Abstract
Objective
The pathophysiology of obsessive-compulsive disorder (OCD) involves increased activity in cortico-striatal circuits connecting the anterior cingulate cortex with other brain regions. The error-related negativity (ERN) is a negative deflection in the event-related potential after an incorrect response that is believed to reflect anterior cingulate cortex activity. This study examined the relation of the ERN to OCD symptom dimensions and other childhood symptom dimensions.
Method
The ERN, correct response negativity, and accuracy were measured during a flanker task to assess performance monitoring in 80 youth with a lifetime diagnosis of OCD and 80 matched healthy comparison participants ranging from 8 to 18 years old. The relation of the ERN to OCD symptom dimension scores and Child Behavior Checklist Syndrome Scale scores was examined in multiple linear regression analyses.
Results
Accuracy was significantly decreased and ERN amplitude was significantly increased in patients compared with controls. ERN amplitude in patients was significantly correlated with accuracy, but not with OCD symptom dimensions, severity, comorbidity, or treatment. In a multiple linear regression analysis using age, accuracy, OCD, and Child Behavior Checklist Syndrome Scale scores as predictors of ERN amplitude, the ERN had significant associations only with Withdrawn/Depressed Scale scores and accuracy.
Conclusion
An enlarged ERN is a neural correlate of pediatric OCD that is independent of OCD symptom expression and severity. The finding of lower accuracy in pediatric cases requires replication. The relation between an enhanced ERN and withdrawn/depressed behaviors warrants further research in youth with OCD and other internalizing disorders.
Keywords: error-related negativity, biomarker, obsessive-compulsive disorder, Child Behavior Checklist, symptom dimensions
Obsessive-compulsive disorder (OCD) is a heterogeneous psychiatric syndrome, with lifetime prevalence estimates ranging from 1% to 3% and a median age at onset of approximately 19 years.1,2 OCD is characterized by recurrent intrusive thoughts and repetitive behaviors or mental acts that vary in their content and are often associated with other psychiatric disorders.2,3 Brain imaging studies have indicated the pathophysiology of OCD involves increased activity in corticostriatal circuits connecting the anterior cingulate cortex with other brain regions.4,5 However, it is unclear whether the phenotypic heterogeneity of OCD reflects distinct or partially distinct disease mechanisms.6 OCD symptom dimensions can have specific relations to genetic variation, comorbid psychiatric disorders, and treatment response.6,7 Hence, further research is warranted on the relation of putative OCD biomarkers to OCD symptom dimensions and other symptom dimensions often associated with OCD.
The error-related negativity (ERN),8 or error negativity,9 is a negative deflection in the response-locked event-related potential that peaks within 100 ms after an incorrect response. It is believed to be generated mainly by the dorsal anterior cingulate cortex and to reflect an alarm signal to increase cognitive control and adjust behavior.10 The ERN has a heritability of 47% in youth, suggesting it might serve as an endophenotype in genetic studies of childhood psychopathology.11 The ERN is a unit of analysis in 3 domains of the Research Domain Criteria project: cognitive systems (cognitive control: performance monitoring), negative valence systems (sustained threat), and positive valence systems (reward learning).12 Its placement in 3 separate domains suggests that it reflects variance in each domain, but further research is required to delineate the behaviors associated with the ERN across the lifespan.12
Increased ERN amplitudes have been demonstrated in most studies of patients with OCD using tasks eliciting response conflict.5,6,12–24 An enlarged ERN has been detected in unaffected first-degree relatives of probands with OCD, indicating that overactive performance monitoring can occur in relatives at risk for developing OCD.18,24 An enhanced ERN has been shown to remain unchanged in patients with OCD, whereas symptom severity has been shown to decrease significantly with cognitive-behavioral therapy, demonstrating that increased error-related brain activity does not necessarily maintain OCD symptoms.21,22 Most studies reporting an enlarged ERN in patients with OCD have detected no correlation between ERN amplitude and OCD symptom severity.5,6,12,13,15–24 A recent study of performance monitoring in adults with OCD found overactive performance monitoring was independent of OCD symptom severity and lifetime symptom dimension scores.6 However, for current symptom dimension scores, an association with mental rituals and superstitious behaviors was found, with higher scores associated with more error-related brain activity. Thus, studies suggest the ERN is a state-independent measurement that could serve as a biomarker or endophenotype for OCD.12,13,18,21,22,24
Because the relation between the ERN and OCD symptom dimensions has not been examined in pediatric OCD, the present study was conducted in 80 youth with a lifetime diagnosis of OCD and 80 age-matched healthy controls using a flanker task.5,23,24 The aims of the study were to examine the relation of the ERN to the OCD symptom dimensions noted earlier and Child Behavior Checklist (CBCL) Syndrome Scales.6,7,25 The CBCL Syndrome Scales were examined because they provide a dimensional classification of psychopathology without reference to traditional categorical diagnoses that might account for a significant amount of the variance in the ERN independent of lifetime OCD diagnosis.12,25
METHOD
Participants
Patients with OCD were recruited from the Department of Psychiatry at the University of Michigan and surrounding community. Comparison participants were recruited from the surrounding community and were matched to patients by age and sex. After a complete description of the study, written informed consent was obtained from at least 1 parent of the participant and written informed assent was obtained from the participant. Participants were paid for their interviews and psychophysiologic recordings. All tasks and procedures were approved by the University of Michigan Medical School Institutional Review Board. Some participants were excluded based on poor electroencephalographic data (n = 2), accuracy level lower than 65% during the task (n = 1), or commission of fewer than 10 errors (n = 3), leaving 160 participants. The final sample consisted of 67 boys and 93 girls 8.0 to 18 years old (mean 13.5, standard deviation 3.0), with an ethnic and racial breakdown that was 86.9% Caucasian, 1.9% Black, 4.4% Latino, 3.7% Asian, and 3.1% Native American.
All 80 patients had a lifetime diagnosis of OCD. Patients were excluded if they had a lifetime diagnosis of autistic disorder, schizophrenia, other psychotic disorder, bipolar disorder, substance-related disorder, or anorexia nervosa. All 80 comparison participants had no history of a specific Axis I disorder. Lifetime and current Axis I diagnoses were made independently by 2 clinicians using all sources of information according to DSM-IV criteria. Participants were excluded if they had a history of intellectual disability, head injury with a loss of consciousness, or chronic neurological disorder other than tics. All participants lived with at least 1 English-speaking biological parent willing to participate in the research.
All 160 participants were interviewed with the Schedule for Schizophrenia and Affective Disorders for School-Aged Children-Present and Lifetime Version26 and the Schedule for Obsessive-Compulsive and Other Behavioral Syndromes (SOCOBS).27 The lifetime and current severity of OCD was assessed in patients with a modified version of the Children’s Yale-Brown Obsessive Compulsive Disorder Scale (CY-BOCS), with patients and their parents providing item scores retrospectively for the most severe episode of OCD and item scores for current severity.28 OCD symptom dimension scores were derived for patients using the SOCOBS checklist, with assignment of items to symptom dimensions based on the largest item-level factor analysis of OCD symptoms.7 The 5 symptom dimensions were taboo, contamination/cleaning, doubt, rituals/superstitions, and hoarding/symmetry. Each patient was described by 5 dimensional scores ranging from 0 to 1 for current and lifetime symptoms, respectively. Parents completed the CBCL25,29 and Social Communication Questionnaire30 about their children. Patients and controls completed the Children’s Depression Inventory31 about themselves.
Table 1 presents the demographic, clinical, behavioral, and event-related brain potential data for the patients with OCD and healthy controls ranging in age from 8 to 18 years. The OCD group had 31 boys and the comparison group had 36 boys (p = .42). Age at onset of OCD symptoms in the patients ranged from 2 to 16 years. Current and lifetime CY-BOCS scores in the patients with OCD ranged from 0 to 37 and 11 to 38, respectively. Although all patients had a lifetime diagnosis of OCD, 54 had a current diagnosis, 26 a past diagnosis with OCD symptoms that no longer met the criteria for diagnosis, and 61 had a history of at least 1 other specific Axis I disorder. Because studies have found that treatment with a serotonin reuptake inhibitor has no effect on the ERN,12,13,16,18,21 34 patients were enrolled taking a stable dose of a serotonin reuptake inhibitor but no other psychotropic medications.
TABLE 1.
Demographic, Clinical, Behavioral, and Brain Potential Data in Pediatric Patients With Obsessive-Compulsive Disorder (OCD) and Healthy Comparison Participants
| Variable | Patients With OCD (n = 80) |
Health Controls (n = 80) |
Patients With OCD vs. Healthy Controls
|
|||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Test Statistic | p | |
| Demographic and clinical data | ||||||
| Age (y) | 13.5 | 3.0 | 13.6 | 3.0 | t158 = 0.28 | .78 |
| Child Behavior Checklist | ||||||
| Obsessive-Compulsive scale | 6.2 | 3.9 | 0.6 | 0.9 | t157 = 12.49 | <.0001 |
| Total score | 36.1 | 21.8 | 7.9 | 7.4 | t157 = 10.92 | <.0001 |
| Internalizing score | 13.8 | 9.1 | 2.4 | 2.4 | t157 = 10.61 | <.0001 |
| Externalizing score | 6.6 | 6.6 | 2.4 | 3.1 | t157 = 5.18 | <.0001 |
| Anxious/Depressed scale | 7.7 | 5.2 | 1.1 | 1.6 | t157 = 10.85 | <.0001 |
| Withdrawn/Depressed scale | 2.8 | 2.5 | 0.7 | 1.2 | t157 = 6.89 | <.0001 |
| Somatic Complaints scale | 3.3 | 3.1 | 0.6 | 0.9 | t157 = 7.60 | <.0001 |
| Social Problems scale | 2.9 | 2.9 | 0.5 | 0.9 | t157 = 6.98 | <.0001 |
| Thought Problems scale | 5.5 | 3.6 | 0.5 | 0.7 | t157 = 12.14 | <.0001 |
| Attention Problems scale | 4.4 | 4.2 | 1.2 | 1.6 | t157 = 6.27 | <.0001 |
| Rule-Breaking Behavior scale | 1.4 | 2.2 | 0.7 | 1.0 | t157 = 2.53 | .0125 |
| Aggressive Behavior scale | 4.9 | 4.9 | 1.4 | 1.8 | t157 = 6.04 | <.0001 |
| Children’s Depression Inventory | 10.8 | 7.4 | 3.0 | 3.0 | t157 = 8.72 | <.0001 |
| Social Communication Questionnaire | 3.5 | 3.2 | 1.8 | 2.3 | t157 = 3.84 | .0002 |
| Age at onset of OCD symptoms (y) | 7.3 | 3.1 | ||||
| Duration of OCD symptoms (y) | 5.9 | 3.6 | ||||
| CY-BOCS lifetime score | 27.4 | 6.9 | ||||
| CY-BOCS current score | 16.1 | 9.4 | ||||
| Behavioral data | ||||||
| Total number of trials | 495.0 | 54.0 | 489.4 | 58.8 | t158 = 0.70 | .48 |
| Total number of error trials | 61.6 | 24.0 | 51.6 | 25.9 | t158 = 2.85 | .005 |
| Accuracy on all trials | 0.87 | 0.07 | 0.89 | 0.06 | t158 = 2.74 | .007 |
| Accuracy on congruent trials | 0.95 | 0.04 | 0.96 | 0.04 | t158 = 2.05 | .042 |
| Accuracy on incongruent trials | 0.79 | 0.10 | 0.83 | 0.08 | t158 = 2.54 | .012 |
| Accuracy after correct trials | 0.87 | 0.06 | 0.90 | 0.05 | t158 = 2.58 | .011 |
| Accuracy after incorrect trials | 0.86 | 0.10 | 0.90 | 0.08 | t158 = 2.48 | .014 |
| Error reaction time (ms) | 452.3 | 203.8 | 451.1 | 176.6 | t158 = 0.04 | .97 |
| Correct reaction time (ms) | 500.3 | 150.0 | 504.2 | 152.9 | t158 = 0.17 | .87 |
| Reaction time on congruent trials (ms) | 476.4 | 141.2 | 476.6 | 138.1 | t158 = 0.01 | .99 |
| Reaction time on incongruent trials (ms) | 545.3 | 167.2 | 552.8 | 179.3 | t158 = 0.27 | .78 |
| Post-error reaction time (ms) | 501.6 | 162.0 | 506.0 | 152.1 | t158 = 0.18 | .86 |
| Event-related brain potential data | ||||||
| Error-related negativity, Cz (μV) | −1.39 | 5.04 | 0.80 | 5.53 | F1,157 = 9.83 | .002 |
| Correct response negativity, Cz (μV) | 3.45 | 4.94 | 3.41 | 4.85 | F1,157 = 0.009 | .92 |
| ΔERN, Cz (μV) | −4.84 | 5.63 | −2.62 | 5.67 | F1,157 = 7.05 | .009 |
| Error-related negativity, FCz (μV) | −3.39 | 4.98 | −2.21 | 5.16 | F1,157 = 3.73 | .055 |
| Correct response negativity, FCz (μV) | 2.61 | 4.40 | 2.35 | 4.11 | F1,157 = 0.17 | .68 |
| ΔERN, FCz (μV) | −5.99 | 5.51 | −4.57 | 6.02 | F1,157 = 2.72 | .10 |
Note: Parent and self-report questionnaires were not completed for 1 patient with OCD at the time of event-related potential data collection. CY-BOCS = Children’s Yale-Brown Obsessive Compulsive Scale; ΔERN = error-related negativity amplitude minus correct response negativity amplitude; OCD = obsessive-compulsive disorder; SD = standard deviation.
Task and Procedure
Participants performed a modified Eriksen flanker task in which arrows appeared on a computer display with congruent (e.g., →→→→→) and incongruent (e.g., →→←→→) conditions.32 They were instructed to respond by pressing 1 of 2 buttons indicating the direction of the central arrow (i.e., right versus left) while ignoring the adjacent arrows and to respond as quickly and accurately as possible, placing equal emphasis on speed and accuracy. The stimuli remained on the screen for 250 ms, with an interval of 1,500 ms between consecutive stimuli. Each participant was seated 0.65 m directly in front of the computer monitor. After 32 practice trials, each participant completed 8 blocks of 64 trials, with the number of completed trials ranging from 256 to 512. Performance feedback was provided after every block to yield an error rate of approximately 10%, with encouragement to focus on speed if there were fewer than 4 errors or to focus on accuracy if there were more than 10 errors.5,23,24
Electrophysiologic Recording, Data Reduction, and Analysis
The electroencephalogram was recorded from DC-104 Hz with 64 Ag/AgCl scalp electrodes, 2 mastoid electrodes, and 2 vertical and 2 horizontal electro-oculogram electrodes using the BioSemi Active-Two system. Data were digitized at 512 Hz, referenced to a ground formed from a common mode sense active electrode and driven right leg passive electrode (http://www.biosemi.com/faq/cms&drl.htm), and re-referenced offline to the average of the 2 mastoid electrodes. Data were bandpass filtered at 0.1–30 Hz using 0-phase shift filters. Electroencephalographic data were screened using automated algorithms that rejected epochs in which absolute voltage exceeded 500 μV and epochs containing peak-to-peak activity greater than 500 μV within 200 ms, with a 100-ms moving window, for midline channels (Fz, FCz, Cz, CPz, Pz). Then, ocular movement artifacts were corrected using a regression-based algorithm.33 After ocular correction, individual trials were rejected if they contained absolute amplitudes greater than 100 μV, a change greater than 50 μV measured from 1 data point to the next point, or a maximum voltage difference less than 0.5 μV within a trial in any of the midline electrodes.
Behavioral measurements included the number of erroneous and correct trials for each participant and accuracy expressed as a percentage of valid trials. Mean reaction times on error and correct trials were calculated separately, and trials were excluded if their reaction times were more than 3 standard deviations from the mean. Reaction time and accuracy after errors were evaluated to determine whether there were group differences in post-error behavioral adjustments.10 Reaction times were analyzed with group as a between-subject factor and response type as a within-subject factor. The mean number of errors per subject contributing to the analysis was 64.3 (standard deviation 30.2, range 10–139).
The ERN was quantified using mean amplitude measurements relative to a pre-response baseline of −200 to −50 ms. The mean amplitude of the ERN was computed on incorrect response trials in a window from 0 to 80 ms after the incorrect response. The correct response negativity (CRN) consisted of the same measurement computed on correct response trials. The ΔERN was calculated by subtracting the CRN from the ERN because it can isolate activity unique to error processing from activity more broadly related to response monitoring.8,10 Amplitudes were calculated for electrodes FCz and Cz; however, the focus of the present data was the ERN and ΔERN at Cz because prior studies have found larger group differences at Cz.5,14,23,24
Student t tests were used to evaluate group differences in demographic, clinical, and behavioral measurements. Pearson correlation coefficients were used to examine associations of response-related amplitudes with age, behavioral measurements, and clinical measurements. Electrocortical indicators (ERN, CRN, ΔERN) of performance monitoring were analyzed separately using a repeated-measure analysis of covariance with group (patients with OCD, healthy controls) as a between-subject factor, response type (correct, error) as a within-subject factor, and age and accuracy included as covariates.10 Multiple linear regression analyses were used to examine the relation of the ERN and ΔERN to OCD symptom dimensions and CBCL Syndrome Scale scores. Additional analyses of covariance were conducted in patients with OCD with medication status and comorbid diagnoses as between-subject factors. Analyses were performed with JMP 10 software. All tests were 2-tailed with an α value equal to 0.05.
RESULTS
Behavioral Data in Patients With OCD and Healthy Controls
Participants were significantly more accurate on congruent than incongruent trials (paired t159 = 23.39, p < .0001). Controls were significantly more accurate than patients with OCD in all conditions (Table 1). There were no significant group differences in reaction time during correct or incorrect trials or in post-error slowing. Correct responses were significantly slower than incorrect responses (paired t159 = 8.17, p < .0001). No main effect of group or response type for reaction time and no interaction between group and response type for reaction time reached significance (p = .96 and p = .68, respectively). In all participants, age had significant negative correlations with reaction time on correct (r = −0.51, p < .0001) and incorrect (r = −0.38, p = .014) trials and a significant positive correlation with post-error slowing (r = 0.20, p = .013). There was a trend for a correlation in all participants between age and accuracy (p = .09), with age significantly correlated with accuracy in controls (r = 0.27, p = .015), but not in patients (p = .84). In all participants, age was significantly correlated with post-error accuracy (r = 0.23, p = .004), but not with post-correct accuracy (p = .17). There were no significant sex differences for accuracy, post-error or post-correct accuracy, reaction time on correct or incorrect trials, or post-error slowing (p > .08 for all comparisons).
Event-Related Potential Data in Patients With OCD and Healthy Controls
Age in all participants was significantly correlated with CRN and ΔERN amplitudes (r = 0.17, p = .03 and r = −0.26, p = .0009, respectively), but not with ERN amplitudes (p = .13). There was a trend in all participants for a correlation between the ERN and accuracy (r = −0.14, p = .066), with the ERN having a significant correlation with accuracy in in patients (r = −0.32, p = .003) but not in controls (p = .63). Neither the CRN nor ΔERN had significant correlations all participants with accuracy (p > .1 for the 2 comparisons). ERN amplitude in all participants had no significant correlations with reaction times on correct or incorrect trials or with post-error slowing (p > .7 for all comparisons). In contrast, CRN and ΔERN amplitudes had significant correlations in all participants with reaction times on correct (r = −0.42, p < .0001 and r = 0.35, p < .0001, respectively) and incorrect (r = −0.35, p =.0001 and r = 0.27, p = .0004, respectively) trials, but not with post-error slowing (p > .4 for the 2 comparisons).
The ERN amplitude was significantly increased in patients compared with controls (F1,157 = 9.83, p = .002, Cohen d = 0.413), with a significant effect for accuracy (F1,157 = 6.38, p = .013; Table 1; Figure 1). The ERN was significantly enlarged in patients with a current (F1,131 = 6.22, p = .014) or past (F1,103 = 5.34, p = .023) diagnosis of OCD. Similarly, ΔERN amplitude at Cz was significantly increased in patients compared with controls (F1,157 = 7.05, p = .009, Cohen d = 0.394), with a significant effect for age (F1,157 = 12.29, p = .0006; Table 1). The ΔERN was significantly enhanced in patients with a current (F1,131 = 4.81, p = .030) or past (F1,103 = 4.39, p = .039) diagnosis of OCD. CRN amplitude at Cz was not significantly different between patients and controls. There were no significant sex differences in any brain potentials (p > .1 for all comparisons).
FIGURE 1.
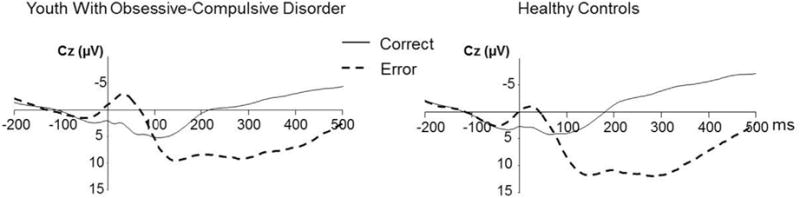
Grand averages of electroencephalographic recordings in youth with obsessive-compulsive disorder and healthy comparison participants. Note: Images depict response-locked grand average waveforms recorded at the central (Cz) electrode for correct and incorrect responses. Responses occurred at 0 ms. The mean amplitude of the error-related negativity (ERN) was computed in a window of 0 to 80 ms after incorrect response trials. CRN = correct response negativity.
CBCL and Event-Related Potential Data in Patients With OCD and Healthy Controls
Separate multiple linear regression analyses were conducted in all participants to examine the relation of the CBCL Syndrome Scales to the ERN and ΔERN.25 Age, accuracy, lifetime OCD diagnosis, and CBCL Syndrome Scale scores were used as predictors with the ERN or ΔERN as the dependent variable. The ERN had significant associations only with the Withdrawn/Depressed scale scores (p = .014) and accuracy (p = .04; Table 2). Similarly, the ΔERN had significant associations only with the Withdrawn/Depressed scale scores (p = .03) and age (p = .002; Table S1, available online). Backward stepwise regression analyses confirmed that no other variable had a significant effect on the ERN or ΔERN and yielded coefficients for predictors in the reduced models consistent with those in the full models (Tables 1 and S1, available online). A correlation matrix with predictor and dependent variables is presented in Table S2 (available online).
TABLE 2.
Multiple Linear Regression Model for Error-Related Negativity at the Central Electrode (Cz) as Dependent Variable and Age, Accuracy, Obsessive-Compulsive Disorder (OCD), and Child Behavior Checklist (CBCL) Syndrome Scales as Predictors
| Full Model | Regression
|
Correlation
|
||||||
|---|---|---|---|---|---|---|---|---|
| R2 | β | β (SE) | t | p | F | r (bivariate) | r (partial) | |
| 0.146 | .013 | 2.29 | ||||||
| Age | −0.08 | 0.15 | −0.56 | .58 | −0.12 | −0.05 | ||
| Accuracy | −17.22 | 7.60 | −2.26 | .025 | −0.15 | −0.16 | ||
| OCD | −1.94 | 1.28 | −1.52 | .13 | −0.20 | −0.12 | ||
| CBCL Anxious/Depressed scale | 0.08 | 0.18 | 0.47 | .64 | −0.18 | 0.04 | ||
| CBCL Withdrawn/Depressed scale | −0.68 | 0.27 | −2.50 | .014 | −0.30 | −0.20 | ||
| CBCL Somatic Complaints scale | −0.09 | 0.22 | −0.43 | .66 | −0.20 | −0.03 | ||
| CBCL Social Problems scale | 0.014 | 0.24 | 0.06 | .95 | −0.12 | 0.003 | ||
| CBCL Thought Problems scale | 0.006 | 0.22 | 0.03 | .98 | −0.18 | 0.0006 | ||
| CBCL Attention Problems scale | 0.14 | 0.16 | 0.83 | .41 | −0.06 | 0.07 | ||
| CBCL Rule-Breaking Behavior scale | −0.07 | 0.33 | −0.20 | .84 | −0.11 | −0.02 | ||
| CBCL Aggressive Behavior scale | 0.03 | 0.15 | 0.17 | .86 | −0.09 | −0.02 | ||
|
| ||||||||
| Reduced Modela |
Regression
|
Correlation
|
||||||
| R2 | β | β (SE) | t | p | F | r (bivariate) | r (partial) | |
|
| ||||||||
| 0.118 | <.0001 | 10.45 | ||||||
| Accuracy | −15.98 | 6.90 | −2.32 | .022 | −0.15 | −0.16 | ||
| CBCL Withdrawn/Depressed scale | −0.72 | 0.18 | −3.98 | <.0001 | −0.30 | −0.30 | ||
Note. A CBCL was not completed for 1 patient with OCD when event-related potential data were collected. Boldface data represent significant results. SE = standard error.
After backward stepwise deletion of nonsignificant variables.
Clinical, Behavioral, and Event-Related Potential Data in Patients With OCD
There were no significant differences in any brain potentials between patients with a current or past diagnosis of OCD (p > .4 for all comparisons). There were no significant correlations in the patients between any brain potentials and current or lifetime CY-BOCS scores (p > .1 for all comparisons) or CBCL Obsessive-Compulsive Scale scores (p > .3 for all comparisons).28,29 However, accuracy had a significant positive correlation in patients with CBCL Obsessive-Compulsive scale scores (r = 0.29, p = .009) and a trend for a correlation with current CY-BOCS scores (r = 0.22, p = .054). There were no significant differences in any brain potentials between patients with and those without a particular comorbid diagnosis (p > .1 for all comparisons). There were no significant differences in any brain potentials between patients taking or not taking a serotonin reuptake inhibitor (p > .1 for all comparisons).
Multiple regression analyses were conducted in patients to examine the relation of current and lifetime OCD symptom dimension scores to the ERN and ΔERN at Cz.6,7 The ERN has no significant associations with the current or lifetime OCD symptom scores (p > .1 for all comparisons; Table 3). Similarly, the ΔERN had no significant associations with the current or lifetime OCD symptoms score (p > .3 for all comparisons; Table S3, available online).
TABLE 3.
Multiple Linear Regression Model for Error-Related Negativity at the Central Electrode (Cz) as the Dependent Variable and Obsessive-Compulsive Disorder (OCD) Symptom Dimensions, Age, and Accuracy as Predictors
| Regression
|
Correlation
|
|||||||
|---|---|---|---|---|---|---|---|---|
| R2 | β | β (SE) | t | p | F | r (bivariate) | r (partial) | |
| Present symptoms | 0.153 | .09 | 1.86 | |||||
| Taboo | −0.25 | 4.86 | −0.05 | .96 | −0.02 | −0.006 | ||
| Cleaning/contamination | −0.76 | 2.63 | −0.29 | .77 | −0.005 | −0.03 | ||
| Doubts | −0.76 | 4.38 | −0.17 | .86 | −0.03 | −0.02 | ||
| Rituals/superstitions | −2.88 | 4.48 | −0.64 | .52 | −0.02 | −0.08 | ||
| Symmetry/hoarding | 5.30 | 3.58 | 1.48 | .14 | 0.06 | 0.17 | ||
| Age | −0.27 | 0.19 | −1.39 | .17 | −0.14 | −0.16 | ||
| Accuracy | −25.87 | 8.38 | −3.09 | .003 | −0.32 | −0.34 | ||
| Lifetime symptoms | 0.189 | .03 | 2.40 | |||||
| Taboo | 4.79 | 3.87 | 1.24 | .22 | −0.004 | 0.14 | ||
| Cleaning/contamination | −2.44 | 2.32 | −1.05 | .30 | −0.14 | −0.12 | ||
| Doubts | −4.09 | 3.61 | −1.13 | .26 | −0.21 | −0.13 | ||
| Rituals/superstitions | −3.08 | 3.23 | 0.95 | .19 | −0.19 | −0.14 | ||
| Symmetry/hoarding | 3.08 | 3.23 | 0.95 | .34 | −0.07 | 0.11 | ||
| Age | −0.14 | 0.19 | −0.76 | .45 | −0.14 | −0.09 | ||
| Accuracy | −23.01 | 8.17 | −2.82 | .006 | −0.33 | −0.32 | ||
Note: Boldface data represent significant results. SE = standard error.
DISCUSSION
The finding of an enlarged ERN in youth with OCD during a task eliciting response conflict is consistent with previous reports of increased performance monitoring in OCD.5,6,12–24 As in most studies of the ERN in OCD, we found no relation between the ERN and OCD symptom severity or current diagnostic status.5,6,12,13,16–24 Contrary to a report that overactive performance monitoring in adults with OCD is associated with current mental rituals and superstitious behaviors,6 we found no relation between the ERN and current or lifetime OCD symptom dimensions. A study of adolescent girls noted the ERN was enlarged primarily in older adolescents with self-reported checking behaviors,34 suggesting that a community sample with a continuous distribution of checking behaviors might detect a relation between performance monitoring and a specific compulsion that might be missed in studies with OCD cases. Contrary to our previous study suggesting the ERN is increased in non–tic-related but not in tic-related OCD,23 we found no evidence that the ERN is influenced significantly by tic history or any other comorbid psychiatric disorder. Overall, our results demonstrate that the ERN in pediatric OCD is independent of OCD symptom severity and expression.
In contrast to 2 studies finding increased accuracy in adults with OCD,6,21 our study found decreased accuracy in youth with OCD compared with healthy controls. However, accuracy in patients was still negatively correlated with the ERN, becoming larger (more negative) as accuracy improved. Accuracy in patients was positively correlated with OCD symptom severity, suggesting that more severe symptoms did not interfere with task performance. The higher error rate is consistent with the hypothesis that OCD involves defects in an error-detection system, which might give rise to repeated doubts about actions and excessive worries about potential mistakes.35 Follow-up studies might determine whether performance on response conflict tasks becomes more accurate in youth with OCD as they mature into adulthood, perhaps in conjunction with a persistently enlarged ERN.
The ERN amplitude was more strongly associated with the CBCL Withdrawn/Depressed scale scores than with any other clinical variable including lifetime OCD diagnosis, demonstrating the utility of including a dimensional classification of psychopathology in psychophysiologic studies.12,34 The finding requires replication in studies of youth with OCD and other internalizing disorders to assess the specificity of this relation across diagnoses. Because the sustained threat construct includes the ERN as a unit of analysis,12,34 persistent obsessions or concerns about mistakes might be endogenous threats, with the ERN possibly reflecting those threats. The Withdrawn/Depressed scale might quantify some of the negative affect or avoidant and anhedonic behaviors associated with the sustained threat construct.
The association between the ERN and withdrawn/depressed behaviors is consistent with the report of an enlarged ERN in adults with OCD or social phobia, suggesting an enlarged ERN could represent a transdiagnostic liability index.20 Increased ERN amplitudes have been found in children with high behavioral inhibition compared with those with low behavioral inhibition, with a large ΔERN related to later childhood social phobia symptoms in children with high behavioral inhibition.36 An increased ΔERN at 6 years of age predicted in another study the onset of new anxiety disorders by 9 years after controlling for baseline anxiety symptoms.37 Longitudinal studies have shown that withdrawn behavior in children has considerable stability throughout childhood that is largely influenced by genetic effects,38 and that withdrawn behavior in childhood is predictive of anxiety disorders and major depression in adolescence and adulthood.39 Epidemiologic studies have noted that social phobia is the most common comorbid anxiety disorder in adults with OCD.2 It is unknown whether an enlarged ERN lies on the causal pathway between genes and OCD or social phobia and is more reflective of the causes than the consequences of either disorder. Even if the ERN is a biomarker rather than an endophenotype, it might still identify a more genetically homogeneous form of OCD that is associated with a higher risk for social phobia.18,24,40
Our study has limitations requiring further consideration. The assessment of lifetime OCD symptom dimensions and symptom severity was performed retrospectively rather than prospectively. Performance monitoring was not assessed prospectively during treatment, so it is unknown whether the ERN might be decreased in patients concurrently with a decrease in OCD and social withdrawal symptoms.
Our study provides further evidence that an enlarged ERN is a neural correlate of pediatric OCD that is independent of OCD symptom severity and expression.5,6,12,13,15–24 Patients were less accurate than controls in their performance despite having an enlarged ERN, and Withdrawn/Depressed scale scores accounted for more of the ERN variance than did a lifetime diagnosis of OCD. The relation between the ERN and withdrawn/depressed behaviors warrants further research in youth with OCD and other internalizing disorders because it could provide a better understanding of anterior cingulate cortex dysregulation in the pathogenesis of severe childhood internalizing disorders and lead to new prevention and treatment strategies.5,12,13,21–24,34–39
Supplementary Material
Acknowledgments
This research was supported by National Institute of Mental Health grants R01 MH085321 (G.L.H.) and R01 MH101493 (G.L.H. and W.J.G.).
Brenda W. Gillespie, PhD, served as the statistical expert for this research.
The authors thank Brenda W. Gillespie, PhD, of the University of Michigan, for statistical consultation.
Footnotes
Supplemental material cited in this article is available online.
Disclosure: Drs. Hanna, Liu, Gehring, Ms. Isaacs, Ms. Ayoub, Mr. Torres, and Mr. O’Hara report no biomedical financial interests or potential conflicts of interest.
Contributor Information
Drs. Gregory L. Hanna, University of Michigan, Ann Arbor.
Yanni Liu, University of Michigan, Ann Arbor.
Yona E. Isaacs, University of Michigan, Ann Arbor.
Mss. Angela M. Ayoub, University of Michigan, Ann Arbor.
Jose J. Torres, University of Michigan, Ann Arbor.
Mr. Nolan B. O’Hara, Wayne State University School of Medicine, Detroit.
William J. Gehring, University of Michigan, Ann Arbor.
References
- 1.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 2.Ruscio AM, Stein DJ, Chiu WT, Kessler RC. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol Psychiatry. 2010;15:53–63. doi: 10.1038/mp.2008.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanna GL. Demographic and clinical features of obsessive-compulsive disorder in children and adolescents. J Am Acad Child Adolesc Psychiatry. 1995;34:19–27. doi: 10.1097/00004583-199501000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Milad MR, Rauch SL. Obsessive-compulsive disorder: beyond segregated cortico-striatal pathways. Trends Cogn Sci. 2012;16:43–51. doi: 10.1016/j.tics.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Y, Hanna GL, Carrasco M, Gehring WJ, Fitzgerald KD. Altered relationship between electrophysiological response to errors and gray matter volumes in an extended network for error processing in pediatric obsessive-compulsive disorder. Hum Brain Mapp. 2014;35:1143–1153. doi: 10.1002/hbm.22240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riesel A, Kathmann N, Endrass T. Overactive performance monitoring in obsessive-compulsive disorder is independent of symptom expression. Eur Arch Psychiatry Clin Neurosci. 2014;264:707–717. doi: 10.1007/s00406-014-0499-3. [DOI] [PubMed] [Google Scholar]
- 7.Katerberg H, Delucchi KL, Stewart SE, et al. Symptom dimensions in OCD: item-level factor analysis and heritability estimates. Behav Genet. 2010;40:505–517. doi: 10.1007/s10519-010-9339-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E. A neural system for error detection and compensation. Psychol Sci. 1993;4:385–390. [Google Scholar]
- 9.Falkenstein M, Hohnsbein J, Hoormann J, Blanke L. Effects of crossmodal divided attention on late ERP components, II: error processing in choice reaction tasks. Electroencephalogr Clin Neurophysiol. 1991;78:447–455. doi: 10.1016/0013-4694(91)90062-9. [DOI] [PubMed] [Google Scholar]
- 10.Gehring WJ, Liu Y, Orr JM, Carp J. The error-related negativity (ERN/Ne) In: Luck SK, Kappenman E, editors. Oxford Handbook of Event-Related Potential Components. New York: Oxford University Press; 2012. pp. 231–291. [Google Scholar]
- 11.Anokhin AP, Golosheykin S, Heath AC. Heritability of frontal brain function related to action monitoring. Psychophysiology. 2008;45:524–534. doi: 10.1111/j.1469-8986.2008.00664.x. [DOI] [PubMed] [Google Scholar]
- 12.Weinberg A, Dieterich R, Riesel A. Error-related brain activity in the age of RDoC: a review of the literature. Int J Psychophysiology. 2015;98:276–299. doi: 10.1016/j.ijpsycho.2015.02.029. [DOI] [PubMed] [Google Scholar]
- 13.Endrass T, Ullsperger M. Specificity of performance monitoring changes in obsessive-compulsive disorder. Neurosci Biobehavior Rev. 2014;46:124–138. doi: 10.1016/j.neubiorev.2014.03.024. [DOI] [PubMed] [Google Scholar]
- 14.Gehring WJ, Himle JA, Nisenson LG. Action-monitoring dysfunction in obsessive-compulsive disorder. Psychol Sci. 2000;11:1–6. doi: 10.1111/1467-9280.00206. [DOI] [PubMed] [Google Scholar]
- 15.Endrass T, Klawohn J, Schuster F, Kathmann N. Overactive performance monitoring in obsessive-compulsive disorder: ERP evidence from correct and erroneous reactions. Neuropsychologia. 2008;46:1877–1887. doi: 10.1016/j.neuropsychologia.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Stern ER, Liu Y, Gehring WJ, et al. Chronic medication does not affect hyperactive error responses in obsessive-compulsive disorder. Psychophysiology. 2010;47:913–920. doi: 10.1111/j.1469-8986.2010.00988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Endrass T, Schuermann B, Kaufmann C, Spielberg R, Kniesche R, Kathmann N. Performance monitoring and error significance in patients with obsessive-compulsive disorder. Biol Psychol. 2010;84:257–263. doi: 10.1016/j.biopsycho.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Riesel A, Endrass T, Kaufmann C, Kathmann N. Overactive error-related brain activity as a candidate endophenotype for obsessive-compulsive disorder: evidence from unaffected first-degree relatives. Am J Psychiatry. 2011;168:317–324. doi: 10.1176/appi.ajp.2010.10030416. [DOI] [PubMed] [Google Scholar]
- 19.Xiao Z, Wang J, Zhang M, et al. Error-related negativity abnormalities in generalized anxiety disorder and obsessive-compulsive disorder. Prog Neuropsychopharm Biol Psychiatry. 2011;35:265–272. doi: 10.1016/j.pnpbp.2010.11.022. [DOI] [PubMed] [Google Scholar]
- 20.Endrass T, Riesel A, Kathmann N, Buhlmann U. Performance monitoring in obsessive-compulsive disorder and social anxiety disorder. J Abnorm Psychol. 2014;123:705–714. doi: 10.1037/abn0000012. [DOI] [PubMed] [Google Scholar]
- 21.Riesel A, Endrass T, Auerbach LA, Kathmann N. Overactive performance monitoring as an endophenotype for obsessive-compulsive disorder: evidence from a treatment study. Am J Psychiatry. 2015;172:665–673. doi: 10.1176/appi.ajp.2014.14070886. [DOI] [PubMed] [Google Scholar]
- 22.Hajcak G, Franklin ME, Foa EB, Simons RF. Increased error-related brain activity in pediatric OCD before and after treatment. Am J Psychiatry. 2008;165:116–123. doi: 10.1176/appi.ajp.2007.07010143. [DOI] [PubMed] [Google Scholar]
- 23.Hanna GL, Carrasco M, Harbin SM, et al. Error-related negativity and tic history in pediatric obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry. 2012;51:902–910. doi: 10.1016/j.jaac.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carrasco M, Harbin SM, Nienhuis JK, Fitzgerald KD, Gehring WJ, Hanna GL. Increased error-related brain activity in youth with obsessive-compulsive disorder and unaffected siblings. Depress Anxiety. 2013;30:39–46. doi: 10.1002/da.22035. [DOI] [PubMed] [Google Scholar]
- 25.Achenbach TM, Rescorla LA. Manual for ASEBA School-Age Forms and Profiles. Burlington: University of Vermont, Research Center for Children, Youth, and Families; 2001. [Google Scholar]
- 26.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 27.Hanna GL. Schedule for Obsessive-Compulsive and Other Behavioral Syndromes (SOCOBS) Ann Arbor: University of Michigan; 2010. [Google Scholar]
- 28.Scahill L, Riddle MA, McSwiggin-Hardin M, et al. Children’s Yale-Brown Obsessive Compulsive Scale: reliability and validity. J Am Acad Child Adolesc Psychiatry. 1997;36:844–852. doi: 10.1097/00004583-199706000-00023. [DOI] [PubMed] [Google Scholar]
- 29.Hudziak JJ, Althoff RR, Stanger C, et al. The Obsessive Compulsive Scale of the Child Behavior Checklist predicts obsessive-compulsive disorder: a receiver operating characteristic curve analysis. J Child Psychol Psychiatry. 2006;47:160–166. doi: 10.1111/j.1469-7610.2005.01465.x. [DOI] [PubMed] [Google Scholar]
- 30.Berument SK, Rutter M, Lord C, Pickles A, Bailey A. Autism screening questionnaire: diagnostic validity. Br J Psychiatry. 1999;175:444–451. doi: 10.1192/bjp.175.5.444. [DOI] [PubMed] [Google Scholar]
- 31.Kovacs M. The Children’s Depression Inventory (CDI) Psychopharm Bull. 1985;21:995–998. [PubMed] [Google Scholar]
- 32.Eriksen CW, Eriksen BA. Effects of noise letters upon the identification of a target letter in a non-search task. Percept Psychophysiol. 1974;16:143–149. [Google Scholar]
- 33.Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalogr Clin Neurophysiol. 1983;54:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- 34.Weinberg A, Meyer A, Hale-Rude E, et al. Error-related negativity and sustained threat: conceptual framework and empirical evaluation in an adolescent sample. Psychophysiology. 2016;53:372–385. doi: 10.1111/psyp.12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pitman RK. A cybernetic model of obsessive-compulsive psychopathology. Compr Psychiatry. 1987;28:334–343. doi: 10.1016/0010-440x(87)90070-8. [DOI] [PubMed] [Google Scholar]
- 36.Lahat A, Lamm C, Chronis-Tuscano A, Pine DS, Henderson HA, Fox NA. Early behavioral inhibition and increased error monitoring predict later social phobia symptoms in childhood. J Am Acad Child Adolesc Psychiatry. 2014;53:447–455. doi: 10.1016/j.jaac.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meyer A, Hajcak G, Torpey-Newman DC, Kujawa A, Klein DN. Enhanced error-related brain activity in children predicts the onset of anxiety disorders between the ages of 6 and 9. J Abnorm Psychol. 2015;124:266–274. doi: 10.1037/abn0000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoekstra RA, Bartels M, Hudziak JJ, Van Beijsterveldt TCEM, Boomsma DI. Genetic and environmental influences on the stability of withdrawn behavior in children: a longitudinal multi-informant twin study. Behav Genet. 2008;38:447–461. doi: 10.1007/s10519-008-9213-4. [DOI] [PubMed] [Google Scholar]
- 39.Goodwin RD, Fergusson DM, Horwood LJ. Early anxious/withdrawn behaviours predict later internalising disorders. J Child Psychol Psychiatry. 2004;45:874–883. doi: 10.1111/j.1469-7610.2004.00279.x. [DOI] [PubMed] [Google Scholar]
- 40.Walters JTR, Owen MJ. Endophenotypes in psychiatric genetics. Mol Psychiatry. 2007;12:886–890. doi: 10.1038/sj.mp.4002068. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


