Abstract
Background
Although high resting heart rates are associated with adverse outcomes in heart failure with reduced ejection, the reports for heart failure with preserved ejection fraction (HFpEF) are conflicting.
Design
A secondary analysis to examine the relationship between resting heart rate and adverse outcomes in 2,705 patients (mean age=68±10 years; 47% men; 88% white) with HFpEF who were in sinus rhythm from the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist Trial (TOPCAT).
Methods
Baseline heart rate was obtained from baseline electrocardiogram data. Outcomes were adjudicated by a clinical end-point committee and included the following: hospitalization, hospitalization for heart failure, death, and cardiovascular death.
Results
Over a median follow-up of 3.4 years (25th–75th percentiles=2.0, 4.9 years), a total of 1,157 hospitalizations, 311 hospitalizations for heart failure, 369 deaths, and 233 cardiovascular deaths occurred. An increased risk (per 5-bpm increase) for hospitalization (HR=1.03, 95%CI=1.004, 1.06), hospitalization for heart failure (HR=1.10, 95%CI=1.05, 1.15), death (HR=1.10, 95%CI=1.06, 1.16), and cardiovascular death HR=1.13, 95%CI=1.07, 1.19), was observed. When the analysis was limited to those who did not report the use of beta blockers, the magnitude of the association for each outcome (per 5-bpm increase) was not materially altered (hospitalization: HR=1.03, 95%CI=0.97, 1.09); hospitalization for heart failure: HR=1.12, 95%CI=0.98, 1.27; death: HR=1.16, 95%CI=1.05, 1.28; cardiovascular death: HR=1.12, 95%CI=0.99, 1.27).
Conclusion
High resting heart rate is a risk factor for adverse outcomes in patients with HFpEF and future studies are needed to determine if reducing heart rate improves outcomes in HFpEF.
Keywords: heart failure, preserved ejection fraction, heart rate
INTRODUCTION
In patients with heart failure with reduced ejection fraction, high resting heart rates represent increased neurohormonal activation, as the heart tries to compensate for its inability to pump effectively.1 Accordingly, heart rate has been shown to be an important predictor for adverse cardiovascular outcomes in patients with reduced ejection fraction.2 Additionally, therapies that aim to reduce resting heart rate are associated with a lower risk of cardiovascular death and hospitalization for heart failure in this high-risk group.3, 4
Although the inherent risk of adverse events associated with high resting heart rate, and the benefit of heart rate reduction, have been clearly demonstrated in heart failure with reduced ejection fraction,3–5 the impact of heart rate on outcomes in heart failure with preserved ejection fraction (HFpEF) is less clear. Studies have suggested that high resting heart rates in HFpEF are associated with an increased risk of mortality,6, 7 while others have reported a null association.8, 9 Similar inconsistencies exist for the relationship between resting heart rate and the risk of hospitalization for heart failure.7, 8 Therefore, a closer examination of heart rate and adverse outcomes in HFpEF is warranted, and we explored this relationship in HFpEF patients from the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist Trial (TOPCAT).
METHODS
Study Design and Patients
TOPCAT was a multi-center, international randomized, double blind, placebo-control study to examine the efficacy of spironolactone in patients with HFpEF. The design, inclusion criteria, and baseline characteristics of the trial have been published previously.10, 11 Briefly, 3,445 patients with symptomatic HFpEF from 270 sites in 6 countries were enrolled between August, 2006 and January, 2012. The primary goal of the trial was to determine if spironolactone was associated with a reduction in the composite outcome of cardiovascular mortality, aborted cardiac arrest, or heart failure hospitalization in patients with HFpEF (e.g., documented ejection fraction ≥45%).
We examined the relationship between resting heart rate obtained on the baseline 12-lead electrocardiogram and the risk of hospitalization, hospitalization for heart failure, death, and cardiovascular death. We excluded TOPCAT participants who had evidence of non-sinus rhythm (e.g., atrial fibrillation) during the baseline electrocardiogram recording.
Baseline Characteristics
Patients who participated in TOPCAT underwent a detailed baseline visit to obtain medical histories and a physical examination was performed.11 Heart rate in beats per minutes (bpm) was obtained from the 12-lead electrocardiogram performed at the baseline study visit, after participants rested in the lying position for 5 minutes. Age, sex, race, and smoking were obtained by self-reported history. Smoking was defined as the current use of cigarettes. Medical history for the following diagnoses were obtained by self-report and medical record review: diabetes, coronary heart disease, stroke, New York Heart Association Class, and prior heart failure hospitalization. Systolic blood pressure and body mass index were obtained by trained staff and laboratory data included serum creatinine. Medication data also were obtained during the initial study visit and the following were included in this analysis: aspirin, beta blockers, angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers, and statins.
Outcomes
Outcomes in TOPCAT were adjudicated by a clinical end-point committee, and the details of this process and definitions for each outcome examined have been described.10, 12 The outcomes examined in this analysis included hospitalization, hospitalization for heart failure, death, and cardiovascular death. Briefly, hospitalization for heart failure was defined as the unexpected presentation to an acute care facility requiring overnight stay with symptoms and physical exam findings consistent with heart failure, and treatment with intravenous vasodilators, inotropes, mechanical fluid removal, or hemodynamic support. Cardiovascular death was defined as death due to one of the following: myocardial infarction, worsening heart failure, sudden death, stroke, pulmonary embolism, death occurring during a cardiovascular-related procedure, or other cardiovascular death. Death included the composite of cardiovascular and non-cardiovascular death.
Statistics
Heart rate was categorized across quartiles of the baseline distribution (Quartile1: <59 bpm; Quartile 2: 59–64 bpm; Quartile 3: 65–73 bpm; Quartile 4: ≥74 bpm). Baseline characteristics were compared across heart rate categories. Categorical variables were reported as frequency and percentage, while continuous variables were recorded as mean ± standard deviation. Statistical significance for categorical variables was tested using the chi-square method and for continuous variables the analysis of variance procedure. Follow-up time was defined as the time from randomization until one of the following: outcome of interest, death, loss to follow-up, or end of follow-up. Kaplan-Meier estimates were used to examine the unadjusted cumulative incidence estimates of each outcome associated with each heart rate category. Incidence rates per 100 person-years were computed for each event. Cox regression was used to examine the risk of each outcome associated with each heart rate category (Referent: Quartile 1). Multivariable models were constructed as follows: Model 1 adjusted for age, sex, and race; Model 2 adjusted for Model 1 covariates plus smoking, systolic blood pressure, diabetes, body mass index, serum creatinine, angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers, beta blockers, statin, randomization group (spironolactone versus placebo), New York Heart Association Class, coronary heart disease, and stroke. The risk of each outcome also was examined using heart rate as a continuous variable (per 5-bpm increase). Several sensitivity analyses were performed. Due to the fact that beta blockers directly influence heart rate, a secondary analysis was performed in which the analysis was stratified by beta blocker use. Data from the Systolic Heart failure treatment with the If inhibitor ivabradine Trial (SHIFT) have suggested that heart failure patients with heart rates ≥70 bpm benefit from further reduction in heart rate.3 Accordingly, a secondary analysis was performed to examine the risk of each outcome in patients with heart rates ≥70 vs. <70 bpm. Additionally, due to differences in the baseline characteristics and event rates observed between patients recruited in Russia and Georgia versus the Americas,13 we examined if our findings varied by region (Russia/Georgia vs. the Americas). Statistical significance was defined as p < 0.05. SAS Version 9.4 (Cary, NC) was used for all analyses.
RESULTS
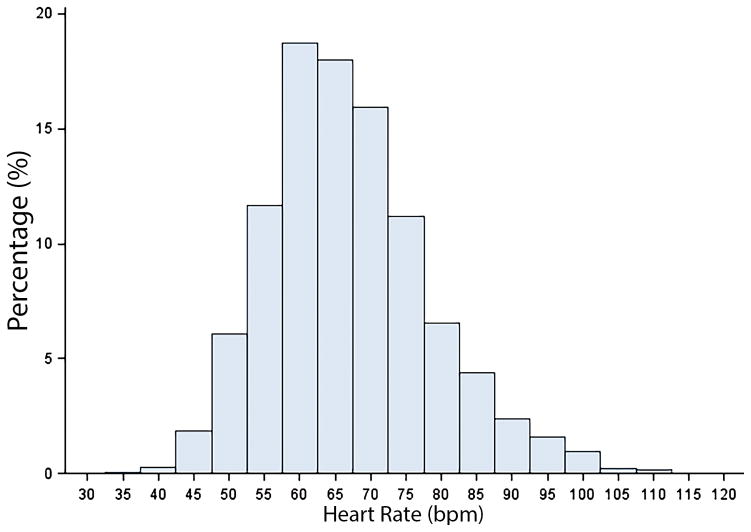
A total of 2,705 patients (mean age=68±10 years; 47% men; 88% white) were included in the final analytical sample. The mean heart rate was 67±11 bpm, and the distribution of heart rate for the study cohort is shown in Figure 1. Baseline characteristics across heart rate quartiles are shown in Table 1.
Figure 1. Distribution of Heart Rate Values.
bpm=beats per minute.
Table 1.
Baseline Characteristics (N=2,705)
| Characteristic | Heart Rate (beats per minute) | P-value* | |||
|---|---|---|---|---|---|
|
| |||||
| <59 (n=608) | 59–64 (n=644) | 65–73 (n=766) | ≥74 (n=687) | ||
| Age, mean ± SD, years | 68 ± 9.5 | 68 ± 9.3 | 67 ± 9.5 | 67 ± 9.7 | <0.001 |
| Male (%) | 310 (51) | 278 (43) | 353 (46) | 319 (46) | 0.049 |
| White (%) | 550 (90) | 568 (88) | 689 (90) | 576 (84) | <0.001 |
| Current smoker (%) | 59 (10) | 65 (10) | 98 (13) | 95 (14) | 0.048 |
| Diabetes (%) | 159 (26) | 210 (33) | 260 (34) | 266 (39) | <0.001 |
| Coronary heart disease (%) | 276 (45) | 259 (40) | 283 (37) | 235 (34) | <0.001 |
| Stroke (%) | 48 (7.9) | 36 (5.6) | 62 (8.1) | 46 (6.7) | 0.25 |
| Systolic blood pressure, mean ± SD, mm Hg | 130 ± 13 | 130 ± 14 | 130 ± 13 | 130 ± 14 | 0.75 |
| Body mass index, mean ± SD, kg/m2 | 31 ± 6.3 | 32 ± 7.2 | 32 ± 7.2 | 33 ± 8.0 | <0.001 |
| Serum creatinine, mean ± SD, mg/dL | 1.09 ± 0.29 | 1.07 ± 0.29 | 1.07 ± 0.30 | 1.07 ± 0.32 | 0.69 |
| New York Heart Association Class III–IV (%) | 164 (27) | 189 (29) | 234 (31) | 233 (34) | 0.052 |
| Prior heart failure hospitalization (%) | 415 (68) | 481 (75) | 563 (74) | 528 (77) | 0.0046 |
| Aspirin use (%) | 447 (74) | 463 (72) | 533 (70) | 480 (70) | 0.35 |
| Beta blockers (%) | 478 (79) | 505 (78) | 597 (78) | 502 (73) | 0.046 |
| ACEi/ARB (%) | 511 (84) | 544 (84) | 652 (85) | 580 (84) | 0.96 |
| Statin (%) | 353 (58) | 337 (52) | 402 (52) | 344 (50) | 0.032 |
| Spironolactone (%) | 311 (51) | 329 (51) | 367 (48) | 351 (51) | 0.52 |
| Russia/Georgia (%) | 317 (52) | 341 (53) | 418 (55) | 336 (49) | 0.18 |
Statistical significance for continuous data was tested using the analysis of variance procedure and categorical data was tested using the chi-square test.
ACEi/ARB= angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers; HDL=high-density lipoprotein; SD=standard deviation.
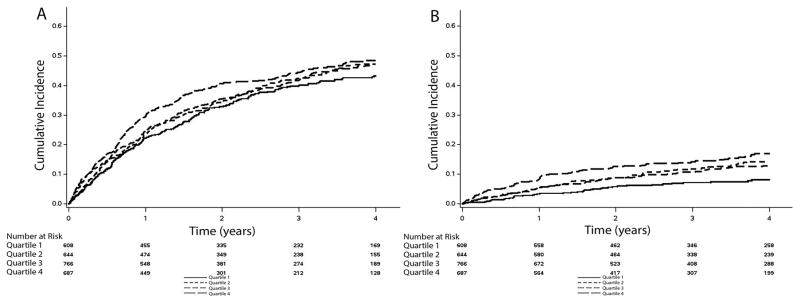
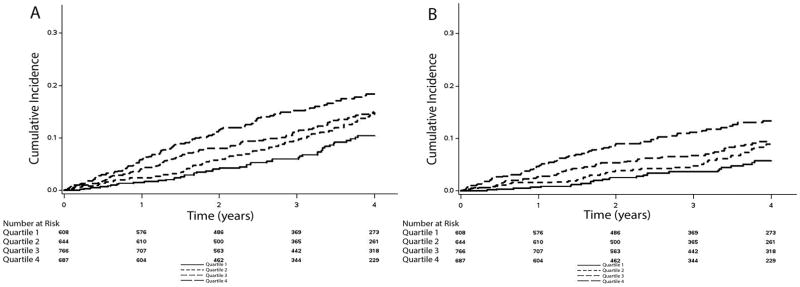
Over a median follow-up of 3.4 years (25th–75th percentiles=2.0, 4.9 years), a total of 1,157 hospitalizations, 311 hospitalizations for heart failure, 369 deaths, and 233 cardiovascular deaths occurred. Higher cumulative incidence estimates for all outcomes were observed in patients as heart rate quartile increased (Table 2). The cumulative incidence estimates for hospitalization and death are depicted in Figures 2 and 3, respectively.
Table 2.
Risk of Hospitalization and Death with Heart Rate (N=2,705)
| Outcome | Events | Incidence rate per 100 person-years | Model 1* HR (95%CI) |
P-value | Model 2† HR (95%CI) |
P-value |
|---|---|---|---|---|---|---|
| Hospitalization | ||||||
| <59 | 249 | 15.6 (13.8, 17.6) | Ref | - | Ref | - |
| 59 to 64 | 280 | 17.3 (15.4, 19.4) | 1.10 (0.93, 1.31) | 0.27 | 1.11 (0.93, 1.31) | 0.25 |
| 65 to 73 | 328 | 17.4 (15.6, 19.4) | 1.17 (0.99, 1.38) | 0.069 | 1.15 (0.97, 1.35) | 0.11 |
| ≥74 | 300 | 19.9 (17.7, 22.2) | 1.23 (1.04, 1.45) | 0.017 | 1.22 (1.03, 1.45) | 0.025 |
| Per 5-bpm increase | 1,157 | 17.5 (16.5, 18.5) | 1.04 (1.01, 1.06) | 0.0046 | 1.03 (1.004, 1.06) | 0.025 |
|
| ||||||
| Hospitalization for Heart Failure | ||||||
| <59 | 46 | 2.2 (1.6, 2.9) | Ref | - | Ref | - |
| 59 to 64 | 82 | 3.9 (3.1, 4.8) | 1.75 (1.22, 2.52) | 0.0024 | 1.71 (1.19, 2.45) | 0.0040 |
| 65 to 73 | 85 | 3.5 (2.8, 4.3) | 1.63 (1.14, 2.34) | 0.0075 | 1.45 (1.01, 2.08) | 0.046 |
| ≥74 | 98 | 5.0 (4.1, 6.1) | 2.21 (1.55, 3.14) | <0.001 | 2.08 (1.45, 2.98) | <0.001 |
| Per 5-bpm increase | 311 | 3.6 (3.2, 4.0) | 1.12 (1.07, 1.17) | <0.001 | 1.10 (1.05, 1.15) | <0.001 |
|
| ||||||
| Death | ||||||
| <59 | 58 | 2.6 (2.0, 3.4) | Ref | - | Ref | - |
| 59 to 64 | 88 | 3.9 (3.2, 4.8) | 1.54 (1.11, 2.15) | 0.010 | 1.55 (1.11, 2.16) | 0.010 |
| 65 to 73 | 109 | 4.2 (3.5, 5.0) | 1.71 (1.24, 2.35) | 0.001 | 1.63 (1.18, 2.25) | 0.0029 |
| ≥74 | 114 | 5.3 (4.4, 6.4) | 2.30 (1.67, 3.16) | <0.001 | 2.20 (1.59, 3.04) | <0.001 |
| Per 5-bpm increase | 369 | 4.0 (3.6, 4.4) | 1.12 (1.07, 1.17) | <0.001 | 1.10 (1.06, 1.16) | <0.001 |
|
| ||||||
| Cardiovascular Death | ||||||
| <59 | 32 | 1.5 (1.0, 2.1) | Ref | - | Ref | - |
| 59 to 64 | 51 | 2.3 (1.7, 3.0) | 1.62 (1.04, 2.53) | 0.032 | 1.66 (1.06, 2.57) | 0.027 |
| 65 to 73 | 71 | 2.7 (2.2, 3.4) | 2.00 (1.32, 3.04) | 0.0011 | 1.98 (1.30, 3.02) | 0.0014 |
| ≥74 | 79 | 3.7 (3.0, 4.6) | 2.80 (1.85, 4.23) | <0.001 | 2.76 (1.82, 4.19) | <0.001 |
| Per 5-bpm increase | 233 | 2.5 (2.2, 2.9) | 1.13 (1.07, 1.19) | <0.001 | 1.13 (1.07, 1.19) | <0.001 |
Adjusted for age, sex, and race.
Adjusted for Model 1 covariates plus smoking, systolic blood pressure, diabetes, body mass index, serum creatinine, angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers, beta blockers, statin, randomization group, New York Heart Association Class, coronary heart disease, and stroke.
bpm=beats per minutes; CI=confidence interval; HR=hazard ratio.
Figure 2. Unadjusted Cumulative Incidence of Hospitalization*.
*The cumulative incidence curves for hospitalization (A: log-rank p=0.16) and hospitalization for heart failure (B: log-rank p<0.001) are shown.
Quartile1: <59 bpm; Quartile 2: 59–64 bpm; Quartile 3: 65–73 bpm; Quartile 4: ≥74 bpm.
bpm=beats per minute.
Figure 3. Unadjusted Cumulative Incidence of Death.
*The cumulative incidence curves for death (A: log-rank p<0.001) and cardiovascular death (B: log-rank p<0.001) are shown.
Quartile1: <59 bpm; Quartile 2: 59–64 bpm; Quartile 3: 65–73 bpm; Quartile 4: ≥74 bpm.
bpm=beats per minute.
An increased risk for hospitalization, hospitalization for heart failure, death, and cardiovascular death, was observed for those with higher compared with lower heart rates (Table 2). Additionally, the results were similar when heart rate was examined as a continuous variable per 5-bpm increase. When the analysis was stratified by beta blocker use, the magnitude of the association for each outcome (per 5-bpm increase) did not vary between groups (Table 3). Also, when we explored the utility of the clinical cut-off point of 70 for resting heart rate, an increased risk for hospitalization for heart failure, death, and cardiovascular death was observed (Table 4). The association between heart rate and each outcome remained similar when the analysis was stratified by country of origin (Supplemental Table 1).
Table 3.
Risk of Hospitalization and Death with Heart Rate by Beta Blockers (N=2,705)*
| Outcome | Events | Incidence rate per 100 person-years | Model 1† HR (95%CI) |
P-value | Model 2‡ HR (95%CI) |
P-value |
|---|---|---|---|---|---|---|
| Beta Blockers (n=2,082) | ||||||
| Hospitalization | 905 | 17.9 (16.8, 19.1) | 1.04 (1.01, 1.07) | 0.0033 | 1.03 (1.01, 1.06) | 0.023 |
| Hospitalization for Heart Failure | 268 | 4.1 (3.6, 4.6) | 1.12 (1.07, 1.18) | <0.001 | 1.09 (1.04, 1.15) | <0.001 |
| Death | 297 | 4.2 (3.7, 4.7) | 1.11 (1.05, 1.16) | <0.001 | 1.09 (1.04, 1.15) | <0.001 |
| Cardiovascular Death | 185 | 2.6 (2.3, 3.0) | 1.13 (1.06, 1.20) | <0.001 | 1.13 (1.06, 1.20) | <0.001 |
|
| ||||||
| No Beta Blockers (n=623) | ||||||
| Hospitalization | 252 | 16.2 (14.3, 18.3) | 1.03 (0.97, 1.09) | 0.34 | 1.03 (0.97, 1.09) | 0.37 |
| Hospitalization for Heart Failure | 43 | 2.1 (1.6, 2.8) | 1.17 (1.03, 1.33) | 0.013 | 1.12 (0.98, 1.27) | 0.099 |
| Death | 72 | 3.4 (2.7, 4.3) | 1.18 (1.07, 1.30) | 0.0012 | 1.16 (1.05, 1.28) | 0.0047 |
| Cardiovascular Death | 48 | 2.3 (1.7, 3.0) | 1.14 (1.01, 1.29) | 0.039 | 1.12 (0.99, 1.27) | 0.081 |
HR presented per 5-bpm increase.
Adjusted for age, sex, and race.
Adjusted for Model 1 covariates plus smoking, systolic blood pressure, diabetes, body mass index, serum creatinine, angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers, statin, randomization group, New York Heart Association Class, coronary heart disease, and stroke.
bpm=beats per minutes; CI=confidence interval; HR=hazard ratio.
Table 4.
Risk of Hospitalization and Death with Heart Rate ≥70 bpm (N=2705)
| Outcome | Events | Incidence rate per 100 person-years | Model 1* HR (95%CI) |
P-value | Model 2† HR (95%CI) |
P-value |
|---|---|---|---|---|---|---|
| Hospitalization | ||||||
| <70 | 727 | 17.0 (15.8, 18.3) | Ref | - | Ref | - |
| ≥70 | 430 | 18.3 (16.7, 20.2) | 1.06 (0.94, 1.19) | 0.36 | 1.06 (0.94, 1.20) | 0.33 |
|
| ||||||
| Hospitalization for Heart Failure | ||||||
| <70 | 184 | 3.3 (2.8, 3.8) | Ref | - | Ref | - |
| ≥70 | 127 | 4.2 (3.5, 5.0) | 1.28 (1.02, 1.60) | 0.036 | 1.27 (1.01, 1.59) | 0.045 |
|
| ||||||
| Death | ||||||
| <70 | 209 | 3.5 (3.1, 4.0) | Ref | - | Ref | - |
| ≥70 | 160 | 4.9 (4.2, 5.7) | 1.50 (1.22, 1.84) | <0.001 | 1.45 (1.17, 1.78) | <0.001 |
|
| ||||||
| Cardiovascular Death | ||||||
| <70 | 126 | 2.1 (1.8, 2.5) | Ref | - | Ref | - |
| ≥70 | 107 | 3.3 (2.7, 4.0) | 1.63 (1.26, 2.11) | <0.001 | 1.59 (1.23, 2.07) | <0.001 |
Adjusted for age, sex, and race.
Adjusted for Model 1 covariates plus smoking, systolic blood pressure, diabetes, body mass index, serum creatinine, angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers, beta blockers, statin, randomization group, New York Heart Association Class, coronary heart disease, and stroke.
bpm=beats per minutes; CI=confidence interval; HR=hazard ratio.
DISCUSSION
In this analysis from TOPCAT, we have demonstrated that higher resting heart rates are associated with an increased risk for adverse outcomes in patients with HFpEF who are in sinus rhythm. Specifically, we have shown that the risk for hospitalization, hospitalization for heart failure, death, and cardiovascular death increases linearly across heart rate values. Overall, our findings suggest that elevated heart rates have important prognostic significance in HFpEF, and careful clinical evaluation is needed in HFpEF patients with heart rates ≥70 bpm.
Despite numerous reports that have demonstrated that high resting heart rates are associated with adverse outcomes among heart failure patients with reduced ejection,2–4 the reports for HFpEF are conflicting. In a subgroup analysis limited to HFpEF patients from the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity Program, resting heart rate (per 10-bpm increase) was not associated with all-cause mortality (HR=1.05, 95%CI=0.98, 1.12).6 A trend for significance was observed between heart rate (per 10-bpm increase) and the composite of cardiovascular death and hospitalization for heart failure (HR=1.06, 95%CI=1.00, 1.12). Data from the irbesartan in patients with heart failure and preserved systolic function trial (I-Preserve) demonstrated that higher resting heart rates per 1-SD increase were associated with cardiovascular death (HR=1.12, 95%CI=1.01, 1.24), but not all-cause mortality (HR=1.08, 95%CI=0.99, 1.18).9 Similarly, data from the Digitalis Investigator Group (DIG) trial demonstrated that higher resting heart rates (Quartile 4 vs. Quartile 2: HR=1.38, 95%CI=0.94, 2.02) were not associated with mortality in HFpEF.8 In contrast, data from the Chronic Heart Failure Analysis and Registry in the Tohoku District 2 (CHART-2) study linked higher resting heart rate with all-cause mortality (Quartile 3 vs. Quartile 1: HR=1.82, 95%CI=1.23, 2.69) and cardiovascular death (Quartile 3 vs. Quartile 1: HR=1.98, 95%CI=1.05, 3.72).7 Similar inconsistencies exist for the relationship between heart rate and the risk of hospitalization for heart failure.7–9
In contrast, the current analysis was able to demonstrate that higher resting heart rates are associated with an increased risk for several outcomes in patients with HFpEF. Due to the robust ascertainment of outcomes in TOPCAT, we were able to examine the individual risk for several outcomes, and this was not done in prior reports. Furthermore, the current analysis was limited to patients with HFpEF, and we did not have to stratify between reduced and preserved ejection fraction, as done in several of the aforementioned studies.6–8 Additionally, compared with prior reports our analysis was limited to participants in sinus rhythm,6 included an international sample,7 and did not precede contemporary heart failure management.8, 9 Therefore, our data likely provide a more accurate assessment of the association between heart rate and outcomes in HFpEF.
Beta blocker therapy has consistently been shown to improve survival in patients with heart failure with reduced ejection fraction, presumably through inhibition of the sympathetic nervous system, and subsequent prevention of adverse myocardial remodeling through reductions in heart rate.5, 14 Recently, data have demonstrated that patients with reduced ejection fraction who have persistent heart rates above 70 bpm, despite adequate beta blocker therapy, benefit from agents that selectively reduce heart rate.3 Accordingly, ivabradine is recommended for symptomatic patients with reduced ejection fraction (<35%) on optimal beta blocker therapy who have heart rates ≥70 bpm in sinus rhythm.15 Although similar recommendations do not exist for HFpEF, our data demonstrate that a significant risk for hospitalization for heart failure, death, and cardiovascular death exists in HFpEF patients with heart rates ≥70 bpm. Therefore, it is possible that a similar benefit in heart rate reductive strategies exists in HFpEF. Additionally, we observed a similar increased risk of adverse events with higher resting heart rates in patients who did and did not receive beta blockers. This would suggest that heart rate goals are more beneficial in HFpEF than the presence of beta blockade therapy. However, the secondary analyses in this report were strictly exploratory and further research, including clinical trials, are needed before changes in clinical practice are recommended.
The current study should be interpreted in the context of certain limitations. The study sample was limited to patients in sinus rhythm and limits the generalizability of our findings. Several baseline characteristics were obtained by self-report and subjected our analysis to recall bias. Similarly, although rigorous methodology was used to ascertain all outcomes, it is possible that events were missed. Heart rate was measured at one visit and it is possible that the results will change with subsequent heart rate recordings. However, the aim of this study was to demonstrate that heart rate measurements at a single point in time are predictive of adverse events in HFpEF. Additionally, significant results were not observed for hospitalization, hospitalization for heart failure, and cardiovascular death in patients who did not report the use of beta blocker therapies. Despite a lack of statistical significance that was related to the small number of patients who did not receive beta blockers, effect estimates were similar to patients who received these therapies, suggesting that a comparable association exists in the 2 groups. Lastly, we attempted to account for differences between study participants in our multivariable models, but acknowledge the possibility of residual confounding.
In conclusion, higher resting heart rates are associated with several adverse events in patients with HFpEF. Further studies are needed to confirm our findings and to explore the possibility of heart rate reduction strategies to improve outcomes in patients with HFpEF.
Supplementary Material
Acknowledgments
This Manuscript was prepared using Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist Trial (TOPCAT) Research Materials obtained from the National Heart, Lung, and Blood Institute Biologic Specimen and Data Repository Information Coordinating Center and does not necessarily reflect the opinions or views of the TOPCAT Study or the National Heart, Lung, and Blood Institute.
FUNDING
Research reported in this publication was supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under award number F32-HL134290. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
AUTHOR CONTRIBUTIONS
All authors contributed to the conception and design of the work. WTO and EZS contributed to the acquisition and analysis of data. All authors contributed to the interpretation of the work, drafting of the manuscript, and critically revising the manuscript. All gave final approval and agree to be accountable for all aspects of work ensuring integrity and accuracy.
DISCLOSURES
None.
References
- 1.Lymperopoulos A, Rengo G, Koch WJ. Adrenergic nervous system in heart failure: pathophysiology and therapy. Circ Res. 2013;113:739–53. doi: 10.1161/CIRCRESAHA.113.300308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pocock SJ, Wang D, Pfeffer MA, et al. Predictors of mortality and morbidity in patients with chronic heart failure. Eur Heart J. 2006;27:65–75. doi: 10.1093/eurheartj/ehi555. [DOI] [PubMed] [Google Scholar]
- 3.Swedberg K, Komajda M, Bohm M, et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010;376:875–85. doi: 10.1016/S0140-6736(10)61198-1. [DOI] [PubMed] [Google Scholar]
- 4.Bohm M, Swedberg K, Komajda M, et al. Heart rate as a risk factor in chronic heart failure (SHIFT): the association between heart rate and outcomes in a randomised placebo-controlled trial. Lancet. 2010;376:886–94. doi: 10.1016/S0140-6736(10)61259-7. [DOI] [PubMed] [Google Scholar]
- 5.Chatterjee S, Biondi-Zoccai G, Abbate A, et al. Benefits of beta blockers in patients with heart failure and reduced ejection fraction: network meta-analysis. BMJ. 2013;346:f55. doi: 10.1136/bmj.f55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castagno D, Skali H, Takeuchi M, et al. Association of heart rate and outcomes in a broad spectrum of patients with chronic heart failure: results from the CHARM (Candesartan in Heart Failure: Assessment of Reduction in Mortality and morbidity) program. J Am Coll Cardiol. 2012;59:1785–95. doi: 10.1016/j.jacc.2011.12.044. [DOI] [PubMed] [Google Scholar]
- 7.Takada T, Sakata Y, Miyata S, et al. Impact of elevated heart rate on clinical outcomes in patients with heart failure with reduced and preserved ejection fraction: a report from the CHART-2 Study. Eur J Heart Fail. 2014;16:309–16. doi: 10.1002/ejhf.22. [DOI] [PubMed] [Google Scholar]
- 8.Maeder MT, Kaye DM. Differential impact of heart rate and blood pressure on outcome in patients with heart failure with reduced versus preserved left ventricular ejection fraction. Int J Cardiol. 2012;155:249–56. doi: 10.1016/j.ijcard.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 9.Bohm M, Perez AC, Jhund PS, et al. Relationship between heart rate and mortality and morbidity in the irbesartan patients with heart failure and preserved systolic function trial (I-Preserve) Eur J Heart Fail. 2014;16:778–87. doi: 10.1002/ejhf.85. [DOI] [PubMed] [Google Scholar]
- 10.Desai AS, Lewis EF, Li R, et al. Rationale and design of the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial: a randomized, controlled study of spironolactone in patients with symptomatic heart failure and preserved ejection fraction. Am Heart J. 2011;162:966–72. e10. doi: 10.1016/j.ahj.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Shah SJ, Heitner JF, Sweitzer NK, et al. Baseline characteristics of patients in the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial. Circ Heart Fail. 2013;6:184–92. doi: 10.1161/CIRCHEARTFAILURE.112.972794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pitt B, Pfeffer MA, Assmann SF, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383–92. doi: 10.1056/NEJMoa1313731. [DOI] [PubMed] [Google Scholar]
- 13.Pfeffer MA, Claggett B, Assmann SF, et al. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation. 2015;131:34–42. doi: 10.1161/CIRCULATIONAHA.114.013255. [DOI] [PubMed] [Google Scholar]
- 14.Kato Y, Suzuki S, Uejima T, et al. The relationship between resting heart rate and peak VO2: A comparison of atrial fibrillation and sinus rhythm. Eur J Prev Cardiol. 2016;23:1429–36. doi: 10.1177/2047487316633885. [DOI] [PubMed] [Google Scholar]
- 15.Yancy CW, Jessup M, Bozkurt B, et al. 2016 ACC/AHA/HFSA Focused Update on New Pharmacological Therapy for Heart Failure: An Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2016;68:1476–88. doi: 10.1016/j.jacc.2016.05.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.