Abstract
We have shown that although the IgG response in fogo selvagem (FS) is mainly restricted to desmoglein (Dsg) 1, other keratinocyte cadherins are also targeted by FS patients and healthy control subjects living in the endemic region of Limão Verde, Brazil (endemic controls). Evaluating nonpathogenic IgG1 and pathogenic IgG4 subclass responses to desmosomal proteins may reveal important differences between pathogenic and nonpathogenic responses, and how these differences relate to the pathogenic IgG4 response and resultant FS. In this study, we tested by ELISA >100 sera from each FS patient, endemic control, and nonendemic control for IgG1 and IgG4 autoantibodies to keratinocyte cadherins besides Dsg1. IgG1 and IgG4 subclass responses in endemic controls are highly correlated between Dsg1 and other keratinocyte cadherins. This correlation persists in the IgG1 response among FS patients, but diminishes in IgG4 response, suggesting that IgG1 binds highly conserved linear epitopes among cadherins, whereas IgG4 binds mainly specific conformational epitopes on Dsg1. A confirmatory test comparing serum samples of 11 individuals before and after their FS onset substantiated our findings that IgG1 recognizes primarily linear epitopes on Dsg1 both before and after disease onset, whereas IgG4 recognizes primarily linear epitopes before disease onset, but recognizes more conformational epitopes on Dsg1 after the onset of disease. This study may provide a mechanism by which a specificity convergence of the IgG4 response to unique Dsg1 epitopes, most likely conformational pathogenic epitopes, leads to the onset of FS disease.
INTRODUCTION
Fogo selvagem (FS) is an endemic form of pemphigus foliaceus (PF) that affects individuals in certain areas of subtropical rural Brazil, such as Limão Verde (1). FS is similar to nonendemic PF in that both are characterized by superficial acantholysis of the subcorneal layer of the epidermis induced by anti–desmoglein 1 (anti-Dsg1) IgG autoantibodies (2). Given the geographic clustering of FS cases, it has been suggested that exposure of genetically susceptible individuals to local environmental stimuli plays a role in the pathogenesis of FS. Genetically, FS exhibits a strong association with the HLA-DRB1*0102, 0404, and 1402 alleles (3). A sand fly salivary gland Ag, LJM11, has been identified as one of the possible environmental triggers for FS because serum autoantibodies and monoclonal anti-Dsg1 autoantibodies from FS patients cross-react to LJM11 environmental Ag (4, 5).
Dsg1 is a member of the desmosomal cadherin family, a group of transmembrane glycoproteins that are essential components of the junctions between keratinocytes in regions known as desmosomes (6). Within this superfamily are seven proteins that are important to the function of the desmosome, including Dsgs (Dsg1–4) and desmocollins (Dscs; Dsc1–3), which have been well characterized in previous studies (7, 8). Also located in close proximity to the desmosome are adherens junctions that are composed of E-cadherin (E-cad). E-cad is similar in both structure and function to the desmosomal cadherins (8, 9).
Previous studies by our group have shown that the anti-Dsg1 response in FS patients consists of IgG1 anti-Dsg1 autoantibodies and IgG4 anti-Dsg1 autoantibodies (10). Whereas the IgG4 anti-Dsg1 response is pathogenic, the IgG1 anti-Dsg1 response tends to be nonpathogenic in passive transfer studies. In addition, we have shown that healthy individuals living in endemic regions of FS also possess IgG1 anti-Dsg1 autoantibodies, and that transition from IgG1 anti-Dsg1 to IgG4 anti-Dsg1 occurs during the onset of disease (11, 12). In fact, the presence of serum IgG4 anti-Dsg1 autoantibodies in healthy individuals living in the endemic region strongly predicts the development of FS (11, 13).
Our group has previously characterized the total IgG response to eight keratinocyte cadherins (Dsg1, Dsg2, Dsg3, Dsg4, Dsc1, Dsc2, Dsc3, and E-cad) in FS patients, endemic controls, and United States controls (14). There are significant differences between the IgG responses to most of the keratinocyte cadherins in FS patients, endemic controls, and United States controls. Interestingly, there was strong correlation between the IgG anti-keratinocyte cadherin responses in endemic controls, whereas the IgG responses in FS patients were less correlated. These findings suggest that a cross-reactive anti-keratinocyte cadherin response is initiated during the preclinical stage of FS in healthy individuals living in endemic areas of FS. Those individuals who go on to develop FS show a more specific IgG response to Dsg1 that is less well correlated to responses against other keratinocyte cadherins (14).
Although the total IgG responses to the eight keratinocyte cadherins has shed some light on the differences in these responses between endemic controls and FS patients, more questions regarding the autoantibody development remain to be answered. For example, the levels of IgG2 and IgG3 autoantibodies in FS patients are either very low or nondetectable (15). The predominance of IgG1 and IgG4 autoantibodies in pemphigus vulgaris (PV) and PF has been studied extensively (16–27) and widely accepted (28). Thus, the activities of total IgG in our previous studies mainly represented those of the IgG1 and IgG4 subclasses. It has been demonstrated that IgG4 anti-Dsg1 autoantibodies are pathogenic, whereas IgG1 anti-Dsg1 autoantibodies are not pathogenic in FS (11, 15). This indicates that the development of IgG1 and IgG4 Abs regarding their specificity or the epitopes they bind are different. In other words, there must be divergent development of IgG1 and IgG4 autoantibodies in individual FS patients. To test this hypothesis, this investigation focused on the specificity of IgG1 and IgG4 subclass responses among FS patients and normal control individuals living in FS endemic regions and non-FS endemic regions. We sought to explore whether these potential pathogenic anti-Dsg1 IgG4 Abs in FS cross-react with other keratinocyte adhesion molecules and whether they differ from other IgG1 subclasses in their specificity evolution among these different groups of individuals. Therefore, the IgG1 and IgG4 responses toeight keratinocytecadherins in FS patients, endemic controls, and United States controls were determined. Our findings demonstrate that among endemic controls, there is a strong correlation among the IgG1 and IgG4 responses to all keratinocyte cadherins. Although the correlation persists in the IgG1 response among FS patients, it significantly diminishes in the IgG4 response against Dsg1. Furthermore, we show that IgG1 recognizes primarily linear epitopes on Dsg1 both in the preclinical phase of disease and after disease onset. IgG4 primarily recognizes linear epitopes in the preclinical phase of disease, but shifts to recognition of conformational epitopes on Dsg1 after the onset of clinical disease. These data strongly suggest that there is an increased specificity of the IgG4 immune response to unique Dsg1 epitopes, likely the pathogenic epitopes, at the onset of FS disease.
MATERIALS AND METHODS
Sources of sera
A total of 313 sera from adult donors was tested for IgG1 and IgG4 autoantibodies against seven desmosomal cadherins and E-cad (101 sera were obtained from FS patients, 106 from healthy controls living in the Limão Verde endemic area of Brazil [endemic controls], and 106 from healthy controls living in the United States). FS sera were collected from patients originating from endemic regions, but hospitalized at the time of drawing blood in three Brazilian hospitals dedicated to treat these patients: Hospital das Clinicas, Sao Paulo (n = 25); Hospital de Doenças Tropicaes, Goiania (n = 31); and Hospital Adventista de Penfigo, Campo Grande (n = 45). The disease was confirmed by clinical, histological, and immunofluorescent findings. The patients were in different clinical stages of evolution, and many were undergoing systemic steroid therapy. However, these factors do not affect the autoantibody specificity, and thus do not impact the correlation analyses of the IgG1 and IgG4 responses to different cadherins in this investigation. The indirect immunofluorescence studies showed anti-epidermal intercellular substance autoantibodies in titers>1:80in 94patients, 1:40 in five patients, 1:20 in one patient, and negative in one patient. Healthy control sera (defined as complete absence of cutaneous disease) were obtained from blood bank donors from the University of North Carolina Blood Bank and healthy individuals from the endemic region of Limão Verde, Brazil. Sera of 11 patients with samples available both before and after the onset of disease were also used.
The study was approved by the University of North Carolina Institutional Review Board and conducted according to the Declaration of Helsinki Principles. Participants gave their written informed consent.
Construction, production, and purification of recombinant keratinocyte cadherins
We have previously constructed and expressed the entire extracellular domains of human Dsg1, Dsg2, Dsg3, Dsg4, Dsc1, Dsc2, Dsc3, and E-cad using the baculovirus system (14, 29–31). Soluble ectodomains were produced in High Five (Invitrogen, Carlsbad, CA) insect cells by infection with high-titer recombinant baculovirus stocks. Optimal infection conditions for each recombinant protein were determined by time-course studies (32). The average protein yield was 10 μg ml−1 of culture supernatant. The protein was purified by nickel affinity chromatography using the procedure previously described (30).
ELISA
Immunomicrotiter plates (CoStar, Cambridge, MA) were coated with one of the eight purified cadherins (200 ng/well for Dsc1, Dsg1, Dsg3, and E-cad; 100 ng/well for Dsg2 and Dsc2; and 50 ng/well for Dsg4 and Dsc3) at 4°C overnight. After washing five times with TBS containing 5 mM Ca2+ and 0.05% Tween 20 (TBS/Ca2+/T-20), the plate was blocked with 1% BSA in TBS/Ca2+/T-20 at room temperature for 1 h. The plate was then washed five times and incubated with duplicate samples of diluted serum for 1 h at room temperature. After washes, separate plates were incubated with diluted HRP-conjugated goat anti-human IgG1 or IgG4 subclass Ab (Bio-Rad, Hercules, CA) for 1 h (1:1000 for seven desmosomal Ags and 1: 1500 for E-cad). The color development was achieved with the peroxidase substrate o-phenylenediamine.
An FS serum (1:100 dilution) that produced consistent and reproducible positive OD values for each IgG anti-Dsg1 autoantibody subclass was selected as a positive control. We also selected a positive control serum (1:100 dilution) for Dsg2, Dsg3, Dsg4, Dsc1, Dsc2, Dsc3, and E-cad. Serum from a healthy control from the United States was used as a negative control throughout. Results were expressed as index value units as reported (13, 29, 33). The index value was defined in terms of OD as follows:
Negative index values occur when the test sample OD is less than the negative control OD. In case of a logarithmic scaleplot, a simple linear adjustment was made to all index values for a plot:
This linear transformation does not affect the results of statistical inference of the nonparametric method, and thus the outcomes or the interpretation of the data.
The cut points for IgG1 were: anti-Dsg1 = 1 (se:66), anti-Dsg2 = 10 (se:15), ant-Dsg3 = 6 (se:36), anti-Dsg4 = 2 (se:33), anti-Dsc1 = 1 (se:51), anti-Dsc2 = 1 (se:36), anti-Dsc3 = 1 (se:44), and anti–E-cad = 13 (se:25). The cut points for IgG4 were: anti-Dsg1 = 9 (se:92), anti-Dsg2 = 7 (se:21), ant-Dsg3 = 1 (se:16), anti-Dsg4 = 25 (se:12), anti-Dsc1 = 26 (se:10), anti-Dsc2 = 3 (se:51), anti-Dsc3 = 5 (se:15), and anti–E-cad = 3 (se:53).
Testing of IgG1 and IgG4 autoantibodies against native and denatured Dsg1
For native versus denatured Dsg1 ELISA studies, ELISA plates were coated with equal amount of either native recombinant Dsg1 or denatured Dsg1 (using 8 M urea). Sera from 11 individuals before (pre) and after (post) their onset of FS were tested for IgG1 and IgG4 against native and denatured Dsg1. Mouse anti-human IgG1 (clone HP6001) and IgG4 (clone HP 6023) HRP-conjugated mAbs (SouthernBiotech, Birmingham, AL) were used as secondary Abs. The ratios of Ab levels against native Dsg1 to those against denatured Dsg1 were determined (anti-native Dsg1 OD/anti-denatured Dsg1 OD). The p values were determined using Wilcoxon rank sum test.
Statistical analysis
To increase the efficiency or power, as well as robustness, of statistical inference for nonnormal data as found in this study, we used two nonparametric methods: Wilcoxon rank sum test and Spearman correlation.
The Wilcoxon rank sum test was used to test equality of two distributions. The Wilcoxon rank sum test was used to analyze the distribution of index values between groups tested. The Wilcoxon rank sum test (34) is a statistical hypothesis test of equality of two distributions (the distributions in the two groups). In brief, the test is performed by pooling the two samples into one sample of n observations, ranking the values from smallest (rank = 1) to largest (rank = n), computing the mean rank in each group, and then comparing the mean ranks in a formal statistical manner. It is somewhat similar in spirit to the two-sample t test, but it only takes the ordering of the observations into account, not their actual magnitude. Assumption of normal distributions is not required.
Bonferroni adjustment for multiple comparisons was used. The Bonferroni correction was applied throughout to adjust for multiple comparisons and control the overall type I error given the large number of hypothesis tests performed. The Bonferroni method controls the overall type I error by performing each test at the 0.05/K level, where K is the number of tests (35).
Spearman correlation was used to assess the strength of correlation among the indices. The data analysis was done using the R software, version 3.2.2 (36), and the SAS software, version 9.3 (SAS Institute, Cary, NC).
RESULTS
IgG1 and IgG4 anti-keratinocyte cadherin responses differ among FS patients, endemic controls, and United States controls
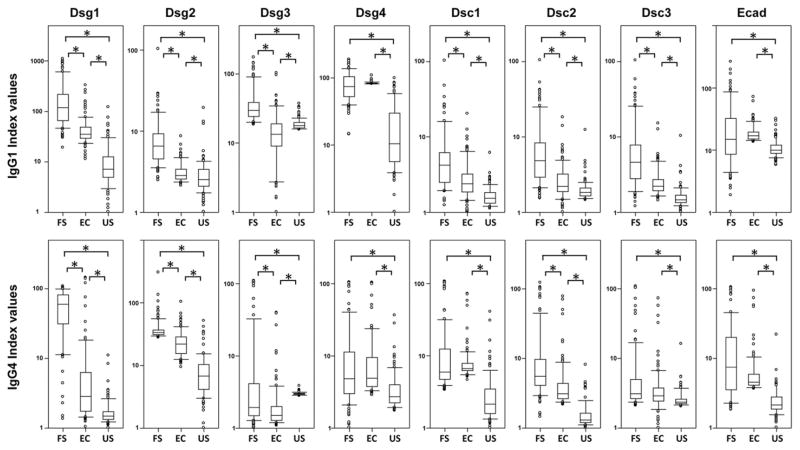
The sera of 102 FS patients, 102 endemic controls, and 106 United States controls were tested for the presence of IgG1 and IgG4 autoantibodies to Dsg1, Dsg2, Dsg3, Dsg4, Dsc1, Dsc2, Dsc3, and E-cad by ELISA and the results reported as index values. The distribution of index values for each response is depicted in box-plot format (Fig. 1). The data were analyzed using a 48 pairwise comparison (three groups of sera compared against each other for 16 different responses). A Bonferroni correction was used to adjust for multiple comparisons among the 48 tests; thus, for an overall α of 0.05, only p < 0.05/48 = 0.001 is considered significant (Supplemental Table I).
FIGURE 1. IgG1 (upper panels) and IgG4 (lower panels) anti-keratinocyte cadherin responses differ among FS patients, endemic controls, and United States controls (US).
Sera from FS patients, endemic controls (EC), and United States controls were tested for IgG1 and IgG4 reactivity to Dsg1, Dsg2, Dsg3, Dsg4, Dsc1, Dsc2, Dsc3, and E-cad by ELISA. Index values were calculated, and the results for each population are displayed as box plots. To use a logarithmic scale, we shifted index values in each plot by an amount specific to that plot to make the lowest value equal 1. Each box extends from the lower to the upper quartile (25–75%), with the median shown within the box. A significant difference between paired groups is marked by an asterisk (*).
As shown in Fig. 1 (upper panels), the index values of IgG1 anti-Dsg1 autoantibodies are significantly higher in FS patients compared with both endemic controls and United States controls (p <0.001 for both). Also, the sera from endemic controls show higher IgG1 anti-Dsg1 index values than United States controls (p < 0.001) (Supplemental Table I). Interestingly, all of the IgG1 index values to the eight keratinocyte cadherins are significantly different between FS patients and United States controls, and between endemic controls and United States controls (Fig. 1, upper panel, Supplemental Table I).
With regard to the IgG4 anti-keratinocyte cadherin responses (Fig. 1, lower panel), the index values of IgG4 anti-Dsg1 autoantibodies are also higher in FS patients compared with both endemic and United States controls, as expected (p < 0.001 for both) (Supplemental Table I). Consistent with our previous report (13), endemic controls show significantly higher IgG4 anti-Dsg1 index values compared with United States controls (p < 0.001). The IgG4 index values to all eight keratinocyte cadherins are significantly different between FS patients and United States controls, and between endemic controls and United States controls (Supplemental Table I).
It should be noted that the index values of IgG1 andIgG4 do not need to reflect the relative titer of the autoantibody levels from these tested individuals. The mean levels of IgG1 against these different adhesion molecules are lower than that of IgG4 (data not shown), similar to the report regarding IgG1 and IgG4 anti-Dsg1 and -Dsg3 levels among PV and PF patients (37). In addition, we did not find that any tested sera cross-react to other Ags used as control in this laboratory (data not shown), indicating that the reactivity of these sera to these adhesion molecules is specific. It is consistent with our previous findings that sera from FS patients, endemic controls, or nonendemic controls do not cross-react with lupus-associated autoantigens, such as ribonucleoprotein, Ro/SSA, La/SSB, and Sm (38).
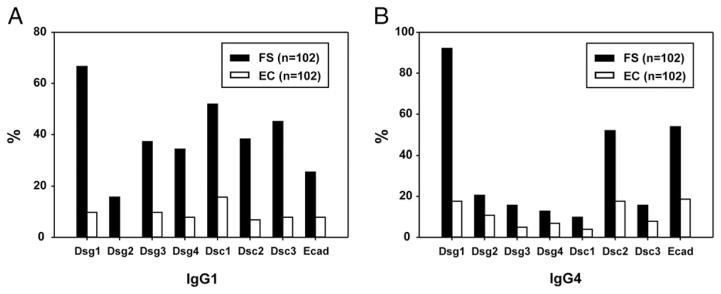
High frequencies of FS patients are serologically positive for IgG1 and IgG4 responses to keratinocyte cadherins other than Dsg1
For each of the IgG1 and IgG4 Ab responses, we used the United States control index values to select a cut point that would generate a specificity of 99%, that is, a false-positive rate of 1% among United States controls. We defined all index values above the cut point as positive and then determined the frequency of FS patients and endemic controls testing positive for each of the eight responses.
Among FS patients, 67% are positive for IgG1 anti-Dsg1 autoantibodies (Fig. 2A). Interestingly, FS patients are positive for IgG1 autoantibodies against the other keratinocyte cadherins, although at lower frequencies ranging from 16% for anti-Dsg2 Abs to 52% for anti-Dsc1 Abs. Among endemic controls, only 10% of the subjects are positive for IgG1 anti-Dsg1 autoantibodies, with the frequency of positive responses to the other keratinocyte cadherins ranging from <1% for anti-Dsg2 to 16% for anti-Dsc1.
FIGURE 2. Frequencies of IgG1 (A) and IgG4 (B) positive individuals among FS patients and endemic controls (ECs).
Bar graphs represent positive sera as defined by the cut points. FS patients (black bars) and ECs (white bars) are shown.
With regard to the IgG4 responses (Fig. 2B), 92% of FS patients are positive for IgG4 anti-Dsg1 autoantibodies, as expected. We also find high frequencies of IgG4 autoantibodies against E-cad (54%) and Dsc2 (52%) in FS patients. Lower frequencies of positive IgG4 autoantibodies (<20%) against other keratinocyte cadherins were also detected in the FS cohort. Among endemic controls, 18% are positive for IgG4 anti-Dsg1 autoantibodies. The frequencies of positive IgG4 responses to all other keratinocyte cadherins tested remain low (<20%).
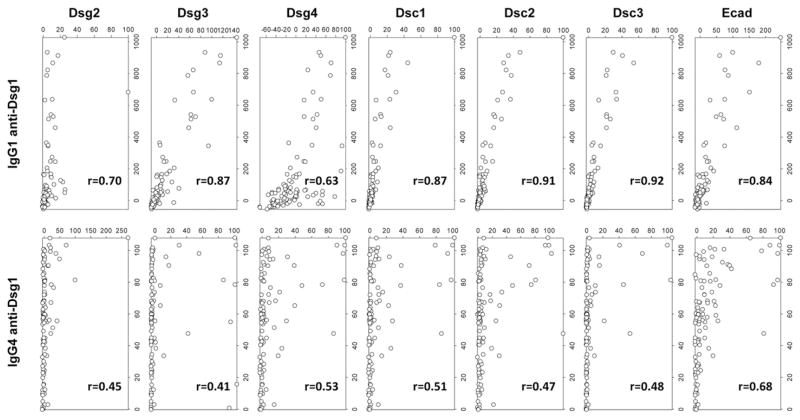
Correlation of anti-Dsg1 responses to other keratinocyte cadherin responses differs between IgG1 and IgG4 responses in FS patients
We next explored the correlation among the responses to the eight keratinocyte cadherins within each group (FS patients, endemic controls, and United States controls). To determine the correlation among responses, we created scatterplots by plotting index values for one response against index values for another response. Spearman correlation coefficients were then computed. For example, FS patients with high index values for IgG1 anti-Dsg1 also tend to have high index values for IgG1 anti–E-cad, generating an r value of 0.84. Similar scatterplots were created for IgG1 anti-Dsg1 index values against each of the other IgG1 responses (Dsg2, Dsg3, Dsg4, Dsc1, Dsc2, and Dsc3) (Fig. 3, upper panels). The r values generated by each of these plots were averaged to give an overall summary of how well the IgG1 anti-Dsg1 responses correlate to the IgG1 responses against the other keratinocyte cadherins in FS patients (average r = 0.82; Table I). Similarly, average r values were generated for the IgG1 anti-Dsg2, -Dsg3, -Dsg4, -Dsc1, -Dsc2, -Dsc3, and –E-cad responses in FS patients to summarize how well they correlate with the other responses within the FS patient group. These average r values are shown in Table I. Following the same method, average r values were generated for each IgG1 response in 22endemic controls and UnitedStates controls and for each IgG4 response in FS patients, endemic controls, and United States controls (Table I). The scatterplots of all pairwise responses and individual r values are shown in Supplemental Fig. 1 (IgG1 responses) and Supplemental Fig. 2 (IgG4 responses).
FIGURE 3. Divergent IgG1 and IgG4 anti-keratinocyte cadherin responses among FS patients.
FS patient IgG1 and IgG4 anti-Dsg1 autoantibody response correlations with their corresponding IgG1 (upper panels) and IgG4 responses (lower panels) to other cadherins are depicted by index value scatterplots. The Spearman correlation coefficient (r value) for each scatterplot is shown in the bottom right corner of each plot.
TABLE I.
Average Spearman correlations (average r values) among IgG1 and IgG4 anti-keratinocyte cadherin responses in FS patients, endemic controls, and United States controls
| IgG1 | IgG4 | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| FS Patients | Endemic Controls | United States Controls | FS Patients | Endemic Controls | United States Controls | |
| Dsg1 | 0.82 | 0.60 | 0.46 | 0.50 | 0.79 | 0.51 |
| Dsg2 | 0.65 | 0.59 | 0.35 | 0.70 | 0.60 | 0.58 |
| Dsg3 | 0.78 | 0.55 | 0.52 | 0.70 | 0.64 | 0.34 |
| Dsg4 | 0.58 | 0.66 | 0.47 | 0.79 | 0.81 | 0.65 |
| Dsc1 | 0.81 | 0.72 | 0.59 | 0.80 | 0.69 | 0.65 |
| Dsc2 | 0.81 | 0.70 | 0.60 | 0.74 | 0.78 | 0.63 |
| Dsc3 | 0.81 | 0.72 | 0.53 | 0.77 | 0.67 | 0.59 |
| E-cad | 0.77 | 0.74 | 0.36 | 0.67 | 0.79 | 0.38 |
The data from which these average Spearman correlations were derived are depicted in Supplemental Fig. 1 and Fig. 2. The r values generated by each of the plots in the figures were averaged to give an overall summary of how well a response, e.g., the IgG1 anti-Dsg1 response, correlates with its responses against the other keratinocyte cadherins.
IgG1 Abs are found to be nonpathogenic in FS patients (11, 15) and are likely the initial anti-keratinocyte cadherin subclass response generated in all individuals living in endemic areas of FS. Among FS patients, the IgG1 anti-Dsg1 response shows a high degree of correlation with the IgG1 responses to each of the other keratinocyte cadherins (Fig. 3, upper panels) (average r = 0.82, Table I). Thus, an FS patient with a high IgG1 index value to Dsg1 tends to have high index values to the other keratinocyte cadherins as well. Among endemic controls, the IgG1 anti-Dsg1 response shows slightly lower average correlation to the other keratinocyte cadherin responses (Supplemental Fig. 1, Table I) (average r = 0.60). However, correlation is very low between the IgG1 anti-Dsg1 response and other IgG1 responses in United States controls (Supplemental Fig. 1, Table I) (average r = 0.46). In general, the average correlations of IgG1 responses range from 0.58 to 0.82 in FS patients, from 0.55 to 0.74 in endemic controls, and from 0.35 to 0.59 in United States controls (Table I).
Because IgG4 responses in FS are known to be pathogenic, we also determined the correlation of the IgG4 responses within each of the three groups (FS patients, endemic controls, and United States controls). As shown in Fig. 3 (lower panels), the IgG4 anti-Dsg1 response in FS patients shows a lower degree of correlation with each of the other IgG4 responses (average r = 0.50, Table I) than the IgG1 anti-Dsg1 response does (average r = 0.82, Table I). However, the average correlations of the IgG4 responses to other keratinocyte cadherins in FS patients remain relatively high, ranging from 0.67 to 0.80 (Table I), suggesting the specificity convergence of IgG4 to Dsg1 is distinctive in FS patients. Among endemic controls, the IgG4 anti-Dsg1 response shows a high degree of correlation with responses to other keratinocyte cadherins (average r = 0.79). In fact, all of the IgG4 anti-keratinocyte cadherin responses in endemic controls show high correlation with each other (average r = 0.60–0.81). Correlation is again low between all IgG4 responses in United States controls (average r = 0.34–0.65) (Supplemental Fig. 2).
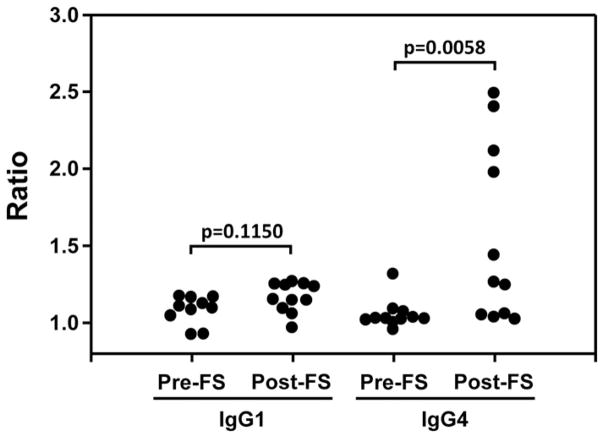
Divergent epitope recognition of IgG1 and IgG4 autoantibodies before and after the onset of FS
The analyses obtained from the study of a large cohort of the FS patients and normal controls suggest that there is a divergence between IgG1 and IgG4 autoantibody development among FS patients; that is, these IgG1 autoantibodies remain to be cross-reactive to multiple cadherin molecules, whereas the specificity of IgG4 gradually focuses on Dsg1. To further examine the convergence of the IgG4 response to unique epitopes on Dsg1 at disease onset, we used sera from a unique cohort of FS patients in which sera are available before the onset of FS (preclinical phase), which can be considered as normal controls by these time points of blood draw, and after the onset of clinical disease (FS patients analogous to the FS patients of the larger cohort present in the first part of this investigation). The sera from these 11 individuals before (pre) and after (post) their onset of FS against were tested for IgG1 and IgG4 reactivity against native (both conformational and linear epitopes are preserved) and denatured (only linear epitopes can be detected) Dsg1 (Fig. 4, Supplemental Table II). The ratios of Ab levels against native Dsg1 to those against denatured Dsg1 show that IgG1 recognizes primarily linear epitopes on Dsg1 both in the preclinical phase and after the onset of disease. In contrast, IgG4 recognizes primarily linear epitopes on Dsg1 in the preclinical phase of the disease, but after the onset of clinical disease recognizes primarily conformational epitopes, corroborating our findings using large cohorts of FS patients and endemic control individuals.
FIGURE 4. Serum IgG1 and IgG4 Ab levels against native Dsg1 in comparison with those against denatured Dsg1.
The IgG1 and IgG4 Abs sera from these 11 individuals before (pre) and after (post) their onset of FS against were tested against native and denatured Dsg1. The ratios of Ab levels against native Dsg1 to those against denatured Dsg1 were calculated (anti-native Dsg1 OD/anti-denatured Dsg1 OD) and plotted. The p values were determined using Wilcoxon rank sum test.
DISCUSSION
FS provides the unique opportunity to understand the impact of environmental stimuli on the development of autoimmune disease in genetically predisposed individuals. Study of the humoral immune responses of FS patients and healthy individuals living in endemic areas of FS (comprising those who are at risk for developing disease) have revealed the following: 1) newborns from mothers of these areas show absence of early autoantibody response to Dsg1 of IgM, IgG, and IgG subclasses (39); 2) the IgM anti-Dsg1 autoantibody response is detected in early childhood and is maintained during adolescence and adult life (29); 3) the IgG anti-Dsg1 response gradually increases from 2.9% in early childhood to 7.3% in young children and reaching 27% in adolescents (29); and 4) a percentage of healthy adults living in endemic areas of FS possess IgG anti-Dsg1 autoantibodies (10). From this large pool of individuals, a small fraction will develop FS, which is heralded by the appearance of IgG4 anti-Dsg1 autoantibodies (13); 5) the IgG response of patients and endemic controls to other desmosomal cadherins reveals significant differences among FS patients, endemic controls, and United States controls (14); 6) there is a parallel response of anti-Dsg1 IgE autoantibodies in FS patients and endemic controls, which correlates with the IgG4 response (40); and 7) monoclonal IgG4 anti-Dsg1 autoantibodies derived from FS patients cross-react with the salivary protein of Lutzomyia longipalpis LJM11 (5), suggesting an environmental trigger of FS. It is hypothesized that exposure of genetically susceptible individuals to salivary Ags delivered by insect bites may be the trigger of autoantibody production in FS (5, 41), although other environmental triggers may contribute to the development of disease as well (4).
In this study, we have focused our attention on the IgG1 and IgG4 subclass autoantibody responses to Dsg1 and a panel of seven additional keratinocyte cadherins including Dsg2, Dsg3, Dsg4, Dsc1, Dsc2, Dsc3, and E-cad in three populations: FS patients, endemic controls, and United States controls. The autoantibody profiles to each of the desmosomal cadherins in each of the three groups yield important information that gives insight into the link between initial nonpathogenic autoantibody responses (as seen in healthy individuals living in endemic areas who are at risk for developing disease, i.e., endemic controls) and pathogenic autoantibody responses (as seen in FS patients).
This large-scale investigation shows that FS patients have significantly higher IgG1 and IgG4 index values to Dsg1 than endemic controls and United States controls as previously reported with a limited number of FS patients and healthy controls (11). Endemic controls have significantly higher IgG1 and IgG4 index values to Dsg1 than United States controls. These significant differences between endemic controls and United States controls mirror our previous findings that endemic controls have significantly higher IgG4 and IgE anti-LJM11 environmental Ag than United States controls (41). These data further illustrate the difference between these two geographic regions in immune response among the individuals and the possible environmental contribution to this difference.
Many FS patients and endemic controls have low-level IgG1 and IgG4 responses to keratinocyte cadherins other than Dsg1 (Fig. 2). Among FS patients, 67% are positive for IgG1 anti-Dsg1 autoantibodies. However, FS patients are also positive for IgG1 autoantibodies against the other keratinocyte cadherins tested, although at lower frequencies (16–52%). Among endemic controls, the subjects are positive for IgG1 anti-Dsg1 autoantibodies, with the frequencies of positive responses to the other keratinocyte cadherins remaining low (<1–16%). As expected, IgG4 responses to Dsg1 were found in 92% of FS patients. We also find high frequencies of IgG4 autoantibodies against E-cad (54%) and Dsc2 (52%) in the sera of FS patients. Low frequencies of FS patients positive for IgG4 autoantibodies (<20%) against other desmosomal cadherins were also detected. The frequencies of positive IgG4 responses to these keratinocyte cadherins tested remain low among endemic controls.
To determine whether there is any association between the responses to different adhesion molecules, we evaluated the correlations between the responses within each population of individuals (FS patients, endemic controls, and United States controls). Even if the magnitudes of the responses are low, high correlation between or among responses within a group could suggest cross-reactivity or a common stimulus that induces multiple similar responses. Correlation coefficients were calculated for each pair of responses in a particular cohort. For example, IgG1 response against Dsg1 versus IgG1 response against E-cad in FS patients yields an r value of 0.84. To better capture the overall correlation within cohorts, an average r was calculated for each response. For example, the average correlation of IgG1 anti-Dsg1 response to other keratinocyte cadherins in the FS patient cohort is 0.82. As shown in this investigation (Fig. 3, Supplemental Figs. 1, 2, Table I), most of the IgG1 and IgG4 responses to different cadherins show a high degree of correlation, except IgG4 anti-Dsg1 response. It is not surprising because all tested adhesion molecules in this investigation belong to the desmosomal cadherins superfamily and they share ~30% primary sequence identity (42). Thus, these correlated responses to different cadherins are more likely caused by the binding of these autoantibodies to epitopes shared by these molecules. It is also evident that FS patients have anti-Dsg3 IgG1 and IgG4 autoantibodies (Fig. 2), but have no clinical symptoms associated with PV in which Dsg3 is the target of IgG4 pathogenic autoantibodies, further suggesting that these possible cross-reactive autoantibodies bind to shared, but nonpathogenic, epitopes on these molecules. IgG1 and IgG4 autoantibody responses among endemic control individuals show correlation against different cadherins, but these individuals have no FS disease. Thus, it is unlikely that there is a global break in B cell tolerance among these individuals living in FS endemic regions, but we cannot completely exclude this possibility. Definitive determination of the cross-reactivity of these autoantibodies requires the generation of mAbs of both IgG1 and IgG4 subclasses derived from FS patients and endemic controls. These mAbs are currently being generated in our laboratory.
In general, IgG1 responses are similar among FS patients and among endemic controls. However, as the autoantibody response to Dsg1 transitions to the IgG4 subclass, the response in FS patients becomes more specific to Dsg1 and has much less correlation with the responses to other cadherins (average r = 0.50), suggesting the transition to unique epitopes on Dsg1 for the majority of the IgG4 autoantibodies among FS patients. This may explain, at least in part, the onset of FS. In addition, the average correlation values of these IgG1 anti-keratinocyte cadherins in both FS and endemic controls are significantly different from those of United States controls.
These findings show that in endemic controls, the IgG1 and IgG4 subclass responses are highly correlated between all keratinocyte cadherins. We speculate that this correlation is due to cross-reactivity of autoantibodies to shared linear epitopes on the keratinocyte cadherins. It is well recognized that FS patients’ IgG autoantibodies are specific for Dsg1 (2) and pathogenic (43, 44). These IgG pathogenic autoantibodies later were found to be of IgG4 subclass and bind specifically to Dsg1 (13, 15), and do not bind other desmosomal cadherins, such as Dsg2, Dsg3, Dsg4, or Dsc1-3 (14, 45–47). In addition, the binding of these IgG4 pathogenic autoantibodies to Dsg1 is Ca2+ dependent (13, 14, 28). To gain more direct evidence that the large-cohort data indeed reflect the divergent development of IgG1 and IgG4 autoantibodies among those individuals who eventually developed FS, we took advantage of the blood bank in which serum samples were collected over the past 30 y. In a small cohort of patients with serum available both before and after the onset of disease, we were able to validate the convergence of the IgG4 response to unique, conformational Dsg1 epitopes. As demonstrated in the Results (Fig. 4, Supplemental Table II), IgG1 primarily recognizes linear epitopes both in the preclinical phase of disease and after disease onset. Although IgG4 also primarily recognizes linear epitopes in the preclinical phase of disease, there is a significant increase in the recognition of conformational epitopes after disease onset. The data confirm our speculation based on the large cohort data that the linear epitopes recognized by IgG1 and by preclinical IgG4 may be conserved across all desmosomal cadherins, explaining the correlation of these responses in IgG1 and IgG4 in endemic controls and in IgG1 in FS patients. Conformational epitopes recognized by IgG4 in FS patients are likely not conserved, explaining the convergence of the IgG4 response. Studies to identify the conserved linear epitopes and unique conformational epitopes in Dsg1 are currently under way.
The broad presence of elevated levels of immune responses directed against self and environmental Ags (5, 41, 48, 49) and different cadherin family proteins in this investigation suggests the existence of indigenous environmental trigger(s), which shares similar epitope(s) with these cadherin adhesion molecules and elicits the IgG responses to these molecules in individuals living in FS endemic regions. These immune responses may eventually proceed to the development of pathogenic IgG4 autoantibodies among these genetically susceptible individuals that are specific to the pathogenic epitope(s) on Dsg1 and result in FS via epitope spreading demonstrated in our previous finding (50).
Collectively, these findings show that among FS patients, the correlation persists in the IgG1 response, but diminishes in the IgG4 response. One explanation for these findings is that the IgG4 response becomes specific to unique conformational or Ca2+-dependent pathogenic epitopes on Dsg1 in FS patients, because the conformational or Ca2+-dependent epitopes are present only on the native form of Dsg. The Ca2+ dependency of the binding between pathogenic autoantibodies and Dsg is well-established for patients with PV and PF/FS (46, 51–56). Based on these and previous findings, it may be hypothesized that the inciting environmental Ag(s) may cross-react with shared nonpathogenic linear epitopes on Dsg1 and other keratinocyte cadherins. This nonpathogenic response develops in all individuals living in the endemic area. In the small cohort who go on to develop disease, the transition to IgG4 is marked by increased specificity for Dsg1, whereas those who remain healthy have an IgG4 response that remains cross-reactive to the other keratinocyte cadherins. This hypothesis needs to be further tested, but the data presented provide an intriguing glimpse into the mechanisms by which autoantibody responses to keratinocyte cadherins evolve among the people living in endemic areas of disease, and specificity convergence of IgG4 autoantibodies leads to the development of FS.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants R01 AR067315 (to Y.Q.) and R01 AR32599 (to L.A.D.), and by a Dermatology Foundation Career Development Award (to D.A.C.).
Abbreviations used in this article
- Dsc
desmocollin
- Dsg
desmoglein
- E-cad
E-cadherin
- FS
fogo selvagem
- PF
pemphigus foliaceus
- PV
pemphigus vulgaris
Footnotes
DISCLOSURES
The authors have no financial conflicts of interest.
The online version of this article contains supplemental material.
References
- 1.Hans-Filho G, dos Santos V, Katayama JH, Aoki V, Rivitti EA, Sampaio SA, Friedman H, Moraes JR, Moraes ME, Eaton DP, et al. Cooperative Group on Fogo Selvagem Research. An active focus of high prevalence of fogo selvagem on an Amerindian reservation in Brazil. J Invest Dermatol. 1996;107:68–75. doi: 10.1111/1523-1747.ep12298213. [DOI] [PubMed] [Google Scholar]
- 2.Stanley JR, Klaus-Kovtun V, Sampaio SA. Antigenic specificity of fogo selvagem autoantibodies is similar to North American pemphigus foliaceus and distinct from pemphigus vulgaris autoantibodies. J Invest Dermatol. 1986;87:197–201. doi: 10.1111/1523-1747.ep12695334. [DOI] [PubMed] [Google Scholar]
- 3.Moraes ME, Fernandez-Vina M, Lazaro A, Diaz LA, Filho GH, Friedman H, Rivitti E, Aoki V, Stastny P, Moraes JR. An epitope in the third hypervariable region of the DRB1 gene is involved in the susceptibility to endemic pemphigus foliaceus (fogo selvagem) in three different Brazilian populations. Tissue Antigens. 1997;49:35–40. doi: 10.1111/j.1399-0039.1997.tb02707.x. [DOI] [PubMed] [Google Scholar]
- 4.Qian Y, Culton DA, Jeong JS, Trupiano N, Valenzuela JG, Diaz LA. Non-infectious environmental antigens as a trigger for the initiation of an autoimmune skin disease. Autoimmun Rev. 2016;15:923–930. doi: 10.1016/j.autrev.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qian Y, Jeong JS, Maldonado M, Valenzuela JG, Gomes R, Teixeira C, Evangelista F, Qaqish B, Aoki V, Hans G, Jr, et al. Cutting edge: Brazilian pemphigus foliaceus anti-desmoglein 1 auto-antibodies cross-react with sand fly salivary LJM11 antigen. J Immunol. 2012;189:1535–1539. doi: 10.4049/jimmunol.1200842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desai BV, Harmon RM, Green KJ. Desmosomes at a glance. J Cell Sci. 2009;122:4401–4407. doi: 10.1242/jcs.037457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomason HA, Scothern A, McHarg S, Garrod DR. Desmosomes: adhesive strength and signalling in health and disease. Biochem J. 2010;429:419–433. doi: 10.1042/BJ20100567. [DOI] [PubMed] [Google Scholar]
- 8.Saito M, Tucker DK, Kohlhorst D, Niessen CM, Kowalczyk AP. Classical and desmosomal cadherins at a glance. J Cell Sci. 2012;125:2547–2552. doi: 10.1242/jcs.066654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Green KJ, Getsios S, Troyanovsky S, Godsel LM. Intercellular junction assembly, dynamics, and homeostasis. Cold Spring Harb Perspect Biol. 2010;2:a000125. doi: 10.1101/cshperspect.a000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Warren SJ, SLin M, Giudice GJ, Hoffmann RG, Hans-Filho G, Aoki V, Rivitti EA, Santos V, Diaz LA Cooperative Group on Fogo Selvagem Research. The prevalence of antibodies against desmoglein 1 in endemic pemphigus foliaceus in Brazil. N Engl J Med. 2000;343:23–30. doi: 10.1056/NEJM200007063430104. [DOI] [PubMed] [Google Scholar]
- 11.Warren SJ, Arteaga LA, Rivitti EA, Aoki V, Hans-Filho G, Qaqish BF, Lin MS, Giudice GJ, Diaz LA. The role of subclass switching in the pathogenesis of endemic pemphigus foliaceus. J Invest Dermatol. 2003;120:104–108. doi: 10.1046/j.1523-1747.2003.12017.x. [DOI] [PubMed] [Google Scholar]
- 12.Dos Santos SNMB, Patrus OA, Fuigueira AL, Diaz LA. Perfil evollutivo das subclasses de immunoglobulinas gama em pacientes de penfigo foliaceo endemico. An Bras Dermatol. 2001;76:561–574. [Google Scholar]
- 13.Qaqish BF, Prisayanh P, Qian Y, Andraca E, Li N, Aoki V, Hans-Filho G, dos Santos V, Rivitti EA, Diaz LA Cooperative Group on Fogo Selvagem Research. Development of an IgG4-based predictor of endemic pemphigus foliaceus (fogo selvagem) J Invest Dermatol. 2009;129:110–118. doi: 10.1038/jid.2008.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flores G, Culton DA, Prisayanh P, Qaqish BF, James K, Maldonado M, Aoki V, Hans-Filho G, Rivitti EA, Diaz LA. IgG autoantibody response against keratinocyte cadherins in endemic pemphigus foliaceus (fogo selvagem) J Invest Dermatol. 2012;132:2573–2580. doi: 10.1038/jid.2012.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rock B, Martins CR, Theofilopoulos AN, Balderas RS, Anhalt GJ, Labib RS, Futamura S, Rivitti EA, Diaz LA. The pathogenic effect of IgG4 autoantibodies in endemic pemphigus foliaceus (fogo selvagem) N Engl J Med. 1989;320:1463–1469. doi: 10.1056/NEJM198906013202206. [DOI] [PubMed] [Google Scholar]
- 16.Jones CC, Hamilton RG, Jordon RE. Subclass distribution of human IgG autoantibodies in pemphigus. J Clin Immunol. 1988;8:43–49. doi: 10.1007/BF00915155. [DOI] [PubMed] [Google Scholar]
- 17.David M, Katzenelson V, Hazaz B, Ben-Chetrit A, Sandbank M. Determination of IgG subclasses in patients with pemphigus with active disease and in remission. Arch Dermatol. 1989;125:787–790. [PubMed] [Google Scholar]
- 18.Kim YH, Geoghegan WD, Jordon RE. Pemphigus immunoglobulin G subclass autoantibodies: studies of reactivity with cultured human keratinocytes. J Lab Clin Med. 1990;115:324–331. [PubMed] [Google Scholar]
- 19.Wilson CL, Wojnarowska F, Dean D, Pasricha JS. IgG subclasses in pemphigus in Indian and UK populations. Clin Exp Dermatol. 1993;18:226–230. doi: 10.1111/j.1365-2230.1993.tb02175.x. [DOI] [PubMed] [Google Scholar]
- 20.Bhol K, Mohimen A, Ahmed AR. Correlation of subclasses of IgG with disease activity in pemphigus vulgaris. Dermatology (Basel) 1994;189(Suppl 1):85–89. doi: 10.1159/000246938. [DOI] [PubMed] [Google Scholar]
- 21.Bhol K, Natarajan K, Nagarwalla N, Mohimen A, Aoki V, Ahmed AR. Correlation of peptide specificity and IgG subclass with pathogenic and nonpathogenic autoantibodies in pemphigus vulgaris: a model for autoimmunity. Proc Natl Acad Sci USA. 1995;92:5239–5243. doi: 10.1073/pnas.92.11.5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dmochowski M, Hashimoto T, Nishikawa T. The analysis of IgG subclasses of anti-intercellular antibodies in pemphigus by an immunoblot technique. Arch Dermatol Res. 1992;284:309–311. doi: 10.1007/BF00372587. [DOI] [PubMed] [Google Scholar]
- 23.Kricheli D, David M, Frusic-Zlotkin M, Goldsmith D, Rabinov M, Sulkes J, Milner Y. The distribution of pemphigus vulgaris-IgG subclasses and their reactivity with desmoglein 3 and 1 in pemphigus patients and their first-degree relatives. Br J Dermatol. 2000;143:337–342. doi: 10.1046/j.1365-2133.2000.03659.x. [DOI] [PubMed] [Google Scholar]
- 24.Futei Y, Amagai M, Ishii K, Kuroda-Kinoshita K, Ohya K, Nishikawa T. Predominant IgG4 subclass in autoantibodies of pemphigus vulgaris and foliaceus. J Dermatol Sci. 2001;26:55–61. doi: 10.1016/s0923-1811(00)00158-4. [DOI] [PubMed] [Google Scholar]
- 25.Hacker MK, Janson M, Fairley JA, Lin MS. Isotypes and antigenic profiles of pemphigus foliaceus and pemphigus vulgaris autoantibodies. Clin Immunol. 2002;105:64–74. doi: 10.1006/clim.2002.5259. [DOI] [PubMed] [Google Scholar]
- 26.Ayatollahi M, Joubeh S, Mortazavi H, Jefferis R, Ghaderi A. IgG4 as the predominant autoantibody in sera from patients with active state of pemphigus vulgaris. J Eur Acad Dermatol Venereol. 2004;18:241–242. doi: 10.1111/j.1468-3083.2004.00708.x. [DOI] [PubMed] [Google Scholar]
- 27.David M, Katzenelson V, Mimouni D, Milner Y. The distribution of pemphigus vulgaris-IgG subclasses in patients with active disease. J Eur Acad Dermatol Venereol. 2006;20:232. doi: 10.1111/j.1468-3083.2006.01393.x. [DOI] [PubMed] [Google Scholar]
- 28.Sitaru C, Mihai S, Zillikens D. The relevance of the IgG subclass of autoantibodies for blister induction in autoimmune bullous skin diseases. Arch Dermatol Res. 2007;299:1–8. doi: 10.1007/s00403-007-0734-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diaz LA, Prisayanh PS, Dasher DA, Li N, Evangelista F, Aoki V, Hans-Filho G, dos Santos V, Qaqish BF, Rivitti EA Cooperative Group on Fogo Selvagem Research. The IgM anti-desmoglein 1 response distinguishes Brazilian pemphigus foliaceus (fogo selvagem) from other forms of pemphigus. J Invest Dermatol. 2008;128:667–675. doi: 10.1038/sj.jid.5701121. [DOI] [PubMed] [Google Scholar]
- 30.Ding X, Aoki V, Mascaro JM, Jr, Lopez-Swiderski A, Diaz LA, Fairley JA. Mucosal and mucocutaneous (generalized) pemphigus vulgaris show distinct autoantibody profiles. J Invest Dermatol. 1997;109:592–596. doi: 10.1111/1523-1747.ep12337524. [DOI] [PubMed] [Google Scholar]
- 31.Ding X, Diaz LA, Fairley JA, Giudice GJ, Liu Z. The anti-desmoglein 1 autoantibodies in pemphigus vulgaris sera are pathogenic. J Invest Dermatol. 1999;112:739–743. doi: 10.1046/j.1523-1747.1999.00585.x. [DOI] [PubMed] [Google Scholar]
- 32.Liebman JM, LaSala D, Wang W, Steed PM. When less is more: enhanced baculovirus production of recombinant proteins at very low multiplicities of infection. Biotechniques. 1999;26:36–38. 40, 42. doi: 10.2144/99261bm05. [DOI] [PubMed] [Google Scholar]
- 33.Amagai M, Komai A, Hashimoto T, Shirakata Y, Hashimoto K, Yamada T, Kitajima Y, Ohya K, Iwanami H, Nishikawa T. Usefulness of enzyme-linked immunosorbent assay using recombinant desmogleins 1 and 3 for serodiagnosis of pemphigus. Br J Dermatol. 1999;140:351–357. doi: 10.1046/j.1365-2133.1999.02752.x. [DOI] [PubMed] [Google Scholar]
- 34.Hollander M, Wolfe DA. Nonparametric Statistical Methods. Wiley & Sons; New York: 1999. [Google Scholar]
- 35.Farcomeni A. A review of modern multiple hypothesis testing, with particular attention to the false discovery proportion. Stat Methods Med Res. 2008;17:347–388. doi: 10.1177/0962280206079046. [DOI] [PubMed] [Google Scholar]
- 36.R Development Core Team. R Foundation for Statistical Computing. R Development Core Team; Vienna, Austria: 2015. R: a language and environment for statistical computing. [Google Scholar]
- 37.Funakoshi T, Lunardon L, Ellebrecht CT, Nagler AR, O’Leary CE, Payne AS. Enrichment of total serum IgG4 in patients with pemphigus. Br J Dermatol. 2012;167:1245–1253. doi: 10.1111/j.1365-2133.2012.11144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Squiquera HL, Diaz LA, Sampaio SA, Rivitti EA, Martins CR, Cunha PR, Lombardi C, Lavrado C, Borges P, Friedman H, et al. Serologic abnormalities in patients with endemic pemphigus foliaceus (Fogo selvagem), their relatives, and normal donors from endemic and non-endemic areas of Brazil. J Invest Dermatol. 1988;91:189–191. doi: 10.1111/1523-1747.ep12464490. [DOI] [PubMed] [Google Scholar]
- 39.Hilario-Vargas J, Vitorio IB, Stamey C, Culton DA, Prisayanh P, Rivitti EA, Aoki V, Filho GH, Dos Santos V, Qaqish B, Diaz LA. Analysis of anti-desmoglein 1 autoantibodies in 68 healthy mother/neonate pairs from a highly endemic region of fogo selvagem in Brazil. J Clin Exp Dermatol Res. 2014;5:209. doi: 10.4172/2155-9554.1000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qian Y, Prisayanh P, Andraca E, Qaqish BF, Aoki V, Hans-Filhio G, Rivitti EA, Diaz LA Cooperative Group on Fogo Selvagem Research. IgE, IgM, and IgG4 anti-desmoglein 1 auto-antibody profile in endemic pemphigus foliaceus (fogo selvagem) J Invest Dermatol. 2011;131:985–987. doi: 10.1038/jid.2010.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qian Y, Jeong JS, Abdeladhim M, Valenzuela JG, Aoki V, Hans-Filhio G, Rivitti EA, Diaz LA Cooperative Group on Fogo Selvagem Research. IgE anti-LJM11 sand fly salivary antigen may herald the onset of fogo selvagem in endemic Brazilian regions. J Invest Dermatol. 2015;135:913–915. doi: 10.1038/jid.2014.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garrod DR, Merritt AJ, Nie Z. Desmosomal cadherins. Curr Opin Cell Biol. 2002;14:537–545. doi: 10.1016/s0955-0674(02)00366-6. [DOI] [PubMed] [Google Scholar]
- 43.España A, Diaz LA, Mascaró JM, Jr, Giudice GJ, Fairley JA, Till GO, Liu Z. Mechanisms of acantholysis in pemphigus foliaceus. Clin Immunol Immunopathol. 1997;85:83–89. doi: 10.1006/clin.1997.4407. [DOI] [PubMed] [Google Scholar]
- 44.Roscoe JT, Diaz L, Sampaio SA, Castro RM, Labib RS, Takahashi Y, Patel H, Anhalt GJ. Brazilian pemphigus foliaceus autoantibodies are pathogenic to BALB/c mice by passive transfer. J Invest Dermatol. 1985;85:538–541. doi: 10.1111/1523-1747.ep12277362. [DOI] [PubMed] [Google Scholar]
- 45.Hisamatsu Y, Amagai M, Garrod DR, Kanzaki T, Hashimoto T. The detection of IgG and IgA autoantibodies to desmocollins 1-3 by enzyme-linked immunosorbent assays using baculovirus-expressed proteins, in atypical pemphigus but not in typical pemphigus. Br J Dermatol. 2004;151:73–83. doi: 10.1111/j.1365-2133.2004.05995.x. [DOI] [PubMed] [Google Scholar]
- 46.Nagasaka T, Nishifuji K, Ota T, Whittock NV, Amagai M. Defining the pathogenic involvement of desmoglein 4 in phigus and staphylococcal scalded skin syndrome. J Clin Invest. 2004;114:1484–1492. doi: 10.1172/JCI20480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ota T, Amagai M, Watanabe M, Nishikawa T. No involvement of IgG autoantibodies against extracellular domains of desmoglein 2 in paraneoplastic pemphigus or inflammatory bowel diseases. J Dermatol Sci. 2003;32:137–141. doi: 10.1016/s0923-1811(03)00072-0. [DOI] [PubMed] [Google Scholar]
- 48.Evangelista F, Dasher DA, Diaz LA, Prisayanh PS, Li N. E-cadherin is an additional immunological target for pemphigus autoantibodies. J Invest Dermatol. 2008;128:1710–1718. doi: 10.1038/sj.jid.5701260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qian Y, Jeong JS, Ye J, Dang B, Abdeladhim M, Aoki V, Hans-Filhio G, Rivitti EA, Valenzuela JG, Diaz LA. Overlapping IgG4 responses to self- and environmental antigens in endemic pemphigus foliaceus. J Immunol. 2016;196:2041–2050. doi: 10.4049/jimmunol.1502233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li N, Aoki V, Hans-Filho G, Rivitti EA, Diaz LA. The role of intramolecular epitope spreading in the pathogenesis of endemic pemphigus foliaceus (fogo selvagem) J Exp Med. 2003;197:1501–1510. doi: 10.1084/jem.20022031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eyre RW, Stanley JR. Human autoantibodies against a desmosomal protein complex with a calcium-sensitive epitope are characteristic of pemphigus foliaceus patients. J Exp Med. 1987;165:1719–1724. doi: 10.1084/jem.165.6.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Amagai M, Klaus-Kovtun V, Stanley JR. Autoantibodies against a novel epithelial cadherin in pemphigus vulgaris, a disease of cell adhesion. Cell. 1991;67:869–877. doi: 10.1016/0092-8674(91)90360-b. [DOI] [PubMed] [Google Scholar]
- 53.Amagai M, Karpati S, Prussick R, Klaus-Kovtun V, Stanley JR. Autoantibodies against the amino-terminal cadherin-like binding domain of pemphigus vulgaris antigen are pathogenic. J Clin Invest. 1992;90:919–926. doi: 10.1172/JCI115968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kowalczyk AP, Anderson JE, Borgwardt JE, Hashimoto T, Stanley JR, Green KJ. Pemphigus sera recognize conformationally sensitive epitopes in the amino-terminal region of desmoglein-1. J Invest Dermatol. 1995;105:147–152. doi: 10.1111/1523-1747.ep12316680. [DOI] [PubMed] [Google Scholar]
- 55.Payne AS, Ishii K, Kacir S, Lin C, Li H, Hanakawa Y, Tsunoda K, Amagai M, Stanley JR, Siegel DL. Genetic and functional characterization of human pemphigus vulgaris monoclonal autoantibodies isolated by phage display. J Clin Invest. 2005;115:888–899. doi: 10.1172/JCI24185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ishii K, Lin C, Siegel DL, Stanley JR. Isolation of pathogenic monoclonal anti-desmoglein 1 human antibodies by phage display of pemphigus foliaceus autoantibodies. J Invest Dermatol. 2008;128:939–948. doi: 10.1038/sj.jid.5701132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.