Abstract
Patients with sickle cell disease (SCD) are occasionally prescribed systemic corticosteroids to treat steroid-responsive conditions. Additionally, use of systemic corticosteroids for sickle cell pain episodes and acute chest syndrome is under investigation. We report 4 patients with SCD who developed severe vaso-occlusive events following the administration of systemic steroids. We also review similar cases from the literature and suggest measures for reducing the potential risk associated with use of systemic corticosteroids in this group of patients. We conclude that corticosteroids should be used with caution in patients with SCD.
Keywords: sickle cell anemia, corticosteroids, adverse events, vaso-occlusion, bone
INTRODUCTION
Although experimental use of high doses of corticosteroids during sickle cell pain episodes and chest syndrome seems to shorten the duration of these complications, systemic corticosteroids may also increase the risk of rebound vaso-occlusive pain events and hemorrhagic stroke in many patients.1–3 We report the clinical features of 4 SCD patients who developed severe sickle cell vaso-occlusive events shortly after the initiation of systemic corticosteroid treatment and review similar cases from the literature.
CASE REPORTS
Case 1
An 8-year-old male with homozygous SCD was started on 1 mg/kg/day of prednisone for the probable diagnosis of sarcoidosis. Ten days later, he was admitted with right leg pain and bibasilar pulmonary infiltrates. Intravenous morphine and antibiotics were started, but pain continued. On day 8 of admission, he developed worsening of pain, redness and warmth of the right lower leg along with leukocytosis [white blood cell (WBC) count 24 × 103/mm3 compared to 13 × 103/mm3 in a steady state of health]. Magnetic resonance imaging (MRI) demonstrated a diffuse right tibial signal abnormality consistent with bone marrow infarction. Bone aspiration cultures were negative. On day 11, the patient developed hypoxia, worsening pleural effusion and progressive anemia. Red-cell transfusion was given. Tapering of prednisone was initiated. He was discharged on day 18. In the year preceding this admission, he had 4 in-patient admissions for new pulmonary infiltrates and pleural effusion, which had led to the lymph node biopsy and diagnosis of sarcoidosis.
Three months later, he was admitted by the rheumatology service for intravenous (IV) methylprednisolone therapy for increasing thoracic lymphadenopathy, worsening pleural effusion and pericardial thickening. He received IV methylprednisolone, 10 mg/kg on day 1 and 5 mg/kg on day 2. On day 3, he developed severe pain in both upper and lower extremities accompanied by leukocytosis (WBC count 29 × 103/mm3). Intravenous morphine and ketorolac were initiated. A few days later, he developed left lower lobe consolidation and hypoxia. He was started on IV antibiotics. He received red-cell transfusion to maintain his hemoglobin at 10 g/dl. On day 7, he developed pulmonary edema and was transferred to the pediatric intensive care unit (ICU). An exchange red-cell transfusion was performed due to progressive hypoxia and increased work of breathing. On day 8, he developed tenderness of the left lower extremity. MRI was suggestive of bone infarction of the left tibia. The previous right tibial lesions had mostly healed. He was discharged on day 12.
Case 2
A 7-year-old male with homozygous SCD was admitted for acute asthma exacerbation. He was started on nebulized albuterol and 2 mg/kg/day dose of oral prednisone. After resolution of the acute asthmatic episode, he was discharged on day 3 on a 5-day course of oral prednisone. The patient was readmitted the next day for severe pain in the back and chest. Corticosteroid therapy was discontinued. The following day, he developed priapism, leukocytosis (WBC count 30 × 103/mm3 compared to 13 × 103/mm3 in steady state) and a decrease in hemoglobin concentration to 5.5 g/dl from 7.0 g/dl. He was transfused with 15 ml/kg of packed red blood cells. A corpora cavernosa aspiration with pseudoephedrine injection was carried out for worsening priapism. On day 3 of the hospitalization, he complained of worsening pain and developed fever with lung infiltrates on chest x-ray. He was started on antibiotics, and red-cell exchange transfusion was performed. The priapism and pain gradually improved, and he was discharged on day 9. Prior to this admission, he was admitted only once for high fever.
Case 3
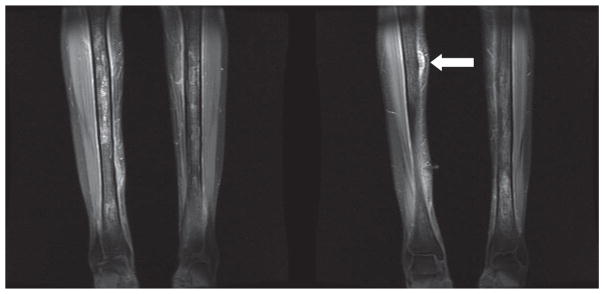
A 31-year-old woman with homozygous SCD and multiple sclerosis was admitted for severe shoulder pain and numbness and tingling of her tongue and lips. Intravenous morphine therapy was instituted. On hospital day 9, intravenous dexamethasone (60 mg followed by 4 mg every 6 hours) was started for a suspected multiple sclerosis exacerbation. On day 12, she developed 10/10 total-body pain and leukocytosis (WBC count of 20 × 103/mm3) and was transferred to the medical ICU for suspected fat embolism. Dexamethasone was discontinued. She developed nodules on her distal anterior lower extremities. The hemoglobin concentration decreased, and serum lactate dehydrogenase concentration rose to 5,700 U/L. MRI demonstrated increased signal intensity in the marrow of both tibias consistent with bone infarction (Figure 1). Quantitative sickle hemoglobin was 81% at admission, and this had declined to 16.3% on hospital day 40 after transfusion with 14 units of packed red cells to maintain the hemoglobin at 10 g/dl. In the past, she had an average of 9 painful episodes per year requiring an emergency department (ED) visit or admission but had only 1 inpatient admission for pain in the 4 months preceding her steroid-related complications.
Figure 1.
T1 with fat saturation postgadolinium MRI images from case 3 with increased signal intensity in the marrow of both tibias, consistent with acute and chronic bone infarction. The arrow in image 2 illustrates one of the nodules noted on clinical exam which is affixed to the periosteum.
Case 4
A 46-year-old male with sickle cell-β+-thalassemia and diabetes mellitus was diagnosed with sarcoidosis by biopsy of a supraclavicular lymph node. His SCD course was mild with only 2 ED visits for painful episodes in the past 5 years. He was started on 40 mg of prednisone daily for chronic shortness of breath attributed to pulmonary sarcoidosis. Two weeks later, he was admitted to his local hospital for pain crisis and bibasilar changes on the chest x-ray, diagnosed as atelectasis. He was discharged after clinical improvement. Prednisone was continued at the same dose. A month later, he was hospitalized with severe pain that progressed to acute chest syndrome and respiratory failure. He also developed expressive aphasia and difficulty following complex commands. Head CT, MRI and lumbar puncture were negative, while the electroencephalogram (EEG) showed diffuse slowing. Prednisone was decreased to 20 mg daily. After receiving an exchange red-cell transfusion, the respiratory failure gradually improved, and he was discharged. Corticosteroid treatment was tapered and discontinued over next 20 days. One year later, cognitive dysfunction and expressive aphasia had not completely resolved.
DISCUSSION
Following systemic corticosteroid therapy in patients with SCD, adverse events range from severe vaso-occlusive episodes to hemorrhagic stroke and death (Table 1). Vasoocclusive events following intra-articular injection have also been described; however, we are not aware of reports of adverse events following topical or inhaled steroids.5
Table 1.
Reports of poor tolerability of steroids in patients with SCD
| Citation | Genotype (#)/Age of the Patients | Indications/Route | Time to Adverse Outcome | Complications | Author’s Conclusion |
|---|---|---|---|---|---|
| Shapiro and Hayes4 | SC (1)/21 years | SLE, Sjogren’s syndrome and lupus cerebritis/PO followed by IV | Few days | Pain crisis, mental confusion, cardio-respiratory arrest followed by death; evidence of bone marrow necrosis and fat embolization on autopsy | Elevated level of IgG and treatment with corticosteroid appeared to be major factor in the death |
| Gladman et al.5 | Sβ+ thal (1), SD (1)/53 and 30 years | Rheumatoid arthritis/IA | 12 hours–2 days | Pain crisis, severe anemia, leukocytosis; recurrence of symptoms in both patients following repeat IA corticosteroid injection | Intra-articular corticosteroid should be used with caution in SCD |
| Huang et al.6 | SC (2)/26 and 49 years | Anterior chamber inflammation following glaucoma surgery/IV Back pain following motor vehicle accident/PO |
3–4 days | Sickling crisis, leukocytosis, increased LDH, anemia, fat embolism syndrome and prolonged coma; extensive bone marrow infarction on bone marrow scan | Corticosteroids may have precipitated the ischemic necrosis of bone and fat embolism syndrome |
| Johnson et al.7 | Sβ+ thal (1)/25 years | Asthma/IV | 3 days | Pain crisis, respiratory arrest, followed by death; evidence of fat embolism, bone marrow necrosis on autopsy | |
| Horton et al.8 | SS (1)/14 years | Nephrotic syndrome, sterile peritonitis, renal failure/PO | 1 month | Systemic fat embolization, bone pain, seizures, coma; diffuse punctate brain lesions, marrow infarction and necrosis on MRI. Patient was treated with exchange transfusions and survived. | Fat embolism must be considered in SCD patient with acute neurologic deterioration |
| Strouse et al.3a | SS (7), Sβ0T (2)/3–18 years | Not available | Within 14 days | Hemorrhagic stroke (9) | Primary hemorrhagic in children with SCD is associated with recent transfusion and use of corticosteroid. |
| Couillard et al2b | SS (9), SC (1)/0.9–18.3 years | Autoimmune disorders/PO | 1 day to 1 year | ACS (2), severe pain episode (6), infection and sepsis(3), intracranial hemorrhage (1), death (1), stroke (1), hypertension (1), avascular necrosis (1) | The steroid treatment is poorly tolerated in SCD. |
| Current report | SS (3), Sβ+T (1)/7–46 years | MS flare/autoimmune disorders/asthma/IV sarcoidosis/PO | 3–45 days | Bone marrow infarction (3), fat embolism (1), ACS (3), ARDS (1) VOC (3), priapism (1), neurologic symptoms (1) | Use of systemic steroid can precipitate severe vaso-occlusive episodes in SCD patients. |
Nine out of 15 patients with hemorrhagic stoke had history of corticosteroid use in last 14 days;
Ten out of 16 patients developed adverse event; SCD: Sickle cell disease; ACS: Acute chest syndrome; PO: Oral, IV: Intravenous; IA: Intra-articular; ARDS: Acute respiratory distress syndrome; VOC: Vaso-occlusive crises; SLE: Systemic lupus erythematosus
On our review of these and other published cases, several considerations suggest that corticosteroids may have played a causative role in the development of these adverse events (Table 1). First, the adverse events were temporally related and occurred following initiation of corticosteroid therapy. Second, vaso-occlusive episodes were unusually severe compared to the precorticosteroid clinical course.6,7 Third, serious adverse events have been reported in patients who received systemic corticosteroids for treating localized conditions not reported to be a trigger for SCD vaso-occlusive events.6 Fourth, we and others have observed recurrence of the adverse event following reinitiation of systemic corticosteroid therapy in some of the patients.5
The potential mechanism(s) involved in systemic steroid-induced vaso-occlusion is unclear. We hypothesize that bone marrow infarction and likely associated fat embolism may have contributed to the adverse events. There is a well-documented association of corticosteroid therapy and bone marrow necrosis, as was reported in 2 of our patients on MRI evaluation. Fat embolism, a potentially serious complication in SCD, is thought to be related to fat emboli arising from the bone marrow necrosis and has been reported in association with use of systemic corticosteroids.7,8 Additionally, we speculate that steroid-induced leukocytosis could be another potential factor. High WBC counts in steady state have been linked to worse clinical course, and polymorphonuclear leukocytes appear to play an active role in vasoocclusive crises. The use of agents that mobilize granulocytes or stimulate their production has been associated with the apparent triggering of sickle-related crises and multiorgan failure in isolated case reports. Interestingly, a patient with hemoglobin-SC disease who developed fatal sickle cell crisis after receiving granulocyte colony-stimulating factor was also on daily dexamethasone.9
The present case series and review of literature suggest that systemic corticosteroids play an important role in the development of severe adverse events in some patients with SCD. Pending better understanding of risk factors, prevalence and pathophysiology of these events, systemic corticosteroids should be used with caution in this population. In our experience, prompt red-cell transfusion or exchange transfusion along with aggressive clinical management can improve the clinical outcome. Furthermore, red blood cell transfusions given with corticosteroid therapy in SCD patients may attenuate the risk of such complications.2,10
Acknowledgments
Financial support: This work was supported in part by contract grants #K12RR17613 and #2MOIRR10284-10 from National Center for Research Resources and #2 R25 HL003679-08 and #1 R01 HL079912-02 from National Heart, Lung and Blood Institute, National Institutes of Health (NIH), Bethesda, MD, and by the intramural research program of NIH.
References
- 1.Bernini JC, Rogers ZR, Sandler ES, et al. Beneficial effect of intravenous dexamethasone in children with mild to moderately severe acute chest syndrome complicating sickle cell disease. Blood. 1998;92:3082–3089. [PubMed] [Google Scholar]
- 2.Couillard S, Benkerrou M, Girot R, et al. Steroid treatment in children with sickle cell disease. Haematologica. 2007;92:425–426. doi: 10.3324/haematol.10800. [DOI] [PubMed] [Google Scholar]
- 3.Strouse JJ, Hulbert ML, DeBaun MR, et al. Primary hemorrhagic stroke in children with sickle cell disease is associated with recent transfusion and use of corticosteroids. Pediatrics. 2006;118:1916–1924. doi: 10.1542/peds.2006-1241. [DOI] [PubMed] [Google Scholar]
- 4.Shapiro MP, Hayes JA. Fat embolism in sickle cell disease: report of a case with brief review of the literature. Arch Intern Med. 1984;144:181–182. [PubMed] [Google Scholar]
- 5.Gladman DD, Bombardier C. Sickle cell crisis following intraarticular steroid therapy for rheumatoid arthritis. Arthritis and Rheum. 1987;9:1065–1068. doi: 10.1002/art.1780300916. [DOI] [PubMed] [Google Scholar]
- 6.Huang JC, Gay R, Khella SL. Sickling crisis, fat embolism, and coma after steroids. Lancet. 1994;344:951–952. doi: 10.1016/s0140-6736(94)92300-0. [DOI] [PubMed] [Google Scholar]
- 7.Johnson K, Stastny JF, Rucknagel DL. Fat embolism syndrome associated with asthma and sickle cell-β+-thalassemia. Am J Hematol. 1994;46:354–357. doi: 10.1002/ajh.2830460418. [DOI] [PubMed] [Google Scholar]
- 8.Horton DP, Ferriero DM, Mentzer WC. Nontraumatic fat embolism syndrome in sickle cell disease. Pediatr Neurol. 1995;95:77–80. doi: 10.1016/0887-8994(94)00108-e. [DOI] [PubMed] [Google Scholar]
- 9.Adler BK, Salzman DE, Carabasi MH, et al. Fatal sickle cell crisis after granulocyte colony-stimulating factor administration. Blood. 2001;97:3313–3314. doi: 10.1182/blood.v97.10.3313. [DOI] [PubMed] [Google Scholar]
- 10.Strouse JJ, Takemoto CM, Keefer JR, et al. Corticosteroids and increased risk of readmission after acute chest syndrome in children with sickle cell disease. Pediatr Blood Cancer. 2008;50:1006–12. doi: 10.1002/pbc.21336. [DOI] [PMC free article] [PubMed] [Google Scholar]