Abstract
Isolated ventricular noncompaction, a rare genetic cardiomyopathy, is thought to be caused by the arrest of normal myocardial morphogenesis. It is characterized by prominent, excessive trabeculation in a ventricular wall segment and deep intertrabecular recesses perfused from the ventricular cavity. The condition can present with heart failure, systematic embolic events, and ventricular arrhythmias. Two-dimensional echocardiography is the typical diagnostic method. We report a case of heart failure in a 35-year-old man who presented with palpitations. Two-dimensional echocardiograms revealed left ventricular noncompaction, which markedly improved after standard heart failure therapy.
Keywords: Cardiomyopathies/diagnosis/prevention & control; heart defects, congenital/diagnostic imaging; heart failure/etiology; heart ventricles/abnormalities/diagnostic imaging; isolated noncompaction of the ventricular myocardium/complications/diagnostic imaging/drug therapy; treatment outcome; ventricular dysfunction, left/etiology
Isolated ventricular noncompaction (IVNC) is a rare genetic cardiomyopathy characterized by excessively prominent ventricular trabeculations and deep intertrabecular recesses. It is thought to arise in utero from arrested compaction of a loose myocardial meshwork. The major clinical presentations of left ventricular noncompaction (LVNC) are heart failure, arrhythmias, and thromboembolism. The diagnosis is established with use of 2-dimensional echocardiography or cardiac magnetic resonance (CMR).
We report the case of a patient with dilated cardiomyopathy and LVNC, and we discuss the effects of standard heart failure therapy on his condition.
Case Report
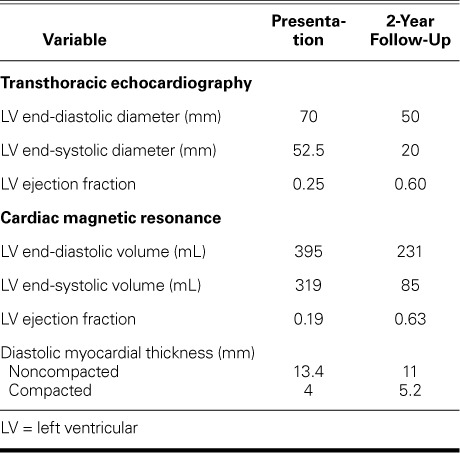
In March 2011, a 35-year-old man presented at a cardiology outpatient clinic with a several-week history of palpitations. His medical history yielded nothing relevant, and routine laboratory test results were normal. His chest radiograph showed mild cardiomegaly and normal lung fields. Physical examination revealed normal heart sounds and a mild systolic murmur heard best at the apex. A 12-lead electrocardiogram (ECG) showed sinus rhythm with poor R-wave progression and T-wave inversion in the precordial leads. A transthoracic echocardiogram (TTE) revealed a dilated left ventricle (LV), a depressed LV ejection fraction (LVEF) of 0.25, and moderate mitral regurgitation. A coronary angiogram revealed normal results. A 24-hour ECG showed frequent premature ventricular complexes and runs of nonsustained ventricular tachycardia.
Results of a CMR study included a markedly dilated LV with an LVEF of 0.19, an end-diastolic volume of 395 mL, and an end-systolic volume of 319 mL (Fig. 1). Prominent trabeculations were seen in the apical segments and lateral wall of the LV (Fig. 2). The ratio of noncompacted-to-compacted myocardium was 3.35:1. The indexed LV mass was 76 g/m2 with no evidence of hypertrophy. Right ventricular (RV) size, function, and wall structure were normal. Delayed myocardial gadolinium enhancement produced subtle mid-wall stripes along the anterior and lateral LV wall and the inferior half of the interventricular septum. There was also marked myocardial wall-thinning, particularly of the apical segments. The left atrial diameter was 36 mm. The diagnosis was LVNC with heart failure. The patient was started on perindopril and carvedilol, with gradual upward titration.
Fig. 1.
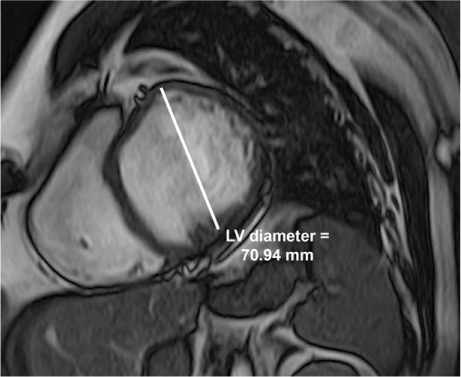
At presentation, a cardiac magnetic resonance image (short-axis, mid-cavity view) at end-diastole reveals the markedly dilated left ventricle (LV).
Fig. 2.
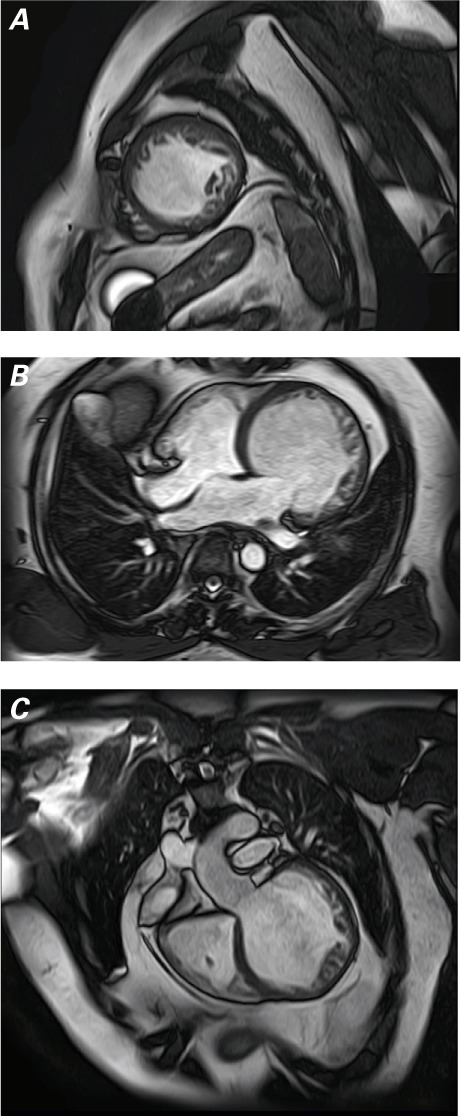
At presentation, cardiac magnetic resonance images show the globally hypokinetic left ventricle and noncompacted myocardium in A) short-axis, white-blood view, B) 4-chamber, white-blood view at the lateral wall and apex, and C) coronal oblique view parallel to the left ventricular outflow tract.
Supplemental motion images are available for Figure 2A, Figure 2B, and Figure 2C.
Because of the patient's ventricular tachycardia and low LVEF, we referred him for implantable cardioverter-defibrillator placement. However, according to the implantation team, the installed device's LV lead showed high impedance with no capture, indicating that it was nonfunctional. The unit was completely explanted, repeat ECG monitoring was suggested, and carvedilol was increased to 6.25 mg twice daily. Three months later, a 24-hour ECG showed sinus rhythm, infrequent premature ventricular complexes, and no ventricular tachycardia. Thereafter, carvedilol was titrated to 25 mg and perindopril to 5 mg, both twice daily.
Ivabradine (5 mg twice daily) was added in 2013. Two years after initial presentation, CMR revealed substantial improvement in LVEF (0.63), end-diastolic volume (231.46 mL), and end-systolic volume (85.3 mL); no LV dilation (Fig. 3A); and a thickness ratio of noncompacted-to-compacted myocardium of 2.1:1, indicating complete myocardial recovery (Fig. 3B and Table I). The indexed LV mass was 63 g/m2. A TTE confirmed the improvement in LV size and function (Table I) and revealed mild mitral regurgitation. The patient's mother, father, sister, paternal aunt, 2 paternal cousins, and a maternal cousin underwent TTE and ECG testing for IVNC, and nothing abnormal was found. As of June 2017, the patient was doing well.
Fig. 3.
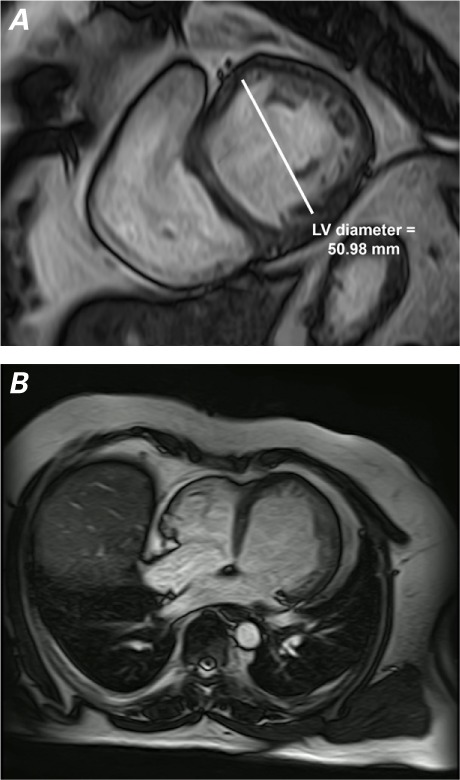
Cardiac magnetic resonance images. A) After 2 years, the short-axis, white-blood view shows decreased dilation and hypokinesia of the left ventricle (LV) at end-diastole. B) The 4-chamber, white-blood view shows improved LV contractility.
Supplemental motion images are available for Figure 3A and Figure 3B.
TABLE I.
Left Ventricular and Hemodynamic Measurements at Presentation and Follow-Up
Discussion
The prevalence of IVNC in adults is 0.05%.1 Although it is usually observed in the LV, the RV can also be affected.2 The condition is thought to be caused by arrested myocardial development in utero. During the first weeks of gestation, prominent myocardial trabeculations communicate with the ventricular cavity to supply blood to the myocardium. After the coronary circulation develops, the myocardial trabeculations disappear, and the spongy myocardium transforms into compact musculature.1,3 Abrupt interruption of this process is thought to cause IVNC.
Anatomically, IVNC is characterized by deep trabeculations in the ventricular wall; defined recesses communicate with the main ventricular cavity. The clinical presentation of IVNC depends upon the extent of the noncompacted cardiac segments. Individuals with IVNC can be asymptomatic, or they might experience heart failure, arrhythmias, or thromboembolism.4–6
Echocardiography is the usual method for diagnosing IVNC.7 The diagnostic criteria are typically those proposed by Jenni and colleagues8 and Frischknecht and associates.9 Jenni and colleagues' criteria include 1) a markedly thickened LV wall of 2 layers (a thin, normally compacted epicardial layer and a markedly thickened endocardial layer with numerous prominent trabeculations and deep recesses); 2) no coexisting cardiac abnormalities; 3) blood flow between the intertrabecular recesses, identified with use of color-flow Doppler echocardiography; and 4) a maximum ratio of noncompacted-to-compacted myocardium of >2:1 at end-systole.
In any image plane, CMR reveals more detailed cardiac morphology than does echocardiography and should be used in the diagnosis of IVNC when echocardiographic windows are poor.10 A >2.3:1 ratio of noncompacted-to-compacted myocardium yields the highest diagnostic sensitivity (86%) and specificity (99%).10,11 Late gadolinium hyperenhancement is related to myocardial fibrosis and scarring in the hypertrabeculated myocardium.12 Differential diagnoses include prominent normal myocardial trabeculations, false tendons, localized LV hypertrophy, LV thrombus, intramyocardial hematoma, arrhythmogenic RV dysplasia, endocardial fibroelastosis, cardiac metastases, and intramyocardial abscesses.
The treatment of IVNC is directed toward preventing thromboembolism, arrhythmias, and heart failure.3,13 Prophylactic anticoagulation is generally indicated in cases of LV systolic dysfunction, previous embolic events, cardiac thrombus, and atrial fibrillation.14,15 Proper therapy for heart failure, involving angiotensin-converting enzyme inhibitors, β-blockers, and appropriate diuretics or dioxin, is essential. Cardiac resynchronization therapy can be helpful in drug-refractory cases. In selected patients who have advanced heart failure, cardiac transplantation should be considered.3
Patients with IVNC should be screened by means of 24-hour ECG recordings to exclude asymptomatic arrhythmias. Implantable cardioverter-defibrillator placement may be considered for managing ventricular arrhythmias; however, its superiority to medical therapy is debatable.7
The long-term prognosis for patients with IVNC depends on the degree and progression of heart failure, the presence of thromboembolic events, and any arrhythmias. Oechslin and colleagues13 reported a mortality rate of 35% during a mean follow-up period of 44 ± 39 months, and another 12% of their patients underwent heart transplantation. In contrast, Murphy and associates16 noted improved prognoses in comparison with previous studies. Better recent prognosis might be attributed to earlier detection of the disease and more aggressive treatment of symptomatic patients. A much better prognosis has been noted in patients with fewer involved LV segments than in those with more.17
Left ventricular geometry—the relationship between LV shape and function and visible LV trabeculations—might explain the reversibility of hypertrabeculation after optimal medical therapy has improved LV function.18 We used the ratio of involvement to LV cavity size as the most important diagnostic criterion in our patient.
Our patient's LV size and function recovered to normal after optimal medical therapy, and the noncompaction became less prominent. The cause of his reversible cardiomyopathy remains unknown. The progress to noncompaction might be a compensatory mechanism for worsening systolic function. To avoid inappropriate and exaggerated diagnoses, we suggest that the diagnosis of suspected myocardial noncompaction be carefully established by means of imaging.
Supplementary Material
References
- 1. Ritter M, Oechslin E, Sutsch G, Attenhofer C, Schneider J, Jenni R.. Isolated noncompaction of the myocardium in adults. Mayo Clin Proc 1997; 72 1: 26– 31. [DOI] [PubMed] [Google Scholar]
- 2. Maron BJ, Towbin JA, Thiene G, Antzelevich C, Corrado D, Arnett D, . et al. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association scientific statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation 2006; 113 14: 1807– 16. [DOI] [PubMed] [Google Scholar]
- 3. Weiford B, Subbarao VD, Mulhern KM.. Noncompaction of the ventricular myocardium. Circulation 2004; 109 24: 2965– 71. [DOI] [PubMed] [Google Scholar]
- 4. Conces DJ Jr, Ryan T, Tarver RD.. Noncompaction of ventricular myocardium: CT appearance. AJR Am J Roentgenol 1991; 156 4: 717– 8. [DOI] [PubMed] [Google Scholar]
- 5. Ichida F, Hamamichi Y, Miyawaki T, Ono Y, Kamiya T, Akagi T, . et al. Clinical features of isolated noncompaction of the ventricular myocardium: long-term clinical course, hemodynamic properties, and genetic background. J Am Coll Cardiol 1999; 34 1: 233– 40. [DOI] [PubMed] [Google Scholar]
- 6. Hook S, Ratliff NB, Rosenkranz E, Sterba R.. Isolated non-compaction of the ventricular myocardium. Pediatr Cardiol 1996; 17 1: 43– 5. [DOI] [PubMed] [Google Scholar]
- 7. Jenni R, Oechslin EN, van der Loo B.. Isolated ventricular non-compaction of the myocardium in adults. Heart 2007; 93 1: 11– 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jenni R, Oechslin E, Schneider J, Attenhofer Jost C, Kaufmann PA.. Echocardiographic and pathoanatomical characteristics of isolated left ventricular non-compaction: a step towards classification as a distinct cardiomyopathy. Heart 2001; 86 6: 666– 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Frischknecht BS, Attenhofer Jost CH, Oechslin EN, Seifert B, Hoigne P, Roos M, Jenni R.. Validation of noncompaction criteria in dilated cardiomyopathy, and valvular and hypertensive heart disease. J Am Soc Echocardiogr 2005; 18 8: 865– 72. [DOI] [PubMed] [Google Scholar]
- 10. Daimon Y, Watanabe S, Takeda S, Hijikata Y, Komuro I.. Two-layered appearance of noncompaction of the ventricular myocardium on magnetic resonance imaging. Circ J 2002; 66 6: 619– 21. [DOI] [PubMed] [Google Scholar]
- 11. Petersen SE, Selvanayagam JB, Wiesmann F, Robson MD, Francis JM, Anderson RH, . et al. Left ventricular non-compaction: insights from cardiovascular magnetic resonance imaging. J Am Coll Cardiol 2005; 46 1: 101– 5. [DOI] [PubMed] [Google Scholar]
- 12. Jassal DS, Nomura CH, Neilan TG, Holmvang G, Fatima U, Januzzi J, . et al. Delayed enhancement cardiac MR imaging in noncompaction of left ventricular myocardium. J Cardiovasc Magn Reson 2006; 8 3: 489– 91. [DOI] [PubMed] [Google Scholar]
- 13. Oechslin EN, Attenhofer Jost CH, Rojas JR, Kaufmann PA, Jenni R.. Long-term follow-up of 34 adults with isolated left ventricular noncompaction: a distinct cardiomyopathy with poor prognosis. J Am Coll Cardiol 2000; 36 2: 493– 500. [DOI] [PubMed] [Google Scholar]
- 14. Udeoji DU, Philip KJ, Morrissey RP, Phan A, Schwarz ER.. Left ventricular noncompaction cardiomyopathy: updated review. Ther Adv Cardiovasc Dis 2013; 7 5: 260– 73. [DOI] [PubMed] [Google Scholar]
- 15. Cevik C, Shah N, Wilson JM, Stainback RF.. Multiple left ventricular thrombi in a patient with left ventricular noncompaction. Tex Heart Inst J 2012; 39 4: 550– 3. [PMC free article] [PubMed] [Google Scholar]
- 16. Murphy RT, Thaman R, Blanes JG, Ward D, Sevdalis E, Papra E, . et al. Natural history and familial characteristics of isolated left ventricular non-compaction. Eur Heart J 2005; 26 2: 187– 92. [DOI] [PubMed] [Google Scholar]
- 17. Cevik C, Stainback RF.. Isolated left ventricular noncompaction in a 90-year-old man. Tex Heart Inst J 2012; 39 2: 255– 7. [PMC free article] [PubMed] [Google Scholar]
- 18. Eurlings LW, Pinto YM, Dennert RM, Bekkers SC.. Reversible isolated left ventricular non-compaction? Int J Cardiol 2009; 136 2: e35– 6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


