Abstract
Dipstick urinalysis is an informative, quick, cost-effective and non-invasive diagnostic tool that is useful in clinical practice for the diagnosis of urinary tract infections (UTIs), kidney diseases, and diabetes. We used dipstick urinalysis as a hands-on microbiology laboratory exercise to reinforce student learning about UTIs with a particular focus on cystitis, which is a common bacterial infection. To avoid exposure to potentially contaminated human urine samples, we prepared artificial urine using easily acquired and affordable ingredients, which allowed less-experienced students to perform urinalysis without the risk of exposure to pathogenic organisms and ensured reliable availability of the urine samples. This practical class taught medical students how to use urinalysis data in conjunction with medical history to diagnose diseases from urine samples and to determine a treatment plan for clinical scenarios.
INTRODUCTION
Human blood and bodily fluids, such as urine, are commonly used in teaching laboratories, but it is desirable to limit students’ exposure to diseased bodily fluids. However, elimination of clinical testing of human bodily fluids from teaching labs might have detrimental effects on learning, because optimal handling of human bodily fluids is important to learn for infection prevention and control practices (1, 2).
There are several limitations to using human bodily fluids for student teaching. These include the requirement for ethics committee review, specific disposal systems, written control plans for minimizing occupational exposure to hazards, and meeting other institutional regulations (1). From a logistics perspective, obtaining real samples from a patient with the desired medical condition may be difficult on the day scheduled for the laboratory exercise. Furthermore, students are inexperienced with handling human specimens but need to gain experience as safely as possible. All of these limitations can be overcome by replacing the human sample with an artificial sample, allowing students to gain essential experience, while protecting them from accidental contamination from human samples.
While it is difficult to perfectly replicate a human bodily fluid sample, it is feasible to create a sample that presents the necessary parameters to be tested in a particular exercise: for example, adding glucose to yellow-stained water for testing hyperglycosuria in a urine sample. Several protocols were available to prepare artificial urine, but no method was suitable for multipurpose use. The available protocols were designed for specific purposes such as to mimic diabetes mellitus (3), to support the growth of a wide range of urinary pathogens (4), for in vitro cellular studies to simulate normal physiological environment of the kidney and urinary tract (5), to study components such as mucous casts, epithelial cells, salt crystals and yeast in urine (7), and in laboratory teaching mimicking glycosuria, proteinuria, ketonuria, pH imbalance, and hemoglobinuria (6). Commercially available artificial urine kits and samples for eliciting normal and positive tests for urinalysis were cost-prohibitive. Moreover, these urine samples or kits were not suitable and appropriate for our application.
Here, we report the generation of an artificial urine suitable for the analysis of standard parameters using urinalysis—an essential test for students to learn, with a particular focus on cystitis. We used cystitis as a lab exercise because it is a very common problem in routine clinical practices. The clinical diagnosis of cystitis is based primarily on the presence of specific symptoms (e.g., pain while urinating, together with symptoms of bladder dysfunction), with urinalysis confirmation. Although urine culture is the gold standard to diagnose cystitis, it is expensive and takes at least 48 hours to complete and obtain results. Dipstick urinalysis is an immediate, informative, easy, and cheap test for predicting cystitis in patients with compatible symptoms (8). Most patients with cystitis do not require any additional laboratory testing and can be treated immediately.
To maintain clinical integration in the microbiology laboratory teaching, we prepared artificial urines to match two different case scenarios, allowing comparison of normal and abnormal urinalysis results.
Intended audience and prerequisite student knowledge
This practical exercise was designed for microbiology laboratory teaching to medical students during their six-year medical program. Entry into the medical program is competitive and is attained by either a high performance during year one of the University of Auckland’s Bachelor of Health Sciences degree or Bachelor of Science (Biomedical Science) degree or by graduate entry following a high performance in the completion of another degree.
The second and third years of the medical program are structured into modules that encompass the anatomy, physiology, and pathology associated with each organ system. At the end of year three, the basic clinical sciences teaching is completed with the “Blood, Immunity and Infection” module. The laboratory exercise described in this paper is preceded by the Genitourinary module (in year two) and by a lecture about cystitis and pyelonephritis within the “Blood, Immunity and Infection” module. Students received training about laboratory safety, microbiology, and laboratory techniques during their microbiology laboratory introduction. The focus of the four microbiology laboratory sessions is to discover the identity and antimicrobial susceptibility of an isolate obtained from one of a series of clinical scenarios and then to apply that knowledge to develop a management plan. We present the current laboratory exercise, even though it is an ancillary exercise, as it demonstrates the development and usage of artificial urine together with its educational applications.
This practical exercise could easily be implemented as a stand-alone laboratory exercise or as an exercise to complement other teaching (see Possible modifications), in order to align with the needs of students. Without modification, the exercise is suitable for microbiology courses for nursing, medical laboratory science, clinical microbiology, and biomedical science students.
Learning time and learning objectives
This practical exercise was scheduled to be completed within a 15-minute period of a two-hour microbiology laboratory. Due to the large class size, we run two lab streams, with 139 students in each stream. The learning objectives for this short exercise are as follows:
Diagnose cystitis by integrating medical symptoms and urinalysis test results.
Devise a cost-effective management plan for patients with symptoms of UTI and decide whether any further tests are required.
Perform dipstick urinalysis as per manufacturer’s instructions.
Interpret annual community laboratory resistance patterns to select appropriate antibiotic treatment.
Learn the common bacteria that cause UTI.
PROCEDURE
Generation of the artificial urine
To allow for comparison, two cases were developed: Case A, representing normal urine, and Case B, representing urine from a patient with cystitis. The components for normal urine (Case A) include readily available, inexpensive materials that form an artificial urine base (Table 2). The artificial urine for the patient with cystitis (Case B) was made from the same urinary base, with the addition of leukocytes (white blood cells, WBC) and erythrocytes (red blood cells, RBC) prepared from human buffy coat, provided by the New Zealand Blood Service and tested to be pathogen-free. Alternatively, buffy coat or leukocyte esterase can be purchased from a variety of suppliers (see Appendix 1). Buffy coat is the fraction of an anticoagulated blood sample obtained by density gradient centrifugation of the blood. It contains red blood cells, white blood cells and platelets. For a more detailed protocol to extract and purify leukocytes from buffy coat (which is required to prepare the Case B urine sample), see Appendix 1. Alternatively, leucocytes and erythrocytes could be prepared from animal blood.
TABLE 2.
Composition of artificial urines along with corresponding case scenarios.
| Urine Sample | Clinical Scenario | Urine Recipe |
|---|---|---|
| Case A (elevation of prostate specific antigen) | A 65-year-old male, a retired plumber, has a history of slow stream of urine and needing to get up to void once or twice in the night. He has been referred to the Urology Outpatient Clinic after a blood test showed an elevated prostate-specific antigen. |
Normal urine base: Potassium chloride, 0.2 g/L Sodium chloride, 8 g/L Di-sodium hydrogen phosphate, 1.14 g/L Potassium dihydrogen phosphate, 0.2 g/L Queen yellow food color, 200 μL/L Milli-Q water up to 1 liter Hydrochloric acid or sodium hydroxide to adjust pH to between 7.5 and 8.0 |
| Case B (dysuria) | An 18-year-old woman presents to the student health clinic with burning discomfort when she passes urine. She has recently started university and has a new boyfriend. They have been having sex without using condoms. |
Normal urine base with the addition of: White blood cells/esterase from buffy coat, 20 – 40 mL/L Whole human blood, 4 μL/L 10 mM sodium nitrite solution, 1.5 mL/L |
Leukocytes are the main marker of inflammation in the urinary tract (9, 10) whereas the presence of erythrocytes (hematuria) or hemoglobin from lysed erythrocytes (hemoglobinuria) are indicators of cellular damage within the urinary tract. We also added sodium nitrite because the presence of blood, leukocytes, and nitrites together is a more specific indicator for cystitis (10). Table 2 shows the composition of each urine sample.
In addition to ease and low cost, another major benefit of our artificial urine base (Case A, Table 2) is stability: a single batch can be made and stored at 4°C over a long time period. To date, we have successfully used a single batch over an eight-year period. However, erythrocytes deteriorate over time (~ eight to nine months) and have to be added each year before the start of the teaching lab. Furthermore, leukocyte and nitrite activity can slowly diminish over the years, and these might need to be replenished as required.
Materials and student instructions
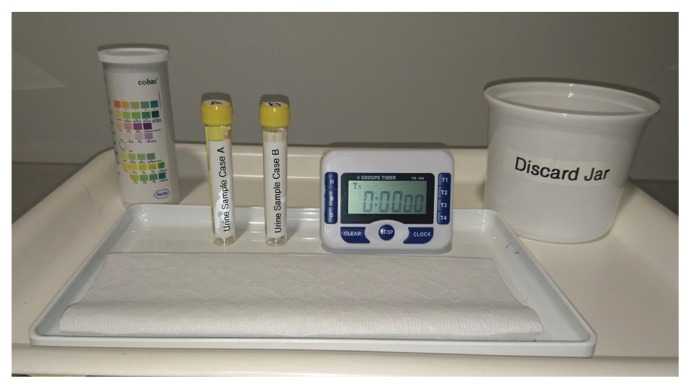
Groups of four students were provided with the two case scenarios together with 10 mL of matching artificial urine samples. These case scenarios are two of the 191 core scenarios that make up the curriculum of the six-year medical program at the University of Auckland. In addition, students received Roche COMBUR-7 TEST strips, a timer, paper towels, and a waste receptacle for used COMBUR-7 TEST strips (Fig. 1). The COMBUR-7 TEST strip contains pads to analyze abnormalities in urinary pH, ketones, glucose, nitrite, leukocytes, protein, and the presence of free hemoglobin or erythrocytes. Full instructions were provided in each student’s laboratory manual (Appendix 2). Demonstrators and lecturers (ratio of 1:12 students) were available to answer questions.
FIGURE 1.
Student materials: 10 mL each of Case A and Case B artificial urine samples, a timer, COMBUR-7 TEST strips, paper towel, and a discard jar for used strips.
Faculty instructions
A list of technical tips for teaching instructors on how to avoid any potential problems with the dipstick test can be found in Appendix 1.
Suggestions for determining student learning
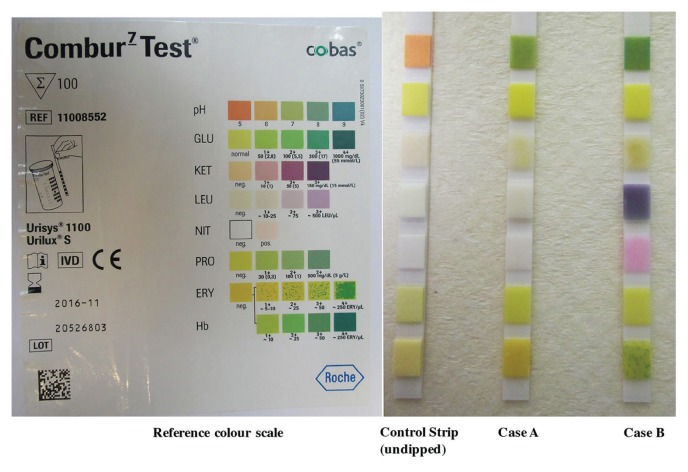
Students were instructed to briefly (about 1 second) dip the test strips into the urine samples. After 60 seconds each color reaction was compared with the reference color scales on the label of the COMBUR-7 TEST strip container (Fig. 2).
FIGURE 2.
COMBUR-7 TEST strips dipped in artificial urine samples for Case A and Case B.
The readings from the artificial urine sample for Case A and Case B are shown in Table 1. Students were instructed to record all test results as outlined in Table 1 and discuss the results within the student group, focusing on questions outlined in Table 3. The accuracy of diagnosis can be increased by combining urinalysis test results with medical/clinical symptoms.
TABLE 1.
Results of urinalysis with COMBUR-7 TEST strip for Case A and Case B.
| Test | Results (Case A) | Results (Case B) | Normal Range |
|---|---|---|---|
| pH | ~ 7.5/8 | ~ 7.5/8 | Approximately 6.0 |
| Glucose | normal | normal | 0 – 0.8 mmol/L |
| Leukocytes | negative | ~3+ or ~500 leukocytes/μL | 0 – 10 leukocytes/μL |
| Ketone | negative | negative | 0 |
| Nitrite | normal | positive | 0 |
| Protein | negative | negative | 0 – 150 mg/dL |
| Blood | negative | ~3+ or approximately 50 erythrocytes/μL | < 3 erythrocytes/μL |
| Hemoglobin | negative | ~ 1.5+ or approximately 20 erythrocytes/μL | 0 |
TABLE 3.
Questions related to Case A and Case B.
| Questions | Model Answer/Instructor’s Key |
|---|---|
| 1. Which urine specimen is most likely to have been provided by the 18-year-old student? | Case B |
| 2. Briefly explain the significance of the results obtained by the dipstick method for each of the two patients. |
Urine Sample Case A indicates only the presence of alkaline pH (Fig. 2, Table 1). Alkaline urinary pH can be associated with: urinary tract obstruction, pyloric obstruction, salicylate intoxication, renal tube acidosis, chronic renal failure, and respiratory diseases that involve hyperventilation. However, the pH alone is not indicative for disease as the patient’s diet and certain medications might also have an influence (11). Urine Sample Case B indicates the presence of nitrite, leukocytes, erythrocytes, and hemoglobin, plus alkaline pH (Fig. 2, Table 1). The presence of nitrite is highly specific for bacteriuria (96.6 – 97.5%) because most bacterial species causing UTI reduce nitrate in the urine to nitrite (9, 10). The presence of leukocytes (an enzyme released by white blood cells) in urine (pyuria) is a good and strong indicator for inflammation and UTI. The presence of red blood cells/erythrocytes indicates glomerular damage or bleeding somewhere in the urinary tract. Presence of hemoglobin in the urine indicates lysis of the red blood cells in urine, renal damage, but could also be due to normal menstrual flow (6). Taken together, the presence of nitrite, leukocyte esterase, and blood in the urine indicates urinary tract infection/cystitis or sexually transmitted infection (cervicitis, urethritis, etc.) |
| 3. What is the likely diagnosis in each case? | Case A: Urinary retention secondary to benign prostatic hyperplasia Case B: Cystitis |
| 4. For Case B, what additional symptoms would you ask about? | Bladder dysfunction: frequency, hesitancy, bladder pain, bladder cramping |
| 5. Describe an appropriate management plan for the student with dysuria (Case B). Are additional tests required? Are follow-up urine tests required? Which antibiotic is appropriate? (Refer to the Table of bacterial susceptibility data derived from the testing of community laboratory isolates in Auckland (Appendix 1).) | Antibiotic therapy will shorten duration of symptoms considerably. Start treatment with nitrofurantoin. Urine culture is not required unless complicating factors are present (fever, flank pain, recent episode of cystitis within one month). If symptoms persist for five days, a mid-stream urine sample should be sent for urine culture—antimicrobial susceptibility testing is required. Culturing the urine to check resolution of an infection is NOT required. |
UTI = urinary tract infection.
Safety issues
In accordance with the University of Auckland’s health and safety regulations, all students were instructed to wear a closed lab coat, closed shoes with covered toes, gloves, and safety glasses before commencing any work within the teaching laboratory and to follow laboratory safety guidelines. While the urine samples provided do not contain any known pathogen and the blood used has been tested to be pathogen-free, the students are not informed that the samples are of artificial urine so they handled the samples according to Infection Prevention and Control Practice guidelines (ASM guidelines).
DISCUSSION
Evidence of student learning
Although the dipstick test results, clinical cases, and answers for the lab-related questions were discussed by students with guidance from laboratory teaching staff, no formal evaluation/assessment was carried out during the lab. However, more than 95% of students answered the questions listed in Table 3 to the satisfaction of the laboratory teaching staff. The biggest challenge for the students was the correct interpretation of the pH and the red blood cell/hemoglobin results. Although an important screening test, changes in pH alone are difficult to interpret and require comparison with other diagnostic information. Similarly, the presence of blood/hemoglobulin in the urine (hematuria/hemoglobinuria) is not necessarily associated with UTIs and could also be detected in urine after excessive exercise and during menstruation.
In order to reinforce the knowledge gained during laboratory teaching, students had to individually complete an online test after the lab that contributed to their course work assessment. Table 4 summarizes evidence of student learning correlated to individual learning objectives. In 2015, 273/278 students (98%) completed the online test, of which 272/273 (~100%) were able to identify the dipstick urinalysis result from a patient with cystitis from a photo, which also included a dipstick of alkaline, but otherwise normal urine (Fig. 2). Furthermore, 259/273 students (95%) correctly answered a multiple-choice question indicating that the management of an 18-year-old woman with dysuria and urinary frequency, whose urine dipstick result was shown (Case B in Fig. 2), included antibiotic treatment without the need for urine culture. Finally, 257/273 (94%) correctly answered a multiple-choice question indicating that Escherichia coli was most commonly isolated from a mid-stream urine (MSU) sample from an otherwise healthy 19-year-old with dysuria, urinary frequency, and pyuria in comparison with other clinical scenarios (e.g., cerebrospinal fluid from a 14-month-old boy with meningitis (Appendix 3)).
TABLE 4.
Assessment method, average class results/scores, and relevant learning goals achieved.
| Learning Objectives/Goal | Assessment Methods | Average Scores/Results |
|---|---|---|
| 1. Diagnose cystitis by integrating medical symptoms and urinalysis test results. | Online test (Appendix 3, MCQ4). Informal assessment during lab (Table 3, Q2 and Appendix 2, Q1 and Q2) | 272/273 students (99.6%) answered the MCQ correctly. |
| 2. Devise a cost effective management plan for patients with symptoms of UTI and decide whether any further tests are required. | Online test (Appendix 3, MCQ5) Informal assessment during lab (Table 3, Q5 and Appendix 2, Q2) | 259/273 students (95%) correctly answered this question |
| 3. Perform dipstick urinalysis according to the manufacturer’s instructions. | Informal assessment during lab | ~98% students performed dipstick urinalysis correctly |
| 4. Interpret annual community laboratory resistance patterns to select appropriate antibiotic treatment. | Informal assessment during lab (Table 3, Q5 and Appendix 2, Q3) | achieved by >95% of students |
| 5. Learn the common bacteria that cause UTI. | Online test (Appendix 3, MCQ1) | 257/273 (94%) correctly answered this question |
MCQ = multiple choice question; UTI: urinary tract infection.
We have developed a long-lasting and economical artificial urine which is made from easily acquired and affordable ingredients and is free of hazardous chemicals and contagious microorganisms, while successfully mimicking a positive dipstick urinalysis test for urine from a patient with cystitis (Fig. 2). With the aid of this artificial urine, we are able to provide a safer teaching environment in the teaching laboratory, allowing medical students to learn and exercise their laboratory skills and perform urinalysis using a cystitis case scenario. This serves to strengthen links between theory and practice, which is relevant for all medical curricula, and builds students’ confidence for real patient encounters. Enabling students to perform a hands-on exercise ensured attentive and engaged students. Training students to take precautions when handling pathogenic samples also provides a basic foundation for a career in clinical medicine (1, 7). Finally, student assessment indicated that acquisition of the knowledge to meet some of our learning objectives was nearly universal.
An advantage of having students performing the experiment in small groups rather than individually is that it fosters the development of teamwork skills; it also increases the speed at which the technical exercises could be completed within a limited timeframe, allowing sufficient time to replicate the tests, if necessary. Working as a group also creates an interactive environment and facilitates the discussion of the interpretation of results, allowing exploration of possible answers to the questions and debate among the group members. It also allowed our technical staff to set up the urinalysis experiment with minimal resources and preparation time, making it very economical to run for large class sizes.
This practical class can easily be adapted for any institution since it does not require expensive equipment and the materials that are needed to make artificial urine are inexpensive and easily sourced. Since our artificial urine base is adaptable and is stable for many years, this also reduces the extra work, time, and cost required to get the regular institutional approvals for use of human urine samples in teaching laboratories. Finally, the artificial urine eliminates the risk of providing infectious urine samples to students.
Possible modifications
Our artificial urine could be adapted to other laboratory exercises and teaching programs. For example, the dipstick test could be simplified for teaching biology in high school classes by using a less alkaline urine pH. Although urine pH is an important screening test for UTIs, other diagnostic information is required for an accurate interpretation, which would be beyond the scope of high school teaching. Alternatively, the dipstick exercise could be modified to suit courses teaching medical laboratory science students or clinical microbiologists. We have found that addition of harmless laboratory strains, such as Escherichia coli K12, just prior to the lab does not affect the dipstick test results. This would allow a further component to be added to the UTI diagnostic exercise—students could culture the artificial urine sample onto agar plates for bacterial enumeration, identification, and antibiotic susceptibility testing (Appendix 1, Fig. A1–II). In our course, these skills are taught during other parts of the microbiology laboratories. Furthermore, protein or glucose could be included in addition to varying the pH (see Appendix 1, Fig. A1–III for more details) to meet the teaching goals for urinalysis in other systemic or renal diseases.
SUPPLEMENTAL MATERIALS
ACKNOWLEDGMENTS
We would like to thank Mark Kilgour (Marketing Manager) at Roche Diagnostic NZ Ltd for giving us permission to use the image of COMBUR-7 TEST strips. The authors declare that there are no conflict of interest.
Footnotes
Supplemental materials available at http://asmscience.org/jmbe
REFERENCES
- 1.Ellis G. Handling human samples is worth the risk. Scientist. 2004;18(7) [Google Scholar]
- 2.Sharp RH, Smailes DL. A simulation of the blood type test. Am Biol Teacher. 1989;51:232–233. doi: 10.2307/4448909. [DOI] [Google Scholar]
- 3.Basso PJ, Tazinafo LF, Silva MF, Rocha MJ. An alternative to the use of animals to teach diabetes mellitus. Adv Physiol Educ. 2014;38:235–238. doi: 10.1152/advan.00051.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brooks T, Keevil CW. A simple artificial urine for the growth of urinary pathogens. Lett Appl Microbiol. 1997;24:203–206. doi: 10.1046/j.1472-765X.1997.00378.x. [DOI] [PubMed] [Google Scholar]
- 5.Chutipongtanate S, Thongboonkerd V. Systematic comparisons of artificial urine formulas for in vitro cellular study. Anal Biochem. 2010;402:110–112. doi: 10.1016/j.ab.2010.03.031. [DOI] [PubMed] [Google Scholar]
- 6.Shmaefsky BR. Artificial urine for laboratory testing. Am Biol Teacher. 1990;52:170–172. doi: 10.2307/4449071. [DOI] [Google Scholar]
- 7.Shmaefsky BR. Artificial urine for laboratory testing: revisited. Am Biol Teacher. 1995;57:428–430. doi: 10.2307/4450032. [DOI] [Google Scholar]
- 8.Dumonceaux M, Gamez M. Rediscovering urine chemistry—and understanding its limitations. Med Lab Observer. 2016 https://www.mlo-online.com/rediscovering-urine-chemistry%E2%80%94and-understanding-its-limitations. [PubMed] [Google Scholar]
- 9.Andriole VT. Urinary tract infections: recent developments. J Infect Dis. 1987;156:865–869. doi: 10.1093/infdis/156.6.865. [DOI] [PubMed] [Google Scholar]
- 10.Mambatta AK, Jayarajan J, Rashme VL, Harini S, Menon S, Kuppusamy J. Reliability of dipstick assay in predicting urinary tract infection. J Fam Med Prim Care. 2015;4:265–268. doi: 10.4103/2249-4863.154672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwalfenberg GK. The alkaline diet: is there evidence that an alkaline pH diet benefits health? J Environ Public Health. 20122012:727630. doi: 10.1155/2012/727630. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.