Summary
Background
Acute hepatitis C has variable modes of presentation and frequently results in chronic infection. Its optimal management has yet to be defined.
Aims
To establish natural history and complications of treatment of acute hepatitis C.
Methods
Data from all patients presenting with acute hepatitis C to the National Institutes of Health between 1994 and 2007 were reviewed.
Results
Twenty-five patients were identified. Symptoms were reported by 80% and jaundice by 40%. Aminotransferase levels and HCV RNA levels fluctuated greatly; 18% of patients were intermittently negative for HCV RNA. Five patients recovered spontaneously whereas 20 developed chronicity or received interferon-based therapy during the acute phase. Among 15 patients treated during the acute phase with peginterferon with or without ribavirin for 24 weeks, all became HCV RNA negative within 4 to 8 weeks, and all except two (HIV-positive) achieved a sustained virological response. Side effects (particularly psychiatric) were common and limited treatment in 30%.
Conclusion
Thus, among 25 patients with acute HCV infection, fluctuating illness was common and spontaneous recovery occurred in only 20%. Antiviral treatment with a 24-week course of peginterferon and ribavirin was highly effective but marked by frequent and severe side effects.
Keywords: Acute hepatitis, hepatitis C virus, interferon, peginterferon, ribavirin, natural history, side-effects, viral fluctuations, treatment
Introduction
Chronic infection with the hepatitis C virus (HCV) is now the leading cause of liver-related morbidity and mortality in the United States and accounts for an estimated 10,000 deaths yearly (1). In contrast, acute HCV infection has become uncommon, new cases having decreased markedly over the last 15 years to a currently historic low level (2). Nevertheless, cases of acute hepatitis C continue to occur and eventuate in chronic infection in 70% to 80% of cases. Among patients who develop chronic hepatitis C, between 20 and 30% develop cirrhosis over the subsequent two to three decades; and likely a higher proportion thereafter (1–6).
Clearance of HCV during the acute phase of infection is typically associated with appearance of a vigorous T cell response against multiple HCV epitopes; whereas evolution to chronicity is associated with poor T cell responses that are limited in scope and depth (7, 8). Jaundice and young age are clinical factors associated with an increased likelihood of clearance of HCV (9). In an individual case, however, there are no features that reliably predict recovery. Even serial testing for HCV RNA can be unreliable as levels of virus may fluctuate widely during the acute course and become transiently undetectable, only to be followed by its reappearance and persistence.
The high rate of chronicity of acute hepatitis C has led to studies of therapy. In a study from Germany, a 24-week course of standard alpha interferon monotherapy was reported to result in sustained viral clearance in 98% of persons treated during the acute phase of hepatitis C (10). This response rate was higher than would be expected to occur spontaneously and far higher than a similar regimen would achieve in chronic hepatitis C. Subsequent studies, however, have reported somewhat lower rates of response (71–94%) even using similar cohorts (9, 11–15).
Acute hepatitis C has been a focus of natural history and immunological studies at the Clinical Center of the National Institutes of Health during the last 15 years. Because of publications on the success of therapy of acute hepatitis C in 2001, subsequent patients were offered therapy during the acute phase of disease using the combination of peginterferon alfa-2a and ribavirin for 24 weeks. This manuscript describes the clinical course of 25 patients with acute hepatitis, some relevant virologic features, and responses to antiviral therapy.
Methods
Between 1994 and 2007, 25 patients with acute hepatitis C were evaluated and followed by the Liver Diseases Branch of the National Institute of Diabetes and Digestive and Kidney Diseases at the Clinical Center of the National Institutes of Health. Patients were enrolled in clinical research protocols that were approved by the NIDDK NIH Institute Review Board, and all patients gave written informed consent. Patients were treated with a “standard of care approach” and results were analyzed retrospectively. Results of immunological studies and virologic outcomes in a subset of 7 patients of this cohort have been published (16). The diagnosis of acute hepatitis C was based upon the detection of HCV RNA in serum and either: (1) documented anti-HCV seroconversion; (2) documented exposure to HCV followed by elevations in serum alanine aminotransferase (ALT) or aspartate aminotransferase (AST) to above 5 times the upper limit of the normal range (ULN) within the subsequent 6 months, or; (3) probable exposure to HCV followed by acute elevations in ALT or AST levels to above 10 times the ULN within the subsequent 6 months. In addition, patients were enrolled in this study only if they had no other obvious cause of acute liver disease (drug-induced liver injury, hepatitis A, hepatitis B, acute alcoholic hepatitis) and gave written informed consent.
All patients underwent an initial history and physical examination and had a battery of blood tests including routine liver tests (ALT, AST, alkaline phosphatase, albumin, direct and total bilirubin, and prothrombin time) as well as serological tests for acute hepatitis A (IgM anti-HAV) and hepatitis B (HBsAg and IgM anti-HBc). Patients were tested for HCV RNA by qualitative polymerase chain reaction (Cobas Amplicor™, Version 2.0, Roche Diagnostics: Branchburg NJ: lower limits of detection of 100 IU/ml) and for anti-HCV by ELISA (Abbott, North Chicago, IL). Selected samples were tested for HCV RNA levels using the Cobas Amplicor Hepatitis C Virus Monitor™ Test, Version 2.0, (Roche Diagnostics: Branchburg NJ: lower limit of detection 600 IU/ml). HCV genotyping was performed by hybridization (InnoLipa; Innogenetics, Ghent, Belgium). Other testing included complete blood counts, erythrocyte sedimentation rate, total immunoglobulin levels, a heterophile test, VDRL, routine urinalysis and abdominal ultrasound. Patients were then followed in the outpatient clinic at 2- to 4-week intervals until either 6 months after treatment ended, or having 3 negative tests for HCV RNA, and at 3- to 6-month intervals thereafter. On each occasion, symptoms of hepatitis were assessed using standardized questionnaires and visual analogue scales.
The time of documented or suspected exposure to HCV was used to calculate incubation period as well as time to seroconversion and recovery. In cases that patients did not recall the specific date of exposure, an approximation was made, based upon best recall.
Prior to 2001, antiviral therapy was not recommended until at least 6 months after exposure and documentation that chronic hepatitis C had been established. However, because therapy failed in a high proportion of chronically infected patients and because of reports of high success rates following treatment early in HCV infection, (10) treatment was subsequently recommended once HCV RNA was found present for at least 16 weeks after exposure. Patients who wanted to be treated earlier than recommended were allowed to begin therapy if HCV RNA was still detectable in serum. From 1994 to mid-2001, patients (n=5) were treated with standard interferon in a dose of 3 million units subcutaneously three times weekly with or without oral ribavirin (1000 mg daily if body weight < 75 kg, and 1200 mg daily if ≥ 75 kg) for 24 or 48 weeks. After mid-2001 and the availability of pegylated forms of interferon, patients (n=15) were treated with peginterferon (either alfa-2a, 180 μg weekly or alfa-2b, 1.5 μg/kg weekly) and ribavirin (1000 or 1200 mg daily) for 24 weeks only. Patients were followed for at least 24 weeks after completing treatment to document whether a sustained virological response (SVR) had been achieved.
Results
Baseline Characteristics (Table 1)
Table 1.
Baseline Characteristics
| Characteristics (N) |
Value (25) |
|---|---|
| Age* - years | 43 ± 13 |
| Female sex – no. | 16 (64%) |
| Icterus – no. | 10 (40%) |
| Race – no. | |
| • Caucasian | 17 (68%) |
| • African American | 6 (24%) |
| • Asian | 2 (8%) |
| Route of exposure – no. | |
| • Needle stick | 9 (36%) |
| • Sexual | 5 (20%) |
| • Occupationala | 3 (12%) |
| • Medical procedureb | 3 (12%) |
| • Injection drug use | 2 (8%) |
| • Household contactc | 2 (8%) |
| • Unknownd | 1 (4%) |
| HIV-HCV Co-infection – no. | 3 (12%) |
| Alanine Aminotransferasee – U/L | 882 ± 640 |
| HCV genotype – no. | |
| • 1 | 18 (72%) |
| • 2 | 1 (4%) |
| • 3 | 1 (4%) |
| • Untypeable | 5 (20%) |
Mean (range)
Occupational exposure: ENT surgeon, ER physician and Nurse.
Medical procedures consisted of dialysis, Cesarean section and rectal dilation.
Household contacts shared razors and their contacts were known to have hepatitis C with the same genotype. No sexual exposure and no history of any other exposure.
Mean ± Standard deviation
Between January 1994 and June 2007, 25 patients (16 females and 9 males) met the diagnostic criteria for acute hepatitis C and were followed. 17 patients were Caucasians (3 Hispanic in ethnicity and one with Native American parentage), 6 African Americans, and 2 Asians. The mean age at time of exposure was 43 years (range: 20–72 years). Genotype distribution included: 72% genotype 1, 4% genotype 2, and 4% genotype 3; in the remaining 20%, the genotype could not be determined. The route of infection is summarized and defined in Table 1; the presumed source of infection was needle-stick injury in 9 (36%), sexual exposure in 5 (20%), occupational exposure in 3 (12%), medical procedures in 3 (12%), razor sharing in 2 (8%), injection drug use in 2 (8%), and was unknown in 1 (4%) – a patient whose only reported parenteral exposure was a professional manicure 4 weeks before presentation. Time of exposure was determined with certainty in 9 patients (needle-stick exposure). Time of exposure was determined within a week in 8 patients. The remaining 8 patients time of exposure was approximated according to presentation and patient’s best recollection.
Most patients (n=20: 80%) were symptomatic and 10 patients (40%) were jaundiced. The most common symptoms were fatigue (68%), dark urine (60%), abdominal pain (60%), low grade fever and chills (44%), loss of appetite (40%), itching (36%), muscle aches (36%), mood disturbances (32%), joint aches (24%), dyspepsia (16%) and diarrhea, and confusion (8% each). The most common clinical sign was icterus, which was reported in 10 (40%) patients. Two patients developed acute liver failure marked by hepatic encephalopathy and ascites, but both recovered symptomatically and subsequently responded to antiviral therapy with clearance of HCV RNA. Acne was reported by 2 patients and maculopapular skin rash by 1. The average time from exposure to onset of signs or symptoms was 4 weeks (range: 2.5 to 8 weeks).
Laboratory testing showed ALT levels greater than 10 times the ULN in 17 patients (70%) and peak bilirubin levels above 2.5 mg/dL in 11 (44%). Prothrombin time elevations occurred in the 2 patients with encephalopathy and ascites. Anti-HCV seroconversion was documented in 20 patients (80%). The remaining 5 (20%) of the patients were anti-HCV positive at the time of presentation. The average time from exposure to seroconversion was 9 weeks (range: 6 to12 weeks). All patients tested positive for HCV RNA. The mean peak HCV RNA level was 5.3 × 106 copies/ml (range <600 to 27,8 ×106 copies/ml). During follow-up and while not on therapy, 4 of 22 patients (18%) who had frequent monitoring of HCV RNA levels were intermittently negative and 15 patients (68%) had greater than one log (ten-fold) fluctuation in viral levels. The calculated mean difference between the peak and nadir pre-treatment viral levels was 2.2 log10 copies/ml (p <0.01). Three patients had extreme fluctuations in viral levels with intermittent negativity (Figure 1). In most patients, fluctuations in viral levels were present only during the first 24 weeks after exposure, but these fluctuations continued beyond 24 weeks in at least one patient, and most others were started on treatment before 24 weeks.
Figure 1.
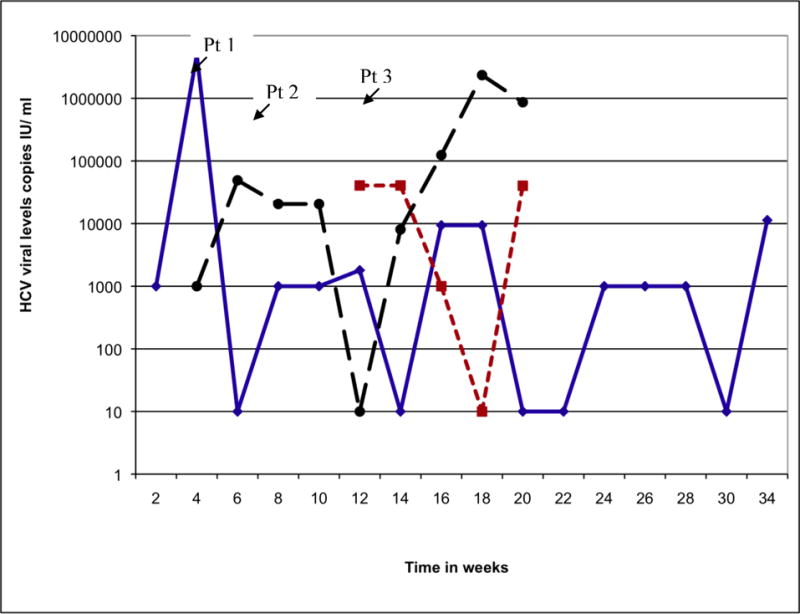
Extreme Fluctuations in Viral Levels in Three Patients Pre-Treatment. Levels below 100 IU/ml were reported as negative.
Outcomes
Five patients cleared HCV RNA spontaneously and remained HCV RNA-negative on multiple occasions thereafter (range 4 to 10) during 4, 13, 24, 31, and 42 months of follow up. One patient refused follow up beyond 4 months. The average time to spontaneous loss of HCV RNA was 19.6 weeks (range: 16–24 weeks). The remaining 20 patients (80%) appeared to be developing chronic infection and were eventually treated with an interferon-based regimen. Because of the evolving nature of therapy of hepatitis C, several regimens were used. Five patients received standard interferon alfa-2b (3 million units three times weekly). The initial two patients received interferon monotherapy; while the next three received interferon and ribavirin (1000 or 1200 mg daily). After 2001, patients were offered therapy with peginterferon (either alfa-2a or alfa-2b) and ribavirin. One patient with concurrent HIV infection received peginterferon monotherapy because of concerns over interactions of ribavirin with antiretroviral agents being used (zidovirine and didanosine) and the excellent results reported with interferon monotherapy. Of the 20 patients treated, all except three achieved an SVR and had normal serum ALT levels and no detectable HCV RNA when last seen (mean = 31.7 months, range = 9 to 79 months after stopping therapy). One patient counted as an SVR received interferon mono-therapy six months after exposure and relapsed when therapy was stopped, but had a long-term SVR in response to re-treatment with standard interferon and ribavirin. The three patients who did not achieve an SVR included one patient who was treated with standard interferon and ribavirin starting 8 months after exposure who never became HCV RNA negative during therapy and two other patients who were HIV-positive and became HCV RNA negative on peginterferon therapy but then had viral breakthrough and did not have a sustained response (one received peginterferon mono-therapy). Thus, the overall SVR rate was 85%. SVR rates were 83% (15/18) for patients with genotype 1, 83% (5/6) among African American patients, but only 33% (1/3) in HIV-positive subjects. One subject was both HIV positive and the single African American non-responder. Among the 12 HIV-negative patients treated during the acute phase of illness with the combination of pegylated or standard interferon and ribavirin for 24 weeks, all became HCV RNA negative within 1 to 8 weeks of initiating therapy (mean = 2.8 weeks) and the SVR rate was 100%.
Side-Effects (Table 2)
Table 2.
Adverse-effects on Therapy in Twenty Patients who Received Treatment
| Adverse-effects | N (%) |
|---|---|
|
| |
| Constitutional | 20 (100) |
| Fatigue | 19 (95) |
| Fever or chills | 17 (85) |
| Myalgias | 15 (75) |
| Insomnia | 12 (60) |
| Anorexia | 11 (55) |
| Alopecia | 8 (40) |
| Headache | 8 (40) |
| Night sweats | 6 (30) |
| 10% Weight loss | 4 (20) |
|
| |
| Psychiatric | 18 (90) |
| Irritability | 15 (75) |
| Depression/anxiety | 12 (60) |
| Despite SSRI prophylaxis | 4 (20) |
| Relapse of injection of illicit drug use | 1 (5) |
|
| |
| Hematologic | 12 (60) |
| Anemia | 10 (50) |
| Neutropenia | 8 (40) |
|
| |
| Autoimmune Diseases | 5 (25) |
| Papilitis | 2 (10) |
| Polymyalgia rheumatica | 1 (5) |
| Hypothyroidism | 1 (5) |
| Hyperthyroidism | 1 (5) |
|
| |
| Gastrointestinal | 13 (65) |
| Nausea | 9 (45) |
| Heartburn | 5 (25) |
| Right upper quadrant pain | 3 (15) |
| Pancreatitis | 1 (5) |
|
| |
| Dermatologic | 8 (40) |
| Itching | 7 (35) |
| Rash | 4 (20) |
| Infection | 3 (15) |
| Urinary tract infection | 2 (10) |
| Upper respiratory tract infection | 1 (5) |
| Otitis media | 1 (5) |
|
| |
| Early Termination of Treatment due to Adverse Events | 6 (30) |
No patient had an exacerbation of liver disease or worsening of serum ALT levels while on therapy. However, typical side effects of interferon and ribavirin were reported in virtually all treated patients and were problematic in many. Psychiatric side effects were particularly troublesome. Selective serotonin reuptake inhibitor (SSRI) prophylaxis was given to 35% of patients, and another 15% initiated SSRI therapy while on treatment in response to depression. One patient had a relapse of injection drug use on therapy. New onset of autoimmune disease occurred in 5 patients (25%); including two cases of papilitis, and one each of polymyalgia rheumatica, hyperthyroidism, and hypothyroidism. Three patients (15%) went on disability while on treatment. Therapy was discontinued early because of side effects in 6 patients (30%) (after 9 to 23 weeks), but all 6 had an SVR. Of the 20 patients treated, one had dose reduction of ribavirin from 1000mg to 800mg because of fatigue. Another patient had a dose reduction of peginterferon to 60μg weekly because of recurrent ear infection. Both tolerated the rest of their treatment without any further dose reduction and both had an SVR. A third patient had initially started at 3 million international units of interferon 3 times a week, but subsequently increased dose to 5 million units of interferon 3 times a week. This was then decreased back to 3 million units of interferon 3 times a week because of fatigue. This patient also had an SVR.
Discussion
Acute hepatitis C is now uncommon in the United States but still presents a challenge in diagnosis, assessment of prognosis, and therapy. The 25 patients seen were not representative of cases of acute hepatitis C occurring in the United States, in that the source of infection in the majority was medical occupation or needle stick accident. In contrast, in the general population, the major risk factor for acquisition of HCV infection is injection drug use, a risk factor identified in only two of the 25 cases described here. Despite this, the clinical course and outcome of cases did appear to be representative of acute hepatitis C. Spontaneous recovery occurred in only 20% of patients, although the rate of recovery could have been higher, because most patients were started on therapy during the acute phase of infection. This rate of 20% is similar to previously published reports (17). Almost half the cases were associated with jaundice and two were severe, fulfilling criteria for acute liver failure. Both of these patients developed mild encephalopathy, ascites and elevations in prothrombin time but did not progress to full hepatic coma and were never listed for liver transplantation. Both patients recovered clinically, but remained HCV RNA positive and were ultimately treated and had an SVR in follow-up. Thus, acute hepatitis C can be severe and protracted, but clinical recovery is common and the major medical concern is not the complications of acute disease, but rather the evolution to chronicity.
A striking finding in monitoring patients during this study was the fluctuating nature of the infection, with marked variation in levels of ALT and AST in association with marked changes in HCV RNA levels. Indeed, several patients had periods during which HCV RNA was undetectable, suggesting that they had recovered. During follow-up, however, HCV RNA and ALT elevations returned. Indeed, two patients were told that they had recovered and were found to be persistently HCV RNA positive only when they returned for routine follow up several months later. These findings are compatible with earlier studies of post-transfusion hepatitis C and indicate that monitoring of patients should continue for at least 6 months after exposure and that a single normal ALT value or absence of HCV RNA does not reliably indicate full recovery and eradication of virus.
In this series of patients, therapy of acute hepatitis C was highly effective when initiated early in the infection. The first five patients seen were given standard interferon alfa-2b, and for most of them, therapy was not initiated until they were documented to be HCV RNA positive for at least 6 months. Using this approach, however, one patient given interferon monotherapy was a non-responder and another had repeated viral breakthrough on interferon monotherapy but subsequently had an SVR after a 48-week course of combination therapy. The remaining three responded to interferon and ribavirin combination therapy but two required treatment for 48-weeks as recommended for chronic hepatitis C. After this experience and after publications reporting the success of therapy initiated during the acute phase of illness, patients were advised to start therapy with peginterferon and ribavirin if they remained HCV RNA positive for 16 weeks. Using this approach, 13 of 15 patients had an SVR in response to treatment; the two without an SVR had a transient response and breakthrough and concurrent HIV infection (one receiving peginterferon monotherapy). While the number of patients treated was small, these results suggest that HIV infection but not viral genotype or race may be factors associated with a lower rate of response. While uncontrolled, these results also indicate that the majority of patients with acute hepatitis C can be successfully treated.
Another striking finding in this study was the number and severity of side effects of antiviral therapy. Antiviral therapy usually resulted in rapid improvements in serum ALT levels and disappearance of detectable HCV RNA. However, virtually all patients had constitutional side effects and specific adverse events were problematic enough to lead to early discontinuation in 30% of patients. This proportion is much higher than what was seen in an acute hepatitis C 126-case prospective study where only 11% of patients discontinued therapy due to severe side effects (18). The frequency of side effects may reflect the focused approach in the presented case series to capturing adverse events and also the patient population, which were often medical personnel. Nevertheless, the severity of side effects is an important reason to embark on therapy only if necessary.
A further important consideration is when to initiate therapy. The decision to recommend waiting for 16 weeks after exposure was a compromise between wanting to avoid therapy of patients who might recover spontaneously and published data to initiate therapy before the disease becomes chronic. However, most patients did not accept this delay in treatment. A recent meta-analysis of acute hepatitis C SVR rates and timing of treatment initiation revealed that highest response rates were seen when treatment was started at 12 weeks of diagnosis (19). It should be noted that time of diagnosis is distinct from time of infection. Choosing to start therapy 16 weeks from point of infection may, in fact, be earlier than the 12 weeks presented in the meta-analysis study. Waiting 12 weeks after diagnosis may be later than the optimal time to initiate therapy. It seems to be that waiting until patients present clinical manifestations may be preferred. The question of how to standardize treatment time course still remains to be elucidated.
Chronicity in hepatitis C is generally defined by the presence of infection or detectable HCV RNA for at least 6 months. This definition is helpful but somewhat arbitrary. The transition from acute to chronic HCV infection most likely represents a change in the interaction between the immune system and the viral infection that does not necessarily occur exactly six months after onset of infection. In this regard, the striking fluctuations in ALT and HCV RNA levels may be a marker for the acute phase of illness, in that they are usually followed by a relatively stable levels of ALT and viral RNA during chronic infection. The cause of this variability in viral levels and disease activity during acute infection is unclear, but it appears to be associated with similar fluctuations in CD4+ and CD8+ T cell responses to HCV antigens (16) and thus may reflect active immunological response to virus infection and thus represent an ideal time to add antiviral treatment to help tip the balance in favor of viral clearance.
Because an SVR was achieved in virtually all patients who were treated within 6 months of exposure and did not have concurrent HIV infection, it is possible that a shorter course of therapy or use of lower doses of peginterferon and/or ribavirin might have been as effective. Recent studies from Egypt and Italy suggest that a 12-week course of peginterferon alone may be adequate, particularly if therapy is started early (11, 12). Indeed, in the current study, 30% of patients stopped therapy early because of side effects, yet still achieved an SVR. Also in support of an abbreviated course of treatment was the number and severity of side effects. However, the possible advantages of an abbreviated course of therapy must be balanced against the possible consequence of failure of therapy. Patients who fail to respond to treatment or relapse during acute infection may need to be re-treated once the disease is chronic, at which point therapy is likely to be less effective and require a longer courses.
Thus, experience in management of acute hepatitis C indicates that the disease can be severe and is likely to result in chronic infection. Initiation of a 24-week course of peginterferon and ribavirin can result in a high rate of ultimate recovery and sustained eradication of virus. Antiviral therapy, however, has problematic side effects and further studies are needed to define markers that will indicate which patients are unlikely to have a spontaneous clearance of virus and whether more abbreviated courses or lower doses of peginterferon and ribavirin can achieve similar high rates of response.
Acknowledgments
The patients are thanked for participating in the study. The assistance of the staff in the department of transfusion medicine, in particular June Germain, are much appreciated. Theresa Kennedy is thanked for expert blood drawing and shipping.
This research was supported by the intramural research program of the National Institute of Diabetes and Digestive and Kidney Diseases (Z01 DK054514-02 LDB) and the Clinical Center, National Institutes of Health, Bethesda, MD.
Abbreviations
- HCV
hepatitis C virus
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- ULN
upper limit of the normal range
- SVR
sustained virological response
- HIV
human immunodeficiency virus
- SSRI
selective serotonin reuptake inhibitors
Footnotes
Conflict of Interest: No conflicts of interest exist.
References
- 1.Kim WR. The burden of hepatitis C in the United States. Hepatology. 2002 Nov;36(5 Suppl 1):S30–4. doi: 10.1053/jhep.2002.36791. [DOI] [PubMed] [Google Scholar]
- 2.Liang TJ, Rehermann B, Seeff LB, Hoofnagle JH. Pathogenesis, natural history, treatment, and prevention of hepatitis C. Ann Intern Med. 2000 Feb 15;132(4):296–305. doi: 10.7326/0003-4819-132-4-200002150-00008. [DOI] [PubMed] [Google Scholar]
- 3.Grant WC, Jhaveri RR, McHutchison JG, Schulman KA, Kauf TL. Trends in health care resource use for hepatitis C virus infection in the United States. Hepatology. 2005 Dec;42(6):1406–13. doi: 10.1002/hep.20941. [DOI] [PubMed] [Google Scholar]
- 4.Seeff LB, Hoofnagle JH. Epidemiology of hepatocellular carcinoma in areas of low hepatitis B and hepatitis C endemicity. Oncogene. 2006 Jun 26;25(27):3771–7. doi: 10.1038/sj.onc.1209560. [DOI] [PubMed] [Google Scholar]
- 5.Seeff LB, Hoofnagle JH. National Institutes of Health Consensus Development Conference: management of hepatitis C: 2002. Hepatology. 2002 Nov;36(5 Suppl 1):S1–2. doi: 10.1053/jhep.2002.36992. [DOI] [PubMed] [Google Scholar]
- 6.Alter MJ. The epidemiology of acute and chronic hepatitis C. Clin Liver Dis. 1997 Nov;1(3):559–68. vi–vii. doi: 10.1016/s1089-3261(05)70321-4. [DOI] [PubMed] [Google Scholar]
- 7.Heller T, Rehermann B. Acute hepatitis C: a multifaceted disease. Semin Liver Dis. 2005 Feb;25(1):7–17. doi: 10.1055/s-2005-864778. [DOI] [PubMed] [Google Scholar]
- 8.Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol. 2005 Mar;5(3):215–29. doi: 10.1038/nri1573. [DOI] [PubMed] [Google Scholar]
- 9.Gerlach JT, Diepolder HM, Zachoval R, Gruener NH, Jung MC, Ulsenheimer A, et al. Acute hepatitis C: high rate of both spontaneous and treatment-induced viral clearance. Gastroenterology. 2003 Jul;125(1):80–8. doi: 10.1016/s0016-5085(03)00668-1. [DOI] [PubMed] [Google Scholar]
- 10.Jaeckel E, Cornberg M, Wedemeyer H, Santantonio T, Mayer J, Zankel M, et al. Treatment of acute hepatitis C with interferon alfa-2b. N Engl J Med. 2001 Nov 15;345(20):1452–7. doi: 10.1056/NEJMoa011232. [DOI] [PubMed] [Google Scholar]
- 11.Wiegand J, Buggisch P, Boecher W, Zeuzem S, Gelbmann CM, Berg T, et al. Early monotherapy with pegylated interferon alpha-2b for acute hepatitis C infection: the HEP-NET acute-HCV-II study. Hepatology. 2006 Feb;43(2):250–6. doi: 10.1002/hep.21043. [DOI] [PubMed] [Google Scholar]
- 12.Kamal SM, Fouly AE, Kamel RR, Hockenjos B, Al Tawil A, Khalifa KE, et al. Peginterferon alfa-2b therapy in acute hepatitis C: impact of onset of therapy on sustained virologic response. Gastroenterology. 2006 Mar;130(3):632–8. doi: 10.1053/j.gastro.2006.01.034. [DOI] [PubMed] [Google Scholar]
- 13.Kamal SM, Ismail A, Graham CS, He Q, Rasenack JW, Peters T, et al. Pegylated interferon alpha therapy in acute hepatitis C: relation to hepatitis C virus-specific T cell response kinetics. Hepatology. 2004 Jun;39(6):1721–31. doi: 10.1002/hep.20266. [DOI] [PubMed] [Google Scholar]
- 14.Corey KE, Ross AS, Wurcel A, Schulze Zur Wiesch J, Kim AY, Lauer GM, et al. Outcomes and treatment of acute hepatitis C virus infection in a United States population. Clin Gastroenterol Hepatol. 2006 Oct;4(10):1278–82. doi: 10.1016/j.cgh.2006.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nomura H, Sou S, Tanimoto H, Nagahama T, Kimura Y, Hayashi J, et al. Short-term interferon-alfa therapy for acute hepatitis C: a randomized controlled trial. Hepatology. 2004 May;39(5):1213–9. doi: 10.1002/hep.20196. [DOI] [PubMed] [Google Scholar]
- 16.Rahman F, Heller T, Sobao Y, Mizukoshi E, Nascimbeni M, Alter H, et al. Effects of antiviral therapy on the cellular immune response in acute hepatitis C. Hepatology. 2004 Jul;40(1):87–97. doi: 10.1002/hep.20253. [DOI] [PubMed] [Google Scholar]
- 17.Page K, Hahn JA, Evans J, Shiboski S, Lum P, Delwart E, et al. Acute hepatitis C virus infection in young adult injection drug users: a prospective study of incident infection, resolution, and reinfection. J Infect Dis. 2009 Oct 15;200(8):1216–26. doi: 10.1086/605947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morin T, Pariente A, Lahmek P, Rabaud C, Silvain C, Cadranel JF. Acute hepatitis C: analysis of a 126-case prospective, multicenter cohort. Eur J Gastroenterol Hepatol. 2010 Feb;22(2):157–66. doi: 10.1097/MEG.0b013e328330a8e8. [DOI] [PubMed] [Google Scholar]
- 19.Corey KE, Mendez-Navarro J, Gorospe EC, Zheng H, Chung RT. Early treatment improves outcomes in acute hepatitis C virus infection: a meta-analysis. J Viral Hepat. 2010 Mar;17(3):201–7. doi: 10.1111/j.1365-2893.2009.01167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


