Abstract
The prochelator BSIH ((E)-N′-(2-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzylidene)isonicotinohydrazide) contains a boronate group that prevents metal coordination until reaction with peroxide releases the iron chelator SIH ((E)-N′-(2-hydroxybenzylidene)isonicotinohydrazide). BSIH exists in aqueous buffer and cell culture media in equilibrium with its hydrolysis products isoniazid and (2-formylphenyl)boronic acid (FBA). The relative concentrations of these species limit the yield of intact SIH available for targeted iron chelation. While the hydrolysis fragments are nontoxic to retinal pigment epithelial cells, these results suggest that modifications to BSIH that improve its hydrolytic stability yet maintain its low inherent cytotoxicity are desirable for creating more efficient prochelators for protection against cellular oxidative damage.
Keywords: Chelating agent, Prochelator, Iron chelation, Oxidative stress, Boronic ester
Graphical abstract
To create your abstract, type over the instructions in the template box below. Fonts or abstract dimensions should not be changed or altered.
The ability of iron to donate and accept electrons while redox cycling between the ferrous (Fe2+) and ferric (Fe3+) oxidation states allows it to play an essential role in a wide range of physiological processes.1 However, the aberrant redox cycling of excess labile Fe can catalyze formation of highly toxic hydroxyl radicals through the Fenton reaction, leading to oxidative damage associated with pathological progression of several diseases, including cardiovascular and neurodegenerative disorders. Metal chelating agents have garnered interest as potential therapeutics for such diseases due to their promising attributes for mitigating metal-promoted oxidative injury.2–7
Aroylhydrazones, introduced by Ponka and colleagues, represent a class of tridentate metal chelators initially investigated for treating iron overload.8 Intracellular chelation of other metals has also been reported for some aroylhydrazones, for example sequestration of Zn2+ as an anticancer strategy.9 Salicylaldehyde isonicotinoyl hydrazone (SIH, Scheme 1a) is an aroylhydrazone analog that binds Fe3+ in a 2:1 complex through the phenolic oxygen, carbonyl oxygen, and aldimine nitrogen. The ability of SIH to scavenge redox-active Fe and exert a cytoprotective effect against oxidative stress is well documented.8,10–14 It has been shown to protect cardiomyocytes and other cell lines from oxidative damage induced by H2O2,11,15 catecholamines16 and tert-butylhydroperoxide.7 Despite these promising effects, high dose or prolonged exposure to SIH is not well tolerated by several cell lines, an effect likely associated with indiscriminate metal depletion or withholding from critical metalloproteins.11,15 Furthermore, the labile aroylhydrazone scaffold of SIH contributes to its short half-life in biological contexts.17
Scheme 1.
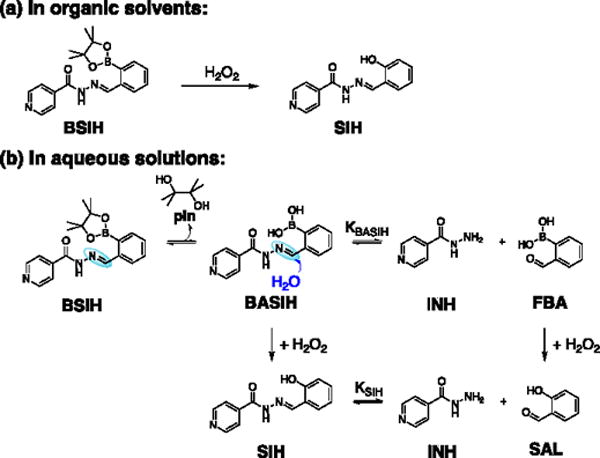
Proposed mechanisms of BSIH activation by H2O2 in organic solvents and aqueous solutions.
To overcome issues of indiscriminate metal chelation, we introduced several years ago the prochelator BSIH (Scheme 1a) as a prodrug version of SIH.11,18,19 BSIH contains a boronic ester group that masks a latent phenol, thereby incapacitating metal binding until the mask is removed by reaction with H2O2.11,18,19 Since localized high concentrations of H2O2 intimate pathological states associated with oxidative stress, this prochelator strategy in principle restricts the release of an active metal chelating agent preferentially to sites where sequestering redox-active Fe can maximize cell protection while minimizing unintended metal depletion. Related prochelator strategies are showing promise in a number of disease models.5,20–24
BSIH has been shown to be nontoxic to human retinal pigment epithelial cells and rat cardiomyocytes, even at concentrations exceeding 500 μM, while concentrations in the 20–80 μM range protect cells against oxidative damage induced by H2O2.11,15 A comparative study of several chelators and boronate-based prochelators revealed BSIH as having the most favorable combination of low inherent toxicity yet effective cytoprotection of rat cardiomyocytes exposed to H2O2.15 Moreover, detectable levels of BSIH in plasma and cell culture media were shown to be stable over time, with a significantly elongated biological half-life compared to that of SIH.11,25
While the cellular outcomes of BSIH are favorable, a lower effective concentration for cytoprotection is desirable. Since the mode of action for prochelators like BSIH derives from their capacity to sequester redox-active metals in oxidatively stressed cells, inefficient conversion of prochelator to chelator will hinder its bioactivity. On this front, we noted that BSIH fails to yield a full complement of SIH in cellular contexts and purely aqueous solutions, although it does react cleanly and quantitatively with H2O2 to yield SIH in organic solvents (Schcme 1a).26 In addition, the degradation byproduct salicylaldehyde (SAL) can be detected from reaction mixtures of BSIH and H2O2 in cell culture media.25
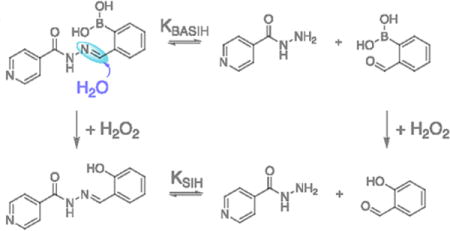
Scheme 1b shows a likely pathway leading to decreased yields of intact SIH from BSIH. The differential reactivity outcomes of BSIH with H2O2 in organic vs. aqueous solutions suggest different equilibrium ratios of the intact aroylhydrazone scaffold of BSIH and its isoniazid (INH) and (2-formylphenyl)boronic acid (FBA) degradation components that result from hydrolysis of the hydrazone C = N bond. In aqueous solution, rapid pinacol dissociation from BSIH produces a preponderance of the boronic acid version BASIH, which may exist in equilibrium with INH and FBA. Upon reaction with H2O2, the intact prochelator BASIH converts to its intact chelator version SIH, while the degradation fragment FBA transforms into byproduct salicylaldehyde (SAL). According to this model, the equilibrium constant KBASIH of BASIH hydrolysis is likely key for determining the effective concentration of intact chelator SIH generated upon peroxide activation. Various conditions such as initial concentrations of BSIH, pH and solvent polarity may affect this equilibrium, which ultimately determines the efficiency of BSIH-to-SIH conversion.
Chemical understanding of the hydrolysis equilibrium and peroxide activation of BSIH may illuminate how these processes influence biological outcomes and thus deserves further investigation. The aim of the current study was to determine the underlying chemistry that leads to the disparate activation outcomes of BSIH by reaction with H2O2 in different solvent systems and biological contexts.
Knowing that chelator SIH suffers from a short biological half-life due to the amino acid-catalyzed hydrolysis of the hydrazone bond in cell culture media,17,27 we monitored the stability of SIH and BSIH over time in phosphate buffered saline (PBS) at pH 7.4 and minimal essential medium (MEM) by UV-Vis spectrophotometry. Whereas the UV-Vis absorbance spectrum of SIH dissolved in PBS was stable over time, the spectrum of solutions prepared in MEM changed significantly over 24 h (Fig. 1a), consistent with prior studies.11,25 While the initial spectrum taken in MEM matched that of SIH recorded in PBS, the appearance of different spectra over time is consistent with degradation of SIH being facilitated by components in the medium. On its own in PBS buffer, however, the uncatalyzed hydrolysis of SIH to SAL and INH is not favorable near neutral pH. The reverse reaction, namely the formation of SIH from the aldehyde SAL and the hydrazide INH, is also unfavorable under these buffered aqueous conditions, as shown in Fig. 1b by the minor changes in spectra that appear for equimolar mixtures of SAL and INH prepared in PBS. Combined, these results indicate that although interconversion between SIH and its components SAL and INH is kinetically slow in buffer, SIH degrades within hours under conditions used for cellular assays.
Figure 1.
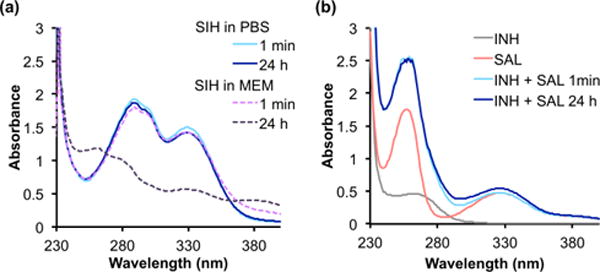
Hydrolytic stability of SIH in PBS and cell culture medium. UV-Vis spectra of (a) freshly prepared vs. 24-h aged solutions of SIH (100 μM) in PBS, pH 7.4 or MEM; (b) reaction mixtures of INH (100 μM) with SAL (100 μM) in PBS, pH 7.4 over the course of 24 h at ambient temp.
In contrast to SIH, spectra of solutions of BSIH or BASIH freshly dissolved in PBS changed significantly during the first 20 min, then remained stable for at least 24 h, the last time point recorded (Fig. 2a and Fig. S1a). Similar results were observed for BSIH dissolved in MEM, with the spectra closely resembling those of BSIH recorded in PBS (Fig. S1b). For comparison, Fig. 2b shows the spectra of equimolar mixtures of INH and FBA in PBS, which stopped changing after 20 min to reach a final spectrum that matches those of BSIH solutions equilibrated in either PBS or MEM. These results suggest that interconversion between the hydrazone of BSIH and its aldehyde FBA and hydrazide INH components is kinetically facile and reaches the same equilibrium mixture regardless from which side of the equilibrium the reaction was initiated. The spectral similarity of BSIH equilibrated in either PBS or MEM suggests the equilibrium reaction is not driven toward hydrolysis by media components as it is with SIH. The susceptibility to decomposition for SIH and BSIH in media is thus very different, which corroborates previously published results obtained using an LC-UV quantification method that demonstrated complete degradation of SIH but persistence of BSIH in cell media.25
Figure 2.
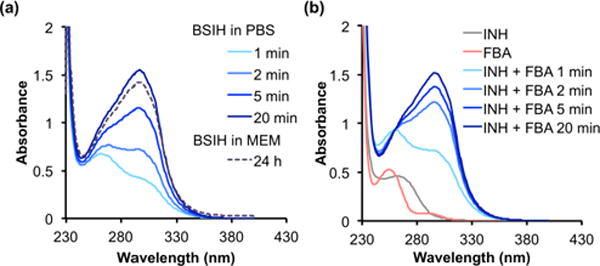
Hydrolysis equilibrium of BSIH with INH and FBA observed by UV-Vis spectrometry. Spectral changes of (a) BSIH (100 μM) in PBS, pH 7.4 or MEM and (b) reaction mixtures of INH (100 μM) with FBA (100 μM) in PBS, pH 7.4 over the course of 20 min or 24 h at ambient temp.
In order to estimate the composition of aqueous BSIH or BASIH solutions, experiments were conducted to obtain the equilibrium constant KBASIH for the hydrolysis reaction defined in Scheme 1b. Absorbance values at 297 nm from the spectra of equilibrated reaction mixtures at various reactant concentrations were used to assess the equilibrium concentrations of BASIH, INH and FBA. These data were used to calculate KBASIH = 1.5 × 10−5 M in PBS, pH 7.4, according to Eq. 2 (see Methods). The percentage of intact prochelator BASIH in an equilibrated mixture can then be estimated. If the initial concentration of BASIH is 100 μM, as in Fig. 2a, ∼68% intact prochelator persists at equilibrium, whereas a 500-μM solution of BASIH will contain ∼84% intact prochelator. Because equilibrated solutions of BSIH match those prepared directly from BASIH, we can assume that the equilibrium ratio of the aldehyde and hydrazide components are governed by the same K value, regardless of the presence of the boronic acid — pinacol boronic ester equilibria that will be present in solutions of BSIH.
To further investigate the hydrolytic stability of the prochelator, 1H NMR spectra were collected for samples of BSIH prepared in both DMSO-d6 and D2O. As shown in Fig. 3a, concentrated solutions of 10 mM BSIH in DMSO-d6 showed a distinct peak at 8.79 ppm consistent with the hydrazone proton “c”. After dilution into D2O, however, the hydrazone peak disappeared, with a concomitant rise of a new peak (δc′ = 9.90 ppm, Fig. 3b) corresponding to the aldehyde proton of the hydrolysis product FBA. Meanwhile, additional peaks were also observed at δa′ = 8.64, δb′ = 7.66 and δd′ = 7.93 ppm, corresponding to the aromatic protons from isoniazid (INH, indicated by red arrows) and FBA (indicated by green arrows). These changes of 1H chemical shifts upon dilution are consistent with hydrolytic susceptibility of the hydrazone bond in the BSIH/BASIH scaffold that partially decomposes into the two fragments, resulting in an equilibrated mixture of BASIH, INH and FBA. Integration of peaks a and a′ provides an estimate of ∼80% intact prochelator vs. ∼20% of hydrolysis products under these solution and concentration conditions, consistent with the UV-vis quantification results obtained above. Moreover, a peak at δ = 1.34 ppm in the full spectrum of BSIH (Fig. S2a) substantiates that the pinacol boronic ester version of BSIH dominates in DMSO-d6. Once diluted in D2O, however, the peak appears at 1.03 ppm, which matches the chemical shift of free pinacol (Fig. S3a), indicating pinacol dissociation from BSIH affords the corresponding boronic acid version BASIH in aqueous solvents.
Figure 3.
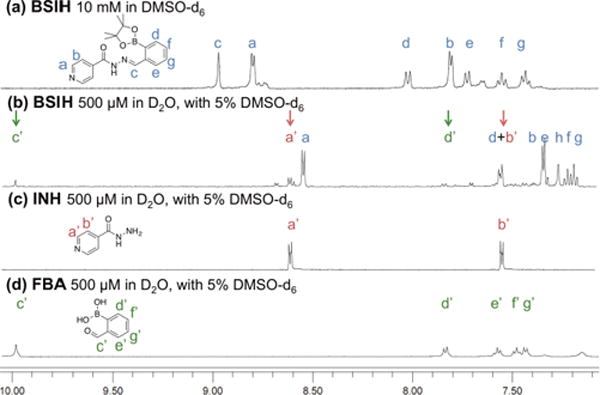
Hydrolysis equilibrium of BSIH with INH and FBA observed by 1H NMR spectroscopy.1H NMR spectra of (a) BSIH (10 mM in DMSO-d6) (b) BSIH (500 μM in D2O with 5% DMSO-d6) (c) INH (500 μM in D2O with 5% DMSO-d6) (d) FBA (500 μM in D2O with 5% DMSO-d6). The arrows indicate the peaks corresponding to the aromatic protons of isoniazid (red) and the aldehyde and aromatic protons of salicylaldehyde (green).
The hydrolysis equilibrium of BSIH is also affected by the pH of the aqueous solution. UV-Vis spectra of 100-μM BSIH solutions equilibrated in Britton-Robinson buffers at various pH values were recorded in order to obtain the corresponding content of intact prochelator. As shown in Fig. 4, the equilibrium favors the hydrolysis products under acidic conditions, whereas intact prochelator dominates the mixtures in neutral to mildly basic solutions.
Figure 4.
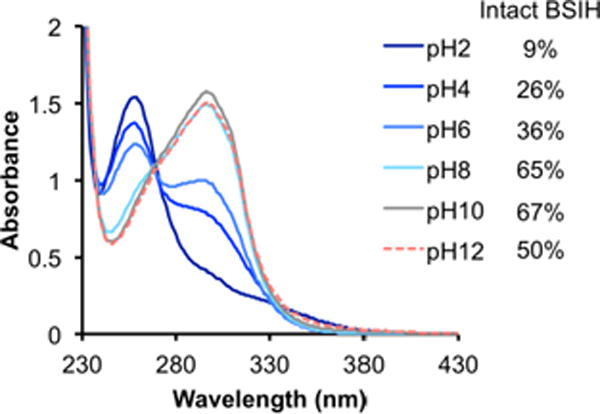
The pH dependence of BSIH hydrolysis in aqueous solution monitored by UV-Vis spectrometry. UV-Vis spectra of equilibrated BSIH solution (100 μM) in Britton-Robinson buffer at indicated pH with the corresponding content of intact prochelator in the solutions.
One of the original motivators for creating BSIH was to test if a prodrug version of SIH might reduce the susceptibility of the aroylhydrazone scaffold to hydrolysis. Previous publications from our group and others have identified BSIH with improved hydrolytic stability compared to SIH, based on the observation of negligible changes in LC-UV signal or UV-Vis absorption spectra of BSIH in aqueous solvents after 24 h.11,25 Indeed, the observation that the signal does not decay over time is a key feature of BSIH and clearly different from SIH. Results shown in the current study, however, reveal that the hydrolysis reaction of BSIH happens as soon as it presents in aqueous solutions and reaches equilibrium in less than 20 min, then the composition of the hydrolysis mixture remains constant over the course of 24 h.
An effective prochelator is expected to respond to the stimulus with fast kinetics and convert into its active chelator version to the maximal extent. The activation process of BSIH triggered by H2O2 is anticipated to occur as shown in Scheme 1a, where peroxide oxidation of the boronate mask reveals the phenol group of SIH as an additional donor arm for tight metal binding. While this BSIH-to-SIH conversion proceeds smoothly and completely in organic solvents like DMSO, reaction of BSIH with H2O2 overnight in aqueous PBS buffer at pH 7.4 resulted in a notable change in the UV-Vis spectrum of BSIH that is recognizably distinct from the spectrum of SIH (Fig. S4). This observation suggests that chelator SIH is not released stoichiometrically from activation of BSIH in aqueous conditions.
To further investigate the product of BSIH activation in aqueous solvents, reaction mixtures of BSIH with H2O2 in PBS were subjected to LC-MS analysis. As shown in Fig. 5a, five major peaks can be observed from the reaction mixture. The peak eluting at 13 min has a corresponding m/z value of 242.1, consistent with that of authentic SIH shown in Fig. 5b. Another major peak eluting at 12 min, however, gave no detectable m/z value on MS. Based on our proposed mechanism in Scheme 1b, peroxide oxidation of intact BSIH releases SIH, while the hydrolysis component FBA likely transforms into salicylaldehyde (SAL). Indeed, the unknown peak in the UV traces eluted with the same retention time as that of authentic SAL (Fig. 5c), confirming it as an activation byproduct. Meanwhile, the other expected fragment, isoniazid (INH) with an m/z value of 138.1, eluted with the solvent front at 2 min (Fig. 5d). The two activation byproducts, INH and SAL, were also identified by 1H NMR spectroscopy (Fig. S5).
Figure 5.
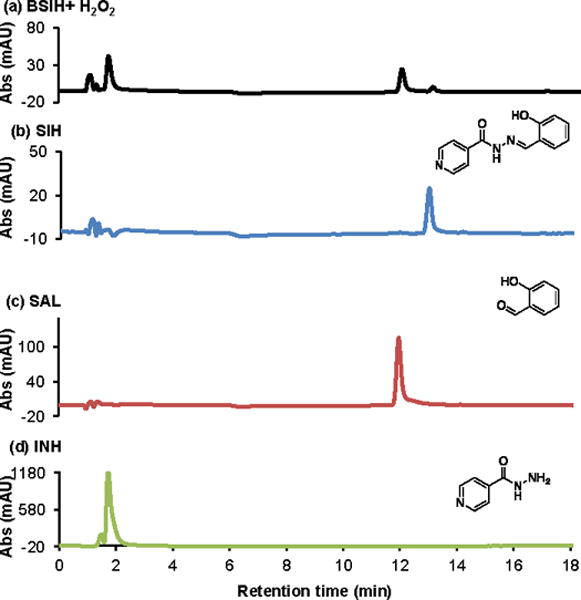
Activation of BSIH by H2O2 in PBS analyzed by LC-MS. LC traces of (a) reaction mixture of BSIH (100 μM) with H2O2 (5 mM) after 24 h (b) SIH (100 μM) (c) salicylaldehyde (SAL, 100 μM) (d) isoniazid (INH, 100 μM) in PBS, pH 7.4 at 254 nm. The peak eluted at 13 min in (a) has a corresponding m/z value of 242.1, matching that of authentic SIH in (b). The peak eluted at 12 min in (a) gives no specific m/z value on MS, but with identical retention time of authentic salicylaldehyde in (c). The peak eluted at 2 min has a corresponding m/z value of 138.1, consistent with authentic isoniazid in (d).
The effect of solvent on SIH vs. SAL product formation was analyzed by LC-MS quantification of samples prepared in varying ratios of buffer to acetonitrile (Fig. S6). The percentage of intact SIH gradually enhanced as the content of acetonitrile increased in the mixed solvent, while the detection of byproduct SAL was reduced. These combined results imply that peroxide activation of BSIH in organic solvents like DMSO and acetonitrile yields equimolar chelator SIH, whereas activation in aqueous solutions produces intact SIH as well as the two byproducts INH and SAL, due to the hydrolysis equilibrium of prochelator BSIH, as depicted in the proposed mechanism in Scheme 1.
While it was previously reported that prochelator BSIH reacts in vitro with H2O2 to generate equimolar chelator SIH, it should be noted that those experiments were conducted in either methanol or a 50/50 mixture of methanol/phosphate buffer.18,19 Activation of a 100-μM BSIH sample by H2O2 in aqueous solvents, however, yields only 25% of intact SIH, with a concomitant formation of byproducts isoniazid and salicylaldehyde. Since SIH was found to be hydrolytically stable in aqueous buffers even with the presence of H2O2 (Fig. S5), the two byproducts INH and SAL are unlikely to originate from hydrolysis of SIH upon peroxide activation. Instead, the formation of byproducts and the low yields of SIH formation have more to do with the hydrolysis equilibrium of prochelator BSIH.
While both SIH and BSIH are susceptible to hydrolysis of the aroylhydrazone framework, their respective hydrolysis reactions are differential in several aspects. Although chelator SIH is relatively stable in aqueous solution, amino acids in the cell media and biological contexts significantly accelerate the degradation of the SIH core, with a much shorter half-life of approximately 2.7 h in cell media.17 This hydrolysis reaction has an equilibrium constant K of 5 × 10−4 M, corresponding to an equilibrated concentration of intact SIH at approximately 15 μM for an initial concentration of 100 μM.17 Prochelator BSIH, on the other hand, undergoes hydrolysis in both aqueous buffer and cell media, achieving equilibrium within 20 minutes. The rapid degradation of BSIH may derive from the installation of the electron-deficient boronate functionality at the ortho- position to the hydrazone carbon, which increases the susceptibility of the hydrazone bond to nucleophilic attack by water. The current study gives an equilibrium hydrolysis constant K of 1.5 × 10−5 M for BSIH, which is 33-fold smaller than that of SIH. In other words, the hydrolysis equilibrium of BSIH favors the intact aroylhydrazone, with an estimation of 68 μM prochelator staying intact at equilibrium in a BSIH solution at initial concentration of 100 μM.
Given that prochelator BSIH exists in equilibrium with its degradation products in aqueous solutions, identifying the cellular activity of each component may facilitate our understanding of the favorable biological profile of BSIH. Cell culture experiments to test the toxicity and protective effect of these BSIH-related components were conducted on retinal pigment epithelial ARPE-19 cells because of their implication in macular degeneration. Prior studies have shown iron chelation to be effective at alleviating oxidative stress in both cell and animal models of macular degeneration.11,28,29 Fig. S7 shows the inherent toxicity of each component in ARPE-19 cells assessed by the CellTiter Blue assay. BSIH, SAL INH and FBA produced no significant reduction of cell viability even after 72-h incubation at concentrations up to 100 μM. High doses of chelator SIH were less well tolerated, with a 30% viability loss observed at the 100 μM dose. This observation indicates that the non-toxic property of degradation products INH and FBA may also account for the favorable tolerability profile of BSIH.
The cytoprotective efficacy of these compounds was further determined in ARPE-19 cells stressed with H2O2. As shown in Fig. 6, prochelator BSIH, chelator SIH as well as degradation byproducts FBA and SAL provided protection in a dose-dependent manner to cells exposed to a toxic bolus of 200 μM H2O2. Chelator SIH and prochelator BSIH were more cytoprotective than FBA and SAL, being effective at concentrations below 5 μM, 50 μM, 100 μM and 100 μM, respectively. In contrast, the hydrolysis fragment INH demonstrated no cytoprotection at concentrations up to 100 μM. These results suggest that the BSIH-to-SIH activation process is the major mode of protective action in peroxide damaged cells, even though the side reaction of FBA-to-SAL conversion contributes partially to the cytoprotective efficacy of BSIH. While this observation is consistent with recently published results that the activation byproduct SAL partially contributes to BSIH’s bioactivity in cardiomyoblasts,26 it is worth noting that FBA and SAL are consistently less effective than chelator SIH regarding the ability to mitigate H2O2-indcued damage. Moreover, both prochelator BSIH and its degradation products INH and FBA are non-toxic to several cell lines, whereas chelator SIH shows moderate inherent toxicity after 72-h exposure or repetitive administration in cells.11,15,26 These combined observations all point to BSIH as a promising therapeutic agent with low inherent cytotoxicity yet maximal iron chelation upon peroxide activation.
Figure 6.
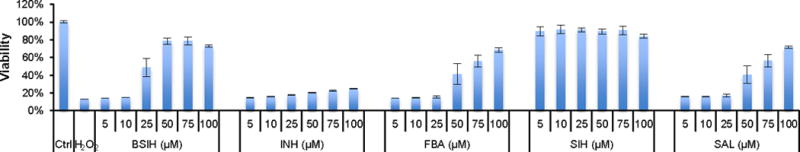
Cytoprotection of BSIH-related components against H2O2 in ARPE-19 human retinal epithelial cells. ARPE-19 cells were pre-incubated with various concentrations of tested compounds for 5 h prior to exposure to H2O2 (200 μM). Cell viability was measured 19 h after peroxide treatment and expressed as percentage of the untreated control group (100%).
The insights of BSIH hydrolysis provide a basis for improving prochelator efficacy by tweaking the molecule to influence the equilibrium hydrolysis constant K to favor the intact prochelator and eventually maximize the release of intact chelator upon reaction with H2O2. Further investigation on structural modification of BSIH is needed to obtain new derivatives that match the low inherent cytotoxicity of BSIH, yet improved hydrolytic stability in aqueous buffered systems and cell culture media.
Supplementary Material
Acknowledgments
We thank the National Institutes of Health (RO1-GM084176) for supporting this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Richardson DR, Kalinowski DS, Lau S, Jansson PJ, Lovejoy DB. Biochim Biophys Acta. 2009;1790:702. doi: 10.1016/j.bbagen.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Boddaert N, Sang K, Rotig A, Leroy-Willig A, Gallet S, Brunelle F, Sidi D, Thalabard JC, Munnich A, Cabantchik ZI. Blood. 2007;110:401. doi: 10.1182/blood-2006-12-065433. [DOI] [PubMed] [Google Scholar]
- 3.Zhou T, Ma Y, Kong X, Hider RC. Dalton Trans. 2012;41:6371. doi: 10.1039/c2dt12159j. [DOI] [PubMed] [Google Scholar]
- 4.Hadziahmetovic M, Song Y, Wolkow N, Iacovelli J, Grieco S, Lee J, Lyubarsky A, Pratico D, Connelly J, Spino M, Harris ZL, Dunaief JL. Invest Ophth Vis Sci. 2011;52:959. doi: 10.1167/iovs.10-6207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perez LR, Franz KJ. Dalton Trans. 2010;39:2177. doi: 10.1039/b919237a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalinowski DS, Richardson DR. Pharmacol Rev. 2005;57:547. doi: 10.1124/pr.57.4.2. [DOI] [PubMed] [Google Scholar]
- 7.Bendova P, Mackova E, Haskova P, Vavrova A, Jirkovsky E, Sterba M, Popelova O, Kalinowski DS, Kovarikova P, Vavrova K, Richardson DR, Simunek T. Chem Res Toxicol. 2010;23:1105. doi: 10.1021/tx100125t. [DOI] [PubMed] [Google Scholar]
- 8.Richardson DR, Ponka P. Am J Hematol. 1998;58:299. doi: 10.1002/(sici)1096-8652(199808)58:4<299::aid-ajh9>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 9.Peterson QP, Goode DR, West DC, Ramsey KN, Lee JJY, Hergenrother PJ. J Mol Biol. 2009;388:144. doi: 10.1016/j.jmb.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simunek T, Boer C, Bouwman RA, Vlasblom R, Versteilen AM, Sterba M, Gersl V, Hrdina R, Ponka P, de Lange JJ, Paulus WJ, Musters RJ. J Mol Cell Cardiol. 2005;39:345. doi: 10.1016/j.yjmcc.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 11.Charkoudian LK, Dentchev T, Lukinova N, Wolkow N, Dunaief JL, Franz KJ. J Inorg Biochem. 2008;102:2130. doi: 10.1016/j.jinorgbio.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horackova M, Ponka P, Byczko Z. Cardiovasc Res. 2000;47:529. doi: 10.1016/s0008-6363(00)00088-2. [DOI] [PubMed] [Google Scholar]
- 13.Kurz T, Gustafsson B, Brunk UT. FEBS J. 2006;273:3106. doi: 10.1111/j.1742-4658.2006.05321.x. [DOI] [PubMed] [Google Scholar]
- 14.Sterba M, Popelova O, Simunek T, Mazurova Y, Potacova A, Adamcova M, Guncova I, Kaiserova H, Palicka V, Ponka P, Gersl V. Toxicol. 2007;235:150. doi: 10.1016/j.tox.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 15.Jansova H, Machacek M, Wang Q, Haskova P, Jirkovska A, Potuckova E, Kielar F, Franz KJ, Simunek T. Free Rad Biol Med. 2014;74:210. doi: 10.1016/j.freeradbiomed.2014.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haskova P, Koubkova L, Vavrova A, Mackova E, Hruskova K, Kovarikova P, Vavrova K, Simunek T. Toxicol. 2011;289:122. doi: 10.1016/j.tox.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 17.Buss JL, Ponka P. Biochim Biophys Acta. 2003;1619:177. doi: 10.1016/s0304-4165(02)00478-6. [DOI] [PubMed] [Google Scholar]
- 18.Charkoudian LK, Pham DM, Franz KJ. J Am Chem Soc. 2006;128:12424. doi: 10.1021/ja064806w. [DOI] [PubMed] [Google Scholar]
- 19.Charkoudian LK, Pham DM, Kwon AM, Vangeloff AD, Franz KJ. Dalton Trans. 2007:5031. doi: 10.1039/b705199a. [DOI] [PubMed] [Google Scholar]
- 20.Oliveri V, Vecchio G. J Inorg Biochem. 2016;162:31. doi: 10.1016/j.jinorgbio.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 21.Zheng H, Youdim MB, Fridkin M. ACS Chem Biol. 2010;5:603. doi: 10.1021/cb900264w. [DOI] [PubMed] [Google Scholar]
- 22.Akam EA, Chang TM, Astashkin AV, Tomat E. Metallomics. 2014;6:1905. doi: 10.1039/c4mt00153b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mertens C, Akam EA, Rehwald C, Brüne B, Tomat E, Jung M. PLOS ONE. 2016;11:e0166164. doi: 10.1371/journal.pone.0166164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thiele NA, Abboud KA, Sloan KB. Eur J Med Chem. 2016;118:193. doi: 10.1016/j.ejmech.2016.04.037. [DOI] [PubMed] [Google Scholar]
- 25.Bures J, Jansova H, Stariat J, Filipsky T, Mladenka P, Simunek T, Kucera R, Klimes J, Wang Q, Franz KJ, Kovarikova P. J Pharm Biomed Anal. 2015;105:55. doi: 10.1016/j.jpba.2014.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jansova H, Bures J, Machacek M, Haskova P, Jirkovska A, Roh J, Wang Q, Franz KJ, Kovarikova P, Simunek T. Toxicol. 2016;350:15. doi: 10.1016/j.tox.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kovarikova P, Klimes J, Sterba M, Popelova O, Mokry M, Gersl V, Ponka P. J Sep Sci. 2005;28:1300. doi: 10.1002/jssc.200500077. [DOI] [PubMed] [Google Scholar]
- 28.Lukinova N, Iacovelli J, Dentchev T, Wolkow N, Hunter A, Amado D, Ying GS, Sparrow JR, Dunaief JL. Invest Ophthalmol Vis Sci. 2009;50:1440. doi: 10.1167/iovs.08-2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hadziahmetovic M, Song Y, Wolkow N, Iacovelli J, Grieco S, Lee J, Lyubarsky A, Pratico D, Connelly J, Spino M, Harris ZL, Dunaief JL. Invest Ophthalmol Vis Sci. 2011;52:959. doi: 10.1167/iovs.10-6207. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


