplementary table S7). 6.1% of patients met the criteria for a direct rule-out (baseline hsTnI below the LoD) and were reclassified as low risk. The modified ADAPT ADP was effective for 49% of patients and reclassified 75% of intermediate-risk patients to low risk. The direct rule-in criteria (baseline hsTnI>52 ng/L) applied to 7.2% of patients.
Strategies considering LoD avoided SSU admissions for 4.9% of patients (−7.5% vs standard care, table 3). The number of ward admissions did not change with hsTnI alone. Using the LoD, ADP or a combination of both resulted in a stepwise and significant reduction of the ward admission rate from 49.6% to 37.1% (−25%; table 3).
Table 3.
Main model outcomes of different troponin-supported assessment strategies
| Indicator | Strategy 1 (standard) | Strategy 2 (hsTnI) |
Strategy 3 (hsTnI+LoD) |
Strategy 4 (hsTnI+ADP) |
Strategy 5 (hsTnI+LoD+ADP) |
Strategy 6 (hsTnI+LoD+ADP+ direct rule- in) |
|
| Short-stay unit admissions*, % | Mean (95% CI) | 65.3 (64.8 to 65.7) | 65.3 (64.8 to 65.7) | 60.4 (59.9 to 60.8) | 65.3 (64.8 to 65.7) | 60.4 (59.9 to 60.8) | 60.4 (59.9 to 60.8) |
| Incremental† (p value) | 0.0 (1.00) | −4.9 (<0.001) | 4.9 (<0.001) | −4.9 (<0.001) | 0.0 (1.00) | ||
| Ward admissions*, % | Mean (95% CI) | 49.7 (49.2 to 50.2) | 49.6 (49.1 to 50.1) | 47.4 (46.9 to 47.9) | 38.4 (37.9 to 38.9) | 37.1 (36.6 to 37.6) | 37.1 (36.6 to 37.6) |
| Incremental† (p value) | −0.1 (0.81) | −2.3 (<0.001) | −9.0 (<0.001) | −1.3 (<0.001) | 0.0 (1.00) | ||
| Overnight stays, % | Mean (95% CI) | 60.3 (59.8 to 60.8) | 42.0 (41.5 to 42.5) | 39.8 (39.3 to 40.3) | 24.4 (24.0 to 24.8) | 23.9 (23.5 to 24.3) | 24.1 (23.7 to 24.5) |
| Incremental† (p value) | −18.3 (<0.001) | −2.2 (<0.001) | −15.4 (<0.001) | −0.5 (0.08) | 0.2 (0.51) | ||
| Referral to ACS management, % | Mean (95% CI) | 32.4 (32.0–32.9) | 32.2 (31.8 to 32.7) | 30.9 (30.5 to 31.4) | 21.0 (20.6 to 21.4) | 20.7 (20.3 to 21.1) | 20.9 (20.5 to 21.3) |
| Incremental† (p value) | −0.2 (0.56) | −1.3 (<0.001) | −9.9 (<0.001) | −0.3 (0.26) | 0.3 (0.37) | ||
| Length of stay, hours | Mean (95% CI) | 34.0 (33.6 to 34.4) | 27.8 (27.4 to 28.2) | 26.8 (26.4 to 27.3) | 20.4 (20.0 to 20.9) | 20.1 (19.6 to 20.5) | 20.4 (19.9 to 20.8) |
| Incremental† (p value) | −6.2 (<0.001) | −1.0 (0.002) | −6.4 (<0.001) | −0.4 (0.23) | 0.3 (0.33) | ||
| Diagnostic accuracy (E), % | Mean (95% CI) | 90.0 (89.7 to 90.3) | 90.0 (89.7 to 90.3) | 90.5 (90.2 to 90.8) | 93.6 (93.4 to 93.8) | 93.7 (93.5 to 93.9) | 94 (93.7 to 94.2) |
| Incremental† (p value) | 0.0 (0.86) | 0.4 (0.04) | 3.1 (<0.001) | 0.1 (0.54) | 0.3 (0.13) | ||
| Index costs per patient, $ | Mean (95% CI) | 3029 (3001 to 3058) | 2923 (2894 to 2952) | 2846 (2816 to 2875) | 2621 (2592 to 2649) | 2568 (2539 to 2596) | 2582 (2553 to 2610) |
| Incremental† (p value) | −106 (<0.001) | −77 (<0.001) | −225 (<0.001) | −53 (0.01) | 14 (0.51) | ||
| Follow-up costs per patient, $ | Mean (95% CI) | 238 (225 to 250) | 211 (199 to 223) | 211 (199 to 223) | 213 (201 to 225) | 213 (201 to 225) | 195 (183 to 206) |
| Incremental† (p value) | −26 (0.003) | 0 (1.00) | 2 (0.82) | 0 (1.00) | −18 (0.03) | ||
| Total costs per patient (C), $ | Mean (95% CI) | 3267 (3236 to 3297) | 3134 (3103 to 3165) | 3057 (3026 to 3088) | 2834 (2804 to 2864) | 2781 (2751 to 2811) | 2776 (2746 to 2807) |
| Incremental† (p value) | −133 (<0.001) | −77 (0.001) | −223 (<0.001) | −53 (0.02) | −5 (0.83) | ||
All stated costs are in A$. (E) and (C) used as main measures of outcome.
*Patients could be admitted to the short-stay unit before being referred to inpatient ward; numbers may not sum up to 100%.
†Incremental values compared with next best alternative to the left.
ACS, acute coronary syndrome; ADP, accelerated diagnostic protocol; hsTnI, highly sensitive cardiac troponin I; LoD, limit of detection.
A 4-hour reduction in protocol time (cTnI vs hsTnI: mean 6.2 hours (range 5.0–10.0 hours) vs 2.3 hours (1.5–5.0 hours)) resulted in earlier management decisions (see online supplementary figure S3). Consequently, strategy 2 led to 30% fewer overnight stays compared with standard care (60.3% vs 42.0%, table 3). Incorporating additional rule-out to hsTnI options further streamlined patient assessment, decreasing overnight stays by up to 43%.
3.2% of patients with a negative or stable cTnI status had a positive hsTnI status indicative of an acute event (see online supplementary table S8). Conversely, 3.0% of patients had an acute cTnI finding but a negative or stable troponin status with hsTnI. In total, the number of referrals to ACS management based on an acute troponin finding did not differ if replacing cTnI with hsTnI (cTnI: 11.9%; hsTnI: 12.1%; p=0.549). Patients with negative or stable troponin conditions were admitted for ACS management and further work-up if such as an exercise stress test or myocardial perfusion scan led to positive findings, resulting in a referral rate of 32% (table 3). Strategies considering the LoD or ADP rules respectively led to 5% or 35% fewer patients referred for ACS management compared with standard care. Additional direct rule-in criteria (strategy 6 vs 5) did not identify more patients requiring ACS management but allowed for earlier cardiac intervention for 46.6% of patients with ACS.
LOS and costs
A significant reduction in LOS was observed if hsTnI replaced cTnI, with a mean saving of 6.2 hours (p<0.001, table 3). Applying LoD or ADP rules to hsTnI saved an additional stay of 1.0 and 7.4 hours, respectively. LOS times for patients with ACS were stable between strategies (see online supplementary table S9). However, applying hsTnI to standard care resulted in a significant reduction of LOS for patients with non-ACS. Substantially decreased 75th percentiles of the LOS for all strategies considering the ADP indicated its considerable streamlining effect. Details for ED and SSU times are given in the supplement (see online supplementary table S10A-B, figure S4A-C).
Significant cost reductions compared with standard care were found with all hsTnI strategies ($133–$491, p<0.001, table 3). This effect was caused by substantial cost reductions for patients with non-ACS. No difference between strategies was observed for patients with ACS (see online supplementary table S9). As stated in table 3, costs during the index stay and follow-up decreased for all hsTnI-supported strategies compared with standard care. The consideration of ADP and LoD alone, or in combination, in addition to hsTnI protocols resulted in further significant cost savings. Applying a direct rule-in strategy (strategy 6) to a combination of hsTnT+ADP+LoD did not result in significant overall costs benefits.
Patient outcome and cost-effectiveness
The introduction of hsTnI into standard care did not alter overall diagnostic accuracy (p=0.86, table 3, figure 2), but tend to increase the number of patients with a false-positive diagnosis of ACS (p=0.056; table 4). While all hsTnI-supported strategies avoided false-negative diagnoses compared with standard care, a statistically significant reduction of the false-positive rate was observed for all strategies using an ADP. Applying LoD and ADP to hsTnI reduced the number of false positives by 6% (p=0.015) and 52% (p<0.001), respectively, whereas no effect was observed on the false-negative rate (table 4).
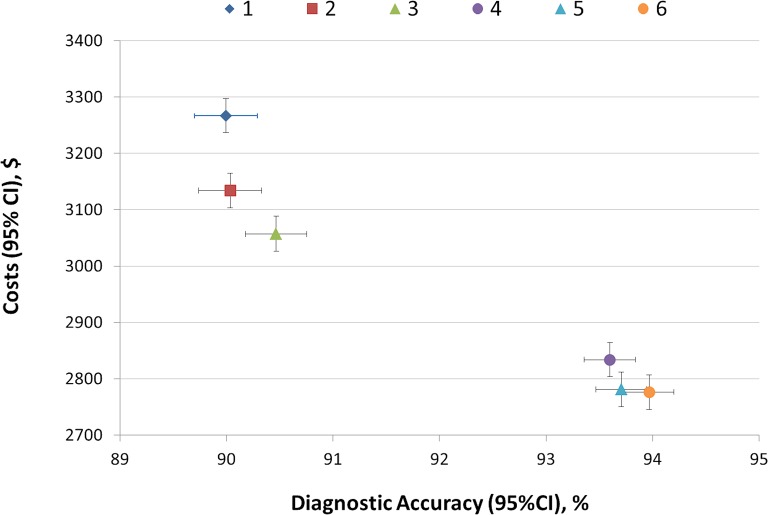
Figure 2.
Cost-effectiveness matrix. Strategy code: (1) standard, (2) hsTnI, (3) hsTnI+LoD, (4) hsTnI+ADP, (5) hsTnI+LoD+ADP, (6) hsTnI+LoD+ADP+direct rule-in. Costs include index costs and 30-day follow-up costs from the hospital perspective. Diagnostic accuracy refers to the adjudicated final diagnosis of acute coronary syndrome within 30 days after presentation to the emergency department. Each data point reflects the strategy specific mean value and 95% CI of 40 000 iterations. ADP, modified ADAPT accelerated diagnostic protocol; hsTnI, high-sensitivity troponin I; LoD, limit of detection.
Table 4.
False-negative and false-positive diagnosis of different assessment strategies
| Strategy | False positives, % | False negatives, % | ||||
| Mean | (95% CI) | p Value | Mean | (95% CI) | p Value | |
| (1) Standard | 6.6 | (6.4 to 6.9) | 3.4 | (3.2 to 3.6) | ||
| (2) hsTnI | 7.0 | (6.7 to 7.2) | 0.06* | 3.0 | (2.8 to 3.2) | 0.002* |
| (3) hsTnI+LoD | 6.5 | (6.3 to 6.8) | 0.62*; 0.02† | 3.0 | (2.8 to 3.2) | 0.002*; 1.00† |
| (4) hsTnI+ ADP | 3.4 | (3.2 to 3.5) | <0.001*,† | 3.0 | (2.9 to 3.2) | 0.005*; 0.84† |
| (5) hsTnI+LoD+ADP | 3.3 | (3.1 to 3.4) | <0.001*,† | 3.0 | (2.9 to 3.2) | 0.005*; 0.84† |
| (6) hsTnI+LoD+ADP+direct rule-in | 3.3 | (3.1 to 3.4) | <0.001*,† | 2.8 | (2.6 to 2.9) | <0.001*; 0.05† |
False positives: Number of patients diagnosed with ACS and a 30 days adjudicated diagnosis of non-ACS.
False negatives: Number of patients not diagnosed with ACS and a 30 days adjudicated diagnosis of ACS.
*p Value versus strategy 1 (standard care)
†p Value versus strategy 2 (hsTnI)
ACS, acute coronary syndrome; ADP, modified ADAPT accelerated diagnostic protocol; hsTnI, high-sensitivity troponin I; LoD, limit of detection.
Strategy 5 (a protocol using hsTnI, ADP and LoD) was found to be the dominant strategy in the study, providing better accuracy at lower costs (figure 2).19 Switching from standard care to strategy 5 saved $486 per patient (p<0.001) and increased the diagnostic accuracy from 90.0% to 94.0% (p<0.001).
Conducting multiple runs in a probabilistic sensitivity analysis revealed consistent benefits confirming the robustness of microsimulation results (see online supplementary figure S5; table S11). hsTnI demonstrated equal or better diagnostic accuracy compared with cTnI in 79% of runs, with a stable average cost saving per patient ranging from $113 to $147. The hsTnI strategy helped to manage 82.6% of individuals at lower costs compared with standard care; 10.2% or 7.1% of patients were treated at equal or higher costs, respectively. More results from testing the model are given in the supplement (see online supplementary figures S6–8).
Discussion
The cost-effectiveness of incorporating hsTnI into management protocols for patients presenting to the ED with chest pain has received increasing attention. HsTnI has been suggested to generate substantial benefits in the ED. ADPs have been found to reduce the average ED LOS in low-risk patients while health outcomes were maintained.5 11 To the best of our knowledge, this is the first study evaluating health economic implications of several hsTnI-enabled assessment algorithms in the ED from a hospital perspective, thus complementing previous research that followed lifetime effects from a health systems perspective.20–24
Complex management algorithms that are based on individual patient attributes, plus the heterogeneity of the ED population, require an individual-level modelling design.25 This allows for more realistic comparisons of different settings, assessment strategies or risk stratification rules. As opposed to other evaluations, costs and all management assumptions in this study were based on actual and individual patient information of a single trial-based cohort. The sampling strategy created a wide spectrum reflecting population heterogeneity and common variation in clinical practice (online supplementary figure S9).26 The clinical picture and additional information from objective testing were also considered in the simulation. We believe that this set the foundation for a consistent evaluation of the benefits that would accrue on the hospital level from implementing hsTnI-enabled algorithms.
We developed a cost prediction model for patients with chest pain presenting to ED, and we conducted a patient-level economic analysis for comparing different hsTnI-enabled algorithms, validated against standard care. The analysis demonstrated that the implementation of hsTnI substantially reduced LOS and costs for patients enrolled in the chest pain pathway compared with standard care. Such benefits occurred without reducing diagnostic accuracy. Moreover, the introduction of hsTnI allows for combining additional validated management rules (LoD, ADP). The overall organisational benefits of the dominant strategy (strategy 5) compared with standard care were caused by two effects: a substantial time reduction in protocol time and significantly improved stratification efficiency.
The significant decrease in overnight stays resulted in downstream effects of accelerated protocols on patient management. A 4-hour reduction in protocol time led to a 6.2-hour saving in LOS. By using the ADP, the timeliness of the second hsTnI result freed an additional 7.4 hours per patient. This strategy saved around 60% of overnights stays and 15% of costs compared with standard care.
In line with the definition for a high-sensitive troponin assay,16 measurable concentrations above the LoD were found for 94% of patients with non-ACS; only 6% of individuals were eligible for a direct rule-out considering the LoD criteria. This proportion appeared to be modest compared with the ADP that captured almost 50% of patients. Nevertheless, switching from strategy 4 (hsTnI+ADP) to strategy 5 (hsTnI+ADP+LOD) resulted in a significant reduction in the number of admissions to the SSU and wards. This was caused by the fact that the LoD rule moved 4.7% of patients from an accelerated rule-out after the second troponin (ADP) to a direct rule-out after the baseline troponin (LoD). In addition, the strategy including LOD classified 1.4% of patients, who were not captured by the ADP, as eligible for a direct rule-out. As a result, total costs were significantly reduced for strategy 5 compared with strategy 4 (p=0.02). The combined strategy of using hsTnI and LoD within the ADP helped to avoid 7.5% of SSU admissions and 25% of unnecessary inpatient ward admissions.
A false-positive troponin status can result in unnecessary referrals. In our study, 12.1% of individuals were categorised with an hsTnI status indicative for an acute event (see online supplementary table S8). Thirty-two per cent of individuals were referred for ACS management; this number was not different between strategy 1 and 2. Most of the referrals were based on a negative troponin finding followed by a positive cardiac work-up. Although we considered a conservative criteria with an absolute delta change between serial hsTnI tests of 2 ng/L, an increase in total referrals for ACS management could not be found. However, a tendency for an increased number of patients with a false-positive diagnosis of ACS was observed. It is however important to note that costs accrued from such interventions were considered in the analysis.
Strategy 6 (including a direct rule-in) did not significantly differ from strategy 5 in terms of costs and diagnostic accuracy. All patients meeting the criteria of a highly elevated baseline hsTnI (≥52 mg/L) were classified as high risk and admitted to inpatient cardiology by all other strategies. Therefore, strategy 6 did not result in a change in admission rates. However, the key value of strategy 6 was the immediate referral to cardiology: 46.6% of patients finally diagnosed with ACS would receive earlier cardiac intervention. Given the fact that all patients in the underlying observational study were managed by standard care, data on potential outcome effects of an earlier cardiac treatment were not available, and thus not captured in the health economic evaluation.
Some limitations deserve attention. The analysis was based on a single-centre cohort, which may limit the generalisability of the findings. Given the nature of a trial-based individual-level simulation, patient attributes were limited to the actual cohort; for example, the impact of variation in ACS prevalence could not be tested in a sensitivity analysis. Follow-up was limited to 30 days. Events happening after 30 days were not considered but may have an impact on the number of false-positive diagnosis. Troponin results must be interpreted in concert with clinical presentation, ECG changes and other available information. Diagnostic accuracy in this study refers to results of the complete assessment pathway consisting of troponin results, ECG and cardiac work-up. In an approach to emphasise safety, we used a conservative dynamic cut-off between serial troponin tests. The impact of different absolute or relative changes was not evaluated. Age or gender-specific troponin reference values may further improve the diagnostic accuracy but were not considered.
Management and cost data extracted from administrative databases may have some inaccuracies. Each of the 719 individuals from the cohort was run through the model on average 55 times with consistent characteristics, but varied in terms of protocol, treatment times, LOS, optional work-up decisions and accrued costs. Thus, the generated cohort of 40 000 individuals reflected heterogeneity in patient management and addressed some of the uncertainty (see online supplementary figure S9). The referral of patients followed strict and standardised assumptions. Deviation from recommended pathways may occur probably due to individual preferences or logistic effects such as access block.27 Some of the potential flow issues were addressed by assuming a wide range in the initial assessment time (6–118 min). The predictors used in the cost model were limited to information about risk assessment and stratification; information about inpatient management other than inpatient time was not available. Patients with a long-term stay were excluded from the analysis to mitigate this potential risk of bias.
Economic implications from breaching specific ED targets or access blocks were not taken into account but may have a significant impact. Based on the findings of this study, it appears likely that considering such aspects would strengthen the results in favour of accelerated protocols. The model compared a sensitive troponin assay at 6 hours with highly sensitive assay at 2 hours. For the models not using the LoD, it is unclear whether a sensitive troponin taken at 2 hours would provide the same benefits outlined here with a highly sensitive assay. The cost prediction did not account for different costs of troponin assays. Compared with the magnitude of the difference between sensitive TnI and hsTnI strategies, this effect was regarded as negligible.
It should be noted that exploiting the value of hsTnI fully relies on the appropriateness of testing and the implementation of adequate protocols.
Conclusion
This trial-based economic modelling study sought to evaluate the impact of different hsTnI protocols on direct costs and diagnostic accuracy compared with standard care. We found that ED assessment strategies using hsTnI are very likely to be cost-effective and provide cost savings on a hospital level when compared with sensitive TnI protocols for patients presenting with symptoms consistent with ACS. This is mainly due to a positive effect on the majority of patients not diagnosed with ACS. In particular, hsTnI-enabled algorithms considering additional rule-out criteria (LoD, ADP) are expected to improve the accuracy of both referral to inpatient wards or safe discharge as appropriate. Implementation of these protocols would provide direct benefits for the hospital in terms of reduced admission rates, avoided overnight stays and improvements in time-based ED performance measures, thereby contributing to streamlined ED processes, more efficient use of resources and overall cost savings.
bmjopen-2016-013653supp002.pdf (37.4KB, pdf)
Supplementary Material
Footnotes
Contributors: All authors had full access to the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis. JHG, LC and WAP led the clinical study design as part of the Asia-Pacific Evaluation of Chest Pain Trial. JHG extracted the dataset required for the modelling study. PJ developed the health economic model and run the analysis. Model design and assumptions were reviewed by all authors. All authors contributed in the interpretation of results, writing the manuscript and critically reviewing each draft of the manuscript. The final version was approved by all authors. The study was supervised by LC.
Funding: Compilation of the dataset for the health economic model was funded by Abbott. Funding for data collection was provided by the Queensland Emergency Medicine Research Foundation (QEMRF). Both Abbott and QEMRF had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; or the decision to submit the manuscript for publication
Disclaimer: The lead author affirms that the manuscript is an honest, accurate and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Competing interests: All authors completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. PJ is a full-time employee of Abbott Diagnostics. JHG has received funding from the Queensland Emergency Medicine Research Foundation. WAP has received funding from the Queensland Emergency Medicine Research Foundation, Abbott Diagnostics, Roche, Alere and Beckmann Coulter for research on diagnostic protocols; and honoraria, travel expenses and consultancy fees from Abbott, AstraZeneca, Hospira and Sanofi-Aventis. LC has received funding from the Queensland Emergency Medical Research Foundation for chest pain clinical trials from Abbott Diagnostics, Roche, Alere, Siemens and Radiometer Pacific for clinical trials; and from Alere, Boehringer-Ingelheim, Pfizer, AstraZeneca, Abbott Diagnostics and Radiometer Pacific for speaking and education
Ethics approval: Royal Brisbane and Women's Hospital Human Research Ethics Committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Statistical code is available from the lead author.
References
- 1. Centers for Disease Control and Prevention (Internet). National Hospital Ambulatory Medical Care Survey: 2011Emergency department summary tables 2011. http://www.cdc.gov/nchs/data/ahcd/nhamcs_emergency/2011_ed_web_tables.pdf (accessed 12 Feb 2016).
- 2. Australian Institute of Health and Welfare. Emergency department care 2014–15: Australian hospital statistics. Canberra: AIHW, 2015. [Google Scholar]
- 3. Than M, Cullen L, Aldous S, et al. 2-Hour accelerated diagnostic protocol to assess patients with chest pain symptoms using contemporary troponins as the only biomarker: the ADAPT trial. J Am Coll Cardiol 2012;59:2091–8. 10.1016/j.jacc.2012.02.035 [DOI] [PubMed] [Google Scholar]
- 4. Cullen L, Mueller C, Parsonage WA, et al. Validation of high-sensitivity troponin I in a 2-hour diagnostic strategy to assess 30-day outcomes in emergency department patients with possible acute coronary syndrome. J Am Coll Cardiol 2013;62:1242–9. 10.1016/j.jacc.2013.02.078 [DOI] [PubMed] [Google Scholar]
- 5. George T, Ashover S, Cullen L, et al. Introduction of an accelerated diagnostic protocol in the assessment of emergency department patients with possible acute coronary syndrome: the Nambour Short Low-Intermediate chest pain project. Emerg Med Australas 2013;25:340–4. 10.1111/1742-6723.12091 [DOI] [PubMed] [Google Scholar]
- 6. Reichlin T, Cullen L, Parsonage WA, et al. Two-hour algorithm for triage toward rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T. Am J Med 2015;128:369–79.e4. 10.1016/j.amjmed.2014.10.032 [DOI] [PubMed] [Google Scholar]
- 7. Reichlin T, Schindler C, Drexler B, et al. One-hour rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T. Arch Intern Med 2012;172:1211–8. 10.1001/archinternmed.2012.3698 [DOI] [PubMed] [Google Scholar]
- 8. Reichlin T, Twerenbold R, Wildi K, et al. Prospective validation of a 1-hour algorithm to rule-out and rule-in acute myocardial infarction using a high-sensitivity cardiac troponin T assay. CMAJ 2015;187:E243–E252. 10.1503/cmaj.141349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rubini Gimenez M, Twerenbold R, Jaeger C, et al. One-hour rule-in and rule-out of acute myocardial infarction using high-sensitivity cardiac troponin I. Am J Med 2015;128:861–70. 10.1016/j.amjmed.2015.01.046 [DOI] [PubMed] [Google Scholar]
- 10. Tanglay Y, Twerenbold R, Lee G, et al. Incremental value of a single high-sensitivity cardiac troponin I measurement to rule out myocardial ischemia. Am J Med 2015;128:638–46. 10.1016/j.amjmed.2015.01.009 [DOI] [PubMed] [Google Scholar]
- 11. Cullen L, Aldous S, Than M, et al. Comparison of high sensitivity troponin T and I assays in the diagnosis of non-ST elevation acute myocardial infarction in emergency patients with chest pain. Clin Biochem 2014;47:321–6. 10.1016/j.clinbiochem.2013.11.019 [DOI] [PubMed] [Google Scholar]
- 12. Rubini Giménez M, Hoeller R, Reichlin T, et al. Rapid rule out of acute myocardial infarction using undetectable levels of high-sensitivity cardiac troponin. Int J Cardiol 2013;168:3896–901. 10.1016/j.ijcard.2013.06.049 [DOI] [PubMed] [Google Scholar]
- 13. Luepker RV, Apple FS, Christenson RH, et al. Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA council on epidemiology and prevention; AHA statistics committee; World heart federation council on epidemiology and prevention; the European society of cardiology working group on epidemiology and prevention; Centers for disease control and prevention; and the national heart, lung, and blood institute. Circulation 2003;108:2543–9. 10.1161/01.CIR.0000100560.46946.EA [DOI] [PubMed] [Google Scholar]
- 14. Chew DP, Aroney CN, Aylward PE, et al. 2011 addendum to the national heart foundation of Australia/Cardiac society of Australia and New Zealand guidelines for the management of acute coronary syndromes (ACS) 2006. Heart Lung Circ 2011;20:487–502. [DOI] [PubMed] [Google Scholar]
- 15. Cullen L, Than M, Brown AF, et al. Comprehensive standardized data definitions for acute coronary syndrome research in emergency departments in Australasia. Emerg Med Australas 2010;22:35–55. 10.1111/j.1742-6723.2010.01256.x [DOI] [PubMed] [Google Scholar]
- 16. Apple FS, Collinson PO. Analytical characteristics of high-sensitivity cardiac troponin assays. Clin Chem 2012;58:54–61. 10.1373/clinchem.2011.165795 [DOI] [PubMed] [Google Scholar]
- 17. Cullen L, Greenslade J, Merollini K, et al. Cost and outcomes of assessing patients with chest pain in an australian emergency department. Med J Aust 2015;202:427–32. 10.5694/mja14.00472 [DOI] [PubMed] [Google Scholar]
- 18. Roffi M, Patrono C, Collet JP, et al. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: task force for the management of acute coronary syndromes in patients presenting without persistent ST-Segment elevation of the European society of cardiology (ESC). Eur Heart J 2016;37:267–315. 10.1093/eurheartj/ehv320 [DOI] [PubMed] [Google Scholar]
- 19. Drummond M, Sculpher MJ, Torrance GW, et al. Methods for the economic evaluation of health care programmes. New York: Oxford University Press, 2005. [Google Scholar]
- 20. Thokala P, Goodacre SW, Collinson PO, et al. Cost-effectiveness of presentation versus delayed troponin testing for acute myocardial infarction. Heart 2012;98:1498–503. 10.1136/heartjnl-2012-302188 [DOI] [PubMed] [Google Scholar]
- 21. Goodacre S, Thokala P, Carroll C, et al. Systematic review, meta-analysis and economic modelling of diagnostic strategies for suspected acute coronary syndrome. Health Technol Assess 2013;17:1–188. 10.3310/hta17010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Westwood M, van Asselt T, Ramaekers B, et al. High-sensitivity troponin assays for the early rule-out or diagnosis of acute myocardial infarction in people with acute chest pain: a systematic review and cost-effectiveness analysis. Health Technol Assess 2015;19:1–234. 10.3310/hta19440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vaidya A, Severens JL, Bongaerts BW, et al. High-sensitive troponin T assay for the diagnosis of acute myocardial infarction: an economic evaluation. BMC Cardiovasc Disord 2014;14:77. 10.1186/1471-2261-14-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Canadian Agency for Drugs and Technologies in Health. High-Sensitivity cardiac troponin for the rapid diagnosis of acute coronary syndrome in the emergency department: a clinical and cost-effectiveness evaluation, 2013. https://www.cadth.ca/media/pdf/OP0511_Troponin_ScienceReport_e.pdf (accessed 12 Sep 2016). [PubMed]
- 25. Brennan A, Chick SE, Davies R. A taxonomy of model structures for economic evaluation of health technologies. Health Econ 2006;15:1295–310. 10.1002/hec.1148 [DOI] [PubMed] [Google Scholar]
- 26. Buisman LR, Rijnsburger AJ, den Hertog HM, et al. Clinical Practice variation needs to be considered in Cost-Effectiveness analyses: a case study of patients with a recent transient ischemic attack or Minor ischemic stroke. Appl Health Econ Health Policy 2016;14:67–75. 10.1007/s40258-015-0167-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pines JM, Hilton JA, Weber EJ, et al. International perspectives on emergency department crowding. Acad Emerg Med 2011;18:1358–70. 10.1111/j.1553-2712.2011.01235.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2016-013653supp001.pdf (846.3KB, pdf)
bmjopen-2016-013653supp002.pdf (37.4KB, pdf)