Abstract
Chemical functionalization broadens carbon nanotube (CNT) applications, conferring new functions, but at the same time potentially altering toxicity. Although considerable experimental data related to CNT toxicity, at the molecular and cellular levels, have been reported, there is very limited information available for the corresponding mechanism involved (e.g. cell apoptosis and genotoxicity). The threshold dose for safe medical application in relation to both pristine and functionalized carbon nanotubes remains ambiguous. In this study, we evaluated the in vitro cytotoxicity of pristine and functionalized (–OH, –COOH) multi-walled carbon nanotubes (MWCNTs) for cell viability, oxidant detection, apoptosis and DNA mutations, to determine the nontoxic dose and influence of functional group in a human lung-cancer cell line exposed to 1–1000 μg/ml MWCNTs for 24, 48 and 72 h. The findings suggest that pristine MWCNTs induced more cell death than functionalized MWCNTs while functionalized MWCNTs are more genotoxic compared to their pristine form. The level of both dose and dispersion in the matrix used should be taken into consideration before applying further clinical applications of MWCNTs.
Keywords: Multi-walled carbon nanotubes, Cytotoxicity, Functionalization, Viability, Reactive oxygen species, Apoptosis, DNA damage
1. Introduction
Carbon nanotubes (CNTs) are a family of nanomaterials made up entirely of carbon. In this family, structurally multi-walled carbon nanotubes (MWCNTs) consist of multiple layers of graphite superimposed and rolled in on themselves to form a tubular shape. MWCNTs are of special interest for industry and have been increasingly utilised as advanced nanovectors in drug/gene delivery systems (Vashist et al., 2011). They possess significant advantages including high surface area, well-defined morphologies and unique optical as well as electrical properties, in addition to their super mechanical strength and thermal conductivity.
Apart from their special physico-chemical properties however, MWCNTs present low bio-compatibility in most biological and chemical environments, already generating some health and environmental concerns. Although the toxicological effects of MWCNTs have been investigated, existing data are far from adequate. It has been suggested that the related factors of MWCNTs such as fibre dose (He et al., 2011), length (Johnston et al., 2010), diameter (Nagai et al., 2011), surface area (Kim et al., 2011), tendency to agglomerate (Kim et al., 2011) and dispersibility in media (Kim et al., 2011) could influence toxicity and reactivity of CNTs in vitro and in vivo. For example, a recent study showed that the pathogenicity observed for asbestos-like long pristine MWCNTs could be obviated if their effective length is decreased (Ali-Boucetta et al., 2013). Elgrabli further reported that CNTs dispersed in bovine serum albumin (BSA) exhibited significantly reduced toxic (Elgrabli et al., 2007). Other researchers observed that agglomerates induced more pronounced cytotoxic effects than asbestos fibres at the same mass concentrations (Wick et al., 2007).
It is worth noting that, as previously reported, the functionalization/modification of the structure of MWCNTs can optimise their solubility and dispersion, allowing innovative applications in materials (Martín et al., 2013), electronics (Castranova et al., 2013), chemical processing (Martín et al., 2013) and energy management (Mauter and Elimelech, 2008). However, the functionalization of MWCNTs produced additional controversy regarding their toxicity. For example, Vashist et al. (2011) and Mali et al. (2011) reported that functionalized CNTs were able to exhibit very low toxicity and higher propensity to cross cell membranes, increasing their potential for drug and gene delivery. On the contrary, Magrez et al. showed that the toxicity of MWCNT, in human lung-tumor cell lines, was increased when carboxyl and hydroxyl groups were present on their surface (Magrez et al., 2006). A study conducted by Patlolla et al. performed on bone marrow cells of mice exposed to –COOH functionalized and pristine MWCNTS, found that functionalized MWCNTs had higher clastogenic and genotoxic potential than non-functionalized CNTs (Patlolla et al., 2010).
Data on genotoxicity induced by pristine and functionalized MWCNTs are still very limited. Until very recently, their effect on DNA damage has been investigated but the accumulation of 8-OHdG in DNA was not found (Ogasawara et al., 2012). Information about apoptosis induced by MWCNT is also incomplete although pristine MWCNTs does not appear to cause apoptosis in lung cells (Ursini et al., 2012).
Hence, in this study, we investigated the effect of dispersion of two most frequently used functionalized multi-walled carbon nanotubes (i.e. OH-MWCNTs and COOH-MWCNTs) on A549 cell line apoptosis and DNA damage, in comparison with pristine MWCNTs. As the primary route of human exposure to MWCNTs is via inhalation, the A549 cell line, a lung cancer cell line, was selected for this study. More importantly and interestingly, cell proliferation and oxidative stress were also investigated in order to provide a more comprehensive understanding of the potential mechanism of toxicity as well as the threshold dose for their safe medical applications.
2. Materials and methods
2.1. Material
Prisitine multi-walled carbon nanotubes (P-MWNCNTs), carboxylic acid, hydroxy functionalized MWCNTs (COOH-MWCNTs, OH-MWCNTs) were purchased at Cheap Tubes Inc. (United States, Sku-030111, 030112, 030113). External diameter: 13–18 nm; functional group content: 7.0% ± 1.5%; length: 1–12 μm; purity: > 99 wt% (Fig. 1).
Fig. 1.
Multi-walled carbon nanotubes (MWCNTs). a, pristine MWCNTs; b, OH-MWCNTs; c, COOH-MWCNTs.
2.2. Preparation of BSA solution
Five milligram nanotubes were suspended in FBS free medium which contains 0.5% BSA at 1000 μg/ml. Then they were diluted into 316, 200, 100, 50, 36, 20, 10 3.6 and 1 μg/ml.
2.3. Cell culture
Human lung epithelial cell line (A549) was obtained as a gift from Dr. Huijun Zhu. Passage number is 76–79 according to ATCC website (http://atcc.custhelp.com/app/answers/detail/a_id/3/~/high-passage-number, 27/06/2014). Before using, bacterial, yeast and fungal contamination were checked under a microscope for mycoplasma using Gibco’s Mycotect kit (Cat.No. 15672017). A549 cells were cultured in F12/DMEM (Dulbecco’s Modified Eagle Medium) (Invitrogen, United Kingdom) supplemented with 10% fetal calf serum (sigma, United Kingdom) at 37 °C in 5% CO2. All the experiments were conducted using the same passage number. Six T-75 flasks of cells were collected and re-suspended in freezing media (90% serum + 10% dimethylsulfoxide), and then they were aliquoted into 1 ml vials. The cells were frozen and kept at −20 °C for 2 h and then at −80 °C overnight. The cells were then moved to a −150 °C freezer the next day. This protocol was repeated in each passage.
2.4. Cell viability
Two hundred microliter of cells were seeded in 96-well plates at 2 × 104 cells/ml per well and treated with carbon nanotubes the following day for 24 h. Before measurement, cells were washed with PBS one time and 100 μl new medium was added per well. The tetrazolium salt WST-1 cell proliferation reagent was added to cells at the recommended concentration (5 μl/well) and incubated at 37 °C in a humidified atmosphere of 5% CO2 for 3 h. Plates were shaken for 1 min and absorbance was measured at 405 nM with a Varioskan Flash Multimode plate reader (Thermo Scientific). Unexposed cells were used as negative control.
2.5. Cell membrane integrity
Lactate dehydrogenase release was measured as an indicator of cell membrane damage using an LDH assay kit (Cytotoxicity Detection Kit, sigma, United Kingdom) of the culture medium of cells exposed to MWCNTs for 24 h. 200 μl cells were seeded in 96-well plates at 2 × 104 cells/ml per well and treated with carbon nanotubes the following day for 24 h. Aliquots (50 μl) of supernatant and reaction mixture were transferred into corresponding wells of an optically clear 96-well plate and incubated for 30 min at 25 °C, protecting the plate from the light. The increase in enzyme activity directly correlates to the amount of formazan produced by reduction of the tetrazolium salt. The absorbance was measured at 490 nm using a Varioskan Flash Multimode plate reader. A background and negative controls were obtained by LDH activity measurement of assay medium and unexposed cell medium, respectively.
2.6. Oxidant detection
The production of oxidants was assessed on intact cells in 96 wells microplates using the dichlorofluorescein (DCF) assay. Oxidation of the hydrolysed product of DCF-DA to DCF is not specific for a particular reactive species, but its oxidation generally correlates with oxidative stress. Cells at 2 × 104/ml were incubated with MWCNTs at concentrations 2, 20 and 50 μg/ml for 6 h firstly. 2′,7′-dichlorofluorescein diacetate (DCFH-DA) (Sigma, United Kingdom) was dissolved in culture medium to give a final concentration of 20 μM. Total DCF fluorescence was detected by incubation with DCFH-DA solution (200 μl) at 37 °C for 30 min, and then washed with PBS (phosphate buffered saline) and resuspended in 100 μl of PBS. The formation of the fluorescence-oxidized derivative of DCF was monitored at an emission wavelength of 520 nm and excitation wavelength of 485 nm with Varioskan Flash Multimode plate reader. DCF fluorescence was expressed as percentage of control.
2.7. Apoptosis detection
A549 cell apoptosis was evaluated by Annexin V–FITC, PI apoptosis detection kit (Ebioscience, United Kingdom). Annexin V binds to phosphatidylserine that moves to the outer leaflet of cells at the beginning of apoptosis. Necrotic cells were stained with both Annexin V and PI.
Cells were cultured in 6 well plate at 1 × 105/ml per well and then exposed for 48 h to MWCNTs culture medium the following day; control cells were incubated without MWCNTs. Before detection, the cell culture medium were removed and washed by cold PBS. Then the detached cells were centrifuged at 1200 rpm and placed on ice. After a 10 min incubation period with 2 μl Annexin-V and 4 μl PI, cells were analysed by flow cytometer (Accuri C6).
2.8. DNA damage
A549 cells were seeded in 6-well plates at 1 × 105/ml exposed to pristine and functionalized MWCNTs at 50 μg/ml for 72 h. Negative control cells were incubated without MWCNTs. The concentration of 8-oxodeoxyguanosine (8-OHdG) in DNA, indicating oxidative DNA damage, was quantified by colorimetric antibody ELISA assay that has been widely used for the 8-OHdG detection.
2.8.1. DNA extraction
DNA isolation from cells treated with MWCNTs was carried out with a DNA extraction kit from QIAGEN (United Kingdom). The assay was performed according to manufacturer’s instruction. After exposure to MWCNTs, cells were treated with cell lysis buffer and then centrifuged at 300 ×g for 3 min, the supernatant discarded, and the tube inverted on a clean piece of absorbent paper. The DNA pellets were cleaned using 600 μg 70% ethanol and then centrifuged at 10000 ×g for 3 min. The supernatant was discarded and the DNA pellets air-dried until dry. The DNA was then dissolved in Tris-EDTA buffer for 30 min at 65 °C. All the DNA samples were stored at −20 °C for later use.
2.8.2. ELISA
The concentration of 8-OHdG was measured by HT 8-oxo-dG kit (TREVIGEN, United Kingdom) based on ELISA. The assay was performed according to manufacturer’s instruction. Briefly, the DNA samples were diluted to 50 μg/ml (Picodrop) and then further diluted 2:3 in Assay Diluent. 25 μg 8-OHdG standards and DNA samples were added to each well, followed by adding 25 μg monoclonal antibody. Then the plate was covered with film sealer and incubated at 25 °C for 1 h. After washing the plate 4 times with PBST, add 50 μl HRP conjugate to each well, followed by 1 h incubation. After aspirating and washing with washing solution, the plate was inverted and blotted against clear paper towels and this process was repeated four times. Each well was added 50 μl of TACS-Sapphire™ gently mixed and incubated at 37 °C for 15 min, then stopped with 50 μl of 1 M hydrochloric acid. Assay plates were read at 450 nm with a plate reader.
2.9. Statistical methods
Three independent experiments were performed. Each concentration was assayed in triplicate, in each independent experiment. The measurements were corrected using absorbance/fluorescence readings from MWCNT addition within a cell-free system. Results were expressed as mean ± standard deviation (SD). Statistical analysis was carried out using analysis of variance (ANOVA), followed by Tukey’s multiple comparison tests. Differences were considered significant from p < 0.05.
3. Results
3.1. Cell viability
In our cell viability study, the cell proliferation reagent WST-1 was used to evaluate cell viability in response to pristine and functionalized MWCNTs. Fig. 2 describes the comparison of the concentration-response curves obtained for each MWCNT [Bottom (minimum effect), IC50 (concentration required for 50% of cell viability inhibition), Top (maximum effect)]. IC50 of pristine MWCNTs (255.2 μg/ml) were significant different from IC50 of –OH and –COOH functionalized MWCNTs (2198, 2456 μg/ml). p < 0.0001.
Fig. 2.
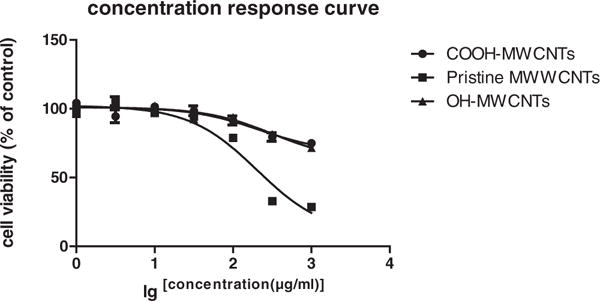
Cell viability curve of A549 cells was evaluated after 24 hour exposure to pristine MWCNTs, OH-MWCNTs and COOH-MWCNTs with concentrations from 1 to 1000 μg/ml. Viability was detected by using WST-1 assay. Data are represented as mean ± SD of triplicate. * p < 0.05, **p < 0.01.
3.2. LDH
LDH release from cells treated with MWCNTs was also assessed. The increase of LDH activity in cell culture medium, indicative of cell membrane damage was detectable as early as 6 h after treatment (data are not shown). Fig. 3 shows the results obtained at 24 h. The effect on LDH leakage was not significant at 20 and 50 μg/ml with all three MWCNTs. However, at 200 μg/ml, functionalized carbon nanotubes exhibited significant membrane damage while the pristine form showed no significant membrane damage compared with untreated cells (Fig. 3).
Fig. 3.
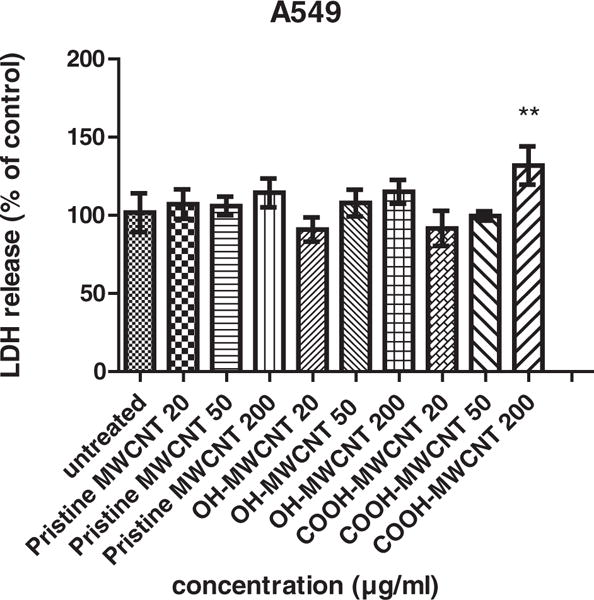
LDH release assay. Cytotoxicity of all the cell lines was evaluated after 24 hour exposure to pristine MWCNTs, OH-MWCNTs and COOH-MWCNTs with concentrations from 20 to 200 μg/ml. Among these groups, cells treated with 200 μg/ml COOH-MWCNTs was significant different. Data are represented as mean ± SD of triplicate. * p < 0.05, **p < 0.01.
3.3. Aggregation
It has been reported that MWCNTs showed a very good dispersion in 0.5% bovine serum albumin (BSA solution) (Elgrabli et al., 2007). A comparison experiment of cell viability test of cells treated with MWCNT and BSA-contained MWCNT suspension, from 0 to 200 μg/ml, has been conducted in order to evaluate toxicity of more thoroughly dispersed CNTs. Fig. 4 shows the pictures of pristine MWCNTs, OH-MWCNTs and COOH-MWCNTs dispersed in cell culture medium without and with BSA, suggesting different extent of aggregation. The samples were suspended at 200 μg/ml and left still for 1 min in order to allow them to settle and form aggregates. From visual observation, pristine carbon nanotubes had smaller aggregates compared to functionalized carbon nanotubes and the suspension with BSA showed better dispersibilty than those without BSA.
Fig. 4.

Photo of MWCNTs in the medium. A–C: pristine MWNCT, OH-MWCNT, COOH-MWCNT in the BSA− medium at 200 μg/ml; D–F: MWNCT, OH-MWCNT, COOH-MWCNT in the BSA+ medium at 200 μg/ml.
The results of comparison of cell viability with and without 0.5% BSA (Fig. 5) showed that the dispersion of carbon nanotubes was of great importance and significantly increased cell viability. At 50 μg/ml, none of the three types of MWCNTs showed significant alterations to cell viability with or without BSA. However, at 200 μg/ml, viability of functionalized carbon nanotubes (OH-MWCNTs & COOH-MWCNTs) treated cells was significantly decreased by adding BSA (the p values of slope difference are 0.01745 and 0.007834 respectively). But with respect to pristine MWCNTs, BSA did not increase cell viability at the same concentration. This indicated that the toxicity of pristine MWCNTs was not changed by adding BSA. These results could be explained by the fact that pristine MWCNTs were well dispersed, so additional BSA did not change the dispersion significantly. Although at 200 μg/ml the absorbance value did not show a significant difference with and without BSA, the p value of slope difference indicates that the adding BSA made functionalised MWCNTs more toxic (Fig. 5b and c). However, the absorbance values corresponding to pristine MWCNTs at both 50 and 200 μg/ml were significantly higher in the presence than in the absence of BSA. In addition, the slopes of the two curves were not significantly different.
Fig. 5.
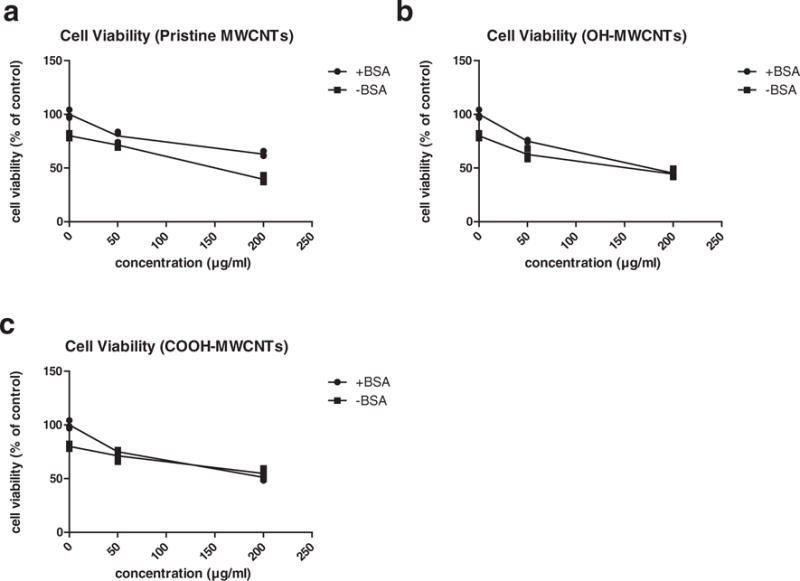
Comparison of cell viability with and without 0.5% BSA in FBS-free medium. The viability of A549 cell was detected by using WST-1 assay following 24 hour incubation with all three types of MWCNTs at concentrations of 0, 50 and 200 μg/ml. a, pristine MWCNTs; b, OH-MWCNTs; c, COOH-MWCNTs. Data are represented as mean ± SD of triplicate.
3.4. DCF fluorescence
Oxidative stress plays a role in toxicity induced by carbon nanotubes (Pichardo et al., 2012). In this report, DCF-DA was used to measures oxidizing potential within cells. The results of MWCNTs-induced DCF fluorescence are summarized in Fig. 6. A549 cells showed responsiveness following 6 h exposure of MWCNTs at each concentration. At 2 μg/ml concentration, all three carbon nanotubes did not induce significant DCF fluorescence while cells treated with 20 μg/ml MWCNTs (pristine, –OH, –COOH) induced 5.35, 4.84 and 5.36 fold greater DCF fluorescence than untreated cells respectively. However, a reduction in DCF fluorescence was observed following exposure to MWCNTs at 50 μg/ml, which may have been caused by either interference of carbon nanotubes with DCF fluorescence or the loss of cell viability (Fig. 2).
Fig. 6.
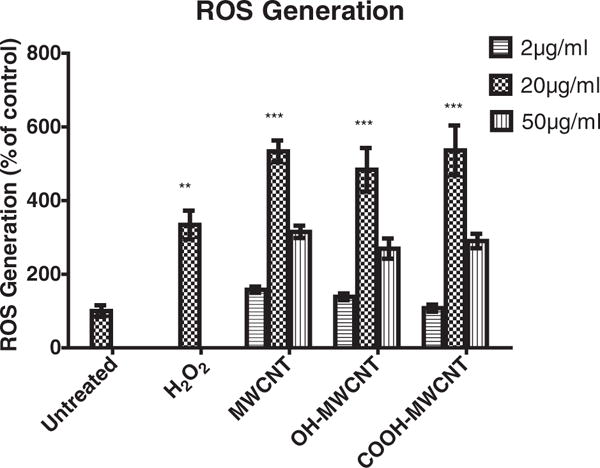
Reactive oxygen species (ROS) generation following 6 hour exposure to various concentrations of MWCNTs in A549 cells. ROS generation was studied using dichlorofluorescin diacetate (DCFH-DA) assay. Positive control: 200 μM H2O2. Data are represented as mean ± SD of triplicate analysis.
3.5. Apoptosis
Flow cytometer analysis of A549 cells treated for 48 h with three types of MWCNTs revealed apoptosis in response to a dose from 20 μg/ml to 200 μg/ml. Functionalized carbon nanotubes elicited a greater dose response and larger apoptotic cell percentage compared with unexposed cells and pristine MWCNTs that was statistically significant starting from 20 μg/ml. At 20 μg/ml, pristine MWCNT induced 8.5% of apoptosis while –OH and –COOH functionalized MWCNT caused 2.58 and 1.59 times that of the pristine MWCNTs, respectively. At 50 μg/ml, the percentage of apoptosis was increased in response to all three different MWCNTs. Similarly, induction of apoptosis by functionalised MWCNTs was more pronounced with –OH and –COOH MWCNTs causing 2.28 and 1.95 times the apoptosis induced by pristine MWCNTs. However, at 200 μg/ml, apoptosis induced by both functionalized MWCNTs decreased while the percentage of apoptosis induced by pristine MWCNTs continued to increase (Table 1). The percentage of necrosis has not been shown here as they were around 1%, which is much lower than the percentage of apoptosis. In addition, they are not significant different among different groups.
Table 1.
Apoptosis induced by MWCNTs in A549 cells after 48 h exposure.
| Non-treated | P-MWCNT | OH-MWCNT | COOH-MWCNT | |
|---|---|---|---|---|
| Mean % apoptotic cells ± SD | ||||
| 5.63% ± 0.66% | 20 μg/ml | 8.5% ± 2.09%a | 21.90% ± 3.80% | 13.50% ± 1.58% |
| 50 μg/ml | 18.8% ± 4.31% | 42.7% ± 4.84%b | 36.8% ± 3.79% | |
| 200 μg/ml | 25.0% ± 1.45% | 29.7% ± 3.40% | 33.9% ± 2.13% |
Denote significant difference (p < 0.05).
3.6. DNA Damage
In order to measure oxidative DNA damage after exposure to MWCNTs, 8-oxo-2′-deoxyguanosine (8-oxo-dG), a frequently used biomarker of oxidative DNA damage, was measured. No significant oxidative of DNA damage was observed following the exposure of all the concentrations of MWCNTs. By 72 h of exposure, all three MWCNTS induced 8-OHdG at 50 μg/ml, which become statistically significant for pristine MWCNTs (0.745 ± 0.017 ng/ml) compared to negative control (0.651 ± 0.014 ng/ml). OH-MWCNTs (0.998 ± 0.008 ng/ml) and COOH-MWCNTs (0.975 ± 0.034 ng/ml) were 1.40 and 1.31 fold more genotoxic than pristine MWCNTs, respectively (Fig. 7).
Fig. 7.
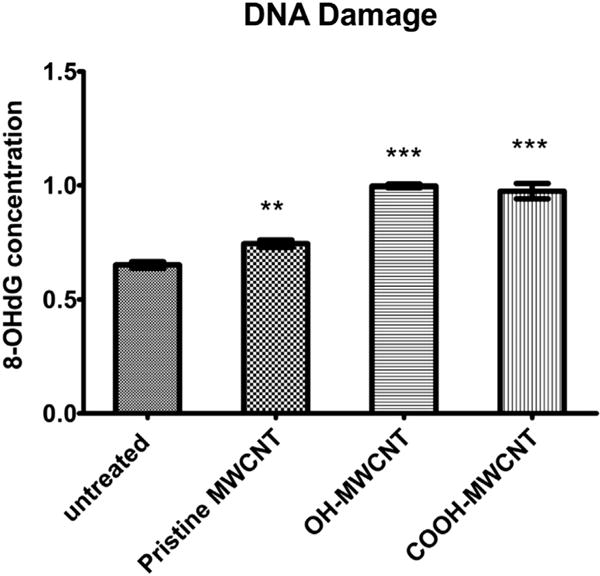
DNA damage induced by MWCNTs following 72 hour exposure at a concentration of 50 μg/ml. DNA damage was studied using DNA samples of untreated and MWCNTs treated A549 cells. 8-OHdG was quantified by colorimetric antibody ELISA assay. Data are represented as mean ± SD of triplicate. **p < 0.01, ***p < 0.001.
4. Discussion
MWCNTs are widely used for a variety of commercial products; however, the biological consequences of MWCNT exposure in the environment are still poorly understood. In this study, the cytotoxic effects of functionalized and pristine MWCNTs (Fig. 1) were investigated using an experimental in vitro model consisting of the human lung A549 epithelial cell line. Several measurements were applied in order to estimate the effects of potential human exposure. Early and late cellular responses to MWCNT were evaluated by measurements of cell cytotoxicity, apoptosis and oxidative stress induced genotoxicity. In addition, non-toxic dose was also investigated in order to evaluate the safety of using such materials therapeutically.
In a study conducted by Srivastava, MWCNTs were found to be non-toxic at concentrations from 0.8 to 10 μg/ml following 24 and 48 h exposure using the less sensitive MTT assay (Srivastava et al., 2011). This finding is consistent with our cell viability results (Fig. 2), suggesting that these concentrations could be applied for biosensor, drug vector or cancer imaging with limited toxicity. Within the concentration range of 50–1000 μg/ml, we found that cell viability was altered by MWCNTs in a dose dependent manner. In addition, the result of IC50 detection showed that pristine MWCNTs were relatively more cytotoxic than functionalized MWCNTs (Fig. 3). This result could be attributed to functionalized MWCNTs exhibiting higher degree of aggregation than pristine MWCNTs in the medium at high concentration (200 μg/ml). At the same concentration, –COOH and –OH functionalized MWCNTs showed a higher level of aggregation than pristine MWCNTs in the medium (F12/DMEM) (Fig. 4), possibly due to the stronger bonding between functional groups (e.g. hydrogen bonding and van der Waals interactions) on MWCNTs.
Agglomeration of CNT (Tagmatarchis and Prato, 2004) could cause problems in the investigation of CNT toxicity. Methods to disperse CNT have been reviewed by Smart et al. (2006). The most commonly used method needs utilization of organic solvents. However organic solvents are not suitable for biological studies as they are toxic. Another method commonly employed is utilization of surfactant such as BSA which is biomolecule present in body fluids. A possible explanation of these results was provided by Casey et al. (2007), in which, a better dispersion was shown to occur in the presence of fetal calf serum and medium. This improved dispersion could be explained by physical absorption of CNT by fetal calf serum-medium proteins including albumin. In order to investigate the link between dispersibility and cytotoxicity, we compared the cell viability of cell exposed to MWCNTs with and without BSA. A study conducted by Li et al. suggested that lower dispersion was more likely to result in settling of the MWCNT at the bottom of the wells (Li et al., 2013), where they make more cellular contact. However, in our experiments, results of functionalized MWCNTs showed that the differences between slopes were significant, demonstrating that carbon nanotubes with better dispersibility actually had increased interactions with cells and thereby decreased cell viability (Fig. 5). These results were also supported by Johnston who suggested that only those carbon nanotubes which are not aggregated and free in the medium are able to reach the cytoplasm and subsequently the nucleus (Johnston et al., 2010).
Apoptosis is one of the major factors, which determine the medical application of nanomaterials. Therefore, it is important to learn which modification of CNTs with functional group would activate apoptosis. The data presented in this study revealed that MWCNTs induced less apoptosis compared with functionalized MWCNTs (Table 1), indicating that the presence of –OH and –COOH on MWCNTs promotes cell apoptosis. However, the extent of apoptosis was reduced when the functionalized MWCNTs’ concentration increased to 200 μg/ml. This reduction may be explained by the aggregation at 200 μg/ml, which altered the mechanism of cell death from apoptosis to membrane disruption (Fig. 3). At lower concentrations, where carbon nanotubes were better dispersed, cell death was mainly due to apoptosis. When concentrations were increased to 200 μg/ml cells underwent necrotic cell death due to aggregates in the medium.
Oxidative stress plays an essential role in toxicity induced by carbon nanotubes (Pichardo et al., 2012). During times of environmental stress, the highly increased production of oxidants can result in significant damage to cells by activating cell death. DNA damage was found in Met-5A and A549 cell lines using the comet assay (Cavallo et al., 2012), (Lindberg et al., 2013) when exposed to MWCNTs. Our study showed that functionalized MWCNTs induced significantly higher amount of 8-oxo-dG indicating that functionalized forms of MWCNTs could lead to genotoxicity by damaging DNA (Fig. 7). However, understanding of the mechanisms underlying this toxicity still remains incomplete. One view is that CNTs were taken up as nanoneedles which could puncture cell membrane directly and then move to the nucleus (Mu et al., 2009). A study carried out by Palomaki further showed that only long, needle-like CNT induced inflammatory activation (Palomäki et al., 2011). Another view is that the induction of oxidative stress is a principle mechanism underlying particle exposure-associated genotoxicity (Van Berlo et al., 2012). DNA damage can occur due to the direct action of hydroxyl radicals (Eastman and Barry, 1992). For MWCNT to produce hydroxyl radicals that could oxidize DNA, they would need to enter the nucleus as hydroxyl radical reacts with targets immediately in the vicinity where it is produced. While in our study, DCF fluorescence was not significantly different among pristine and functionalised MWCNTs (Fig. 6), it must be noted that 8-OHdG is actually a far better indicator of oxidant damage to DNA than the measurement of a dye that has little specificity. In our experiment, functionalized MWNCTs induced more cell damage while DCF fluorescence caused by them was not significantly higher than that caused by the pristine form. It is possible that functionalized MWCNTs have higher propensity to cross cell membranes into the nucleus (Mali et al., 2011), and thereby they increase more genotoxicity as we observed.
5. Conclusions
In conclusion, in this study of pristine and functionalized (–OH, –COOH) multi-walled carbon nanotubes (MWCNTs) caused cell death with a concentration at or above 50 μg/ml with the mechanism of cell death altered from apoptosis to necrosis as concentration increased. After 48 h exposure, both apoptosis and DNA damage could be further observed. Pristine MWCNTs are more cytotoxic while functionalized MWCNTs exerted more genotoxicity compared with the pristine form. Toxic effects of MWCNTs were also dependent on the dispersing agents. Cytotoxicity was increased by the presence of BSA which changed dispersibility of MWCNTs in cell culture medium.
Further work is needed to investigate cellular internalization of carbon nanotubes in order to gain better insight into the mechanism of toxicity. In addition, a normal lung fibroblast cell line as well as other cell lines (e.g. skin cell lines) may be employed for further study since different cell lines may show various sensitivities to nanoparticles (Hasan et al., 2012) and cancer cells might be more resistant. Progress has been made, but it clinical applications of MWCNTs will still be limited until a better understanding their health impact on humans is achieved.
Acknowledgments
We thank Dr. Huijun Zhu for providing the A549 cell line as a gift, and Adeel Irfan and Dr. Natalie Kenny and Dr. Alex Charlton for their great help and advice in practice. We also thank Jake Chandler for his technical advice. This work was performed at Cranfield University. Partial support came from ES023864 from the National Institutes of Health.
Footnotes
Conflict of interest
The authors declare that there are no conflicts of interest.
Transparency document
The http://dx.doi.org/10.1016/j.tiv.2017.04.030 associated with this article can be found, in online version.
References
- Ali-Boucetta H, Nunes A, Sainz R, Herrero MA, Tian B, Prato M, Bianco A, Kostarelos K. Asbestos-like pathogenicity of long carbon nanotubes alleviated by chemical functionalization. Angew Chem Int Ed (n/a-n/a) 2013 doi: 10.1002/anie.201207664. [DOI] [PubMed] [Google Scholar]
- Casey A, Davoren M, Herzog E, Lyng FM, Byrne HJ, Chambers G. Probing the interaction of single walled carbon nanotubes within cell culture medium as a precursor to toxicity testing. Carbon. 2007;45:34–40. [Google Scholar]
- Castranova V, Schulte PA, Zumwalde RD. Occupational nanosafety considerations for carbon nanotubes and carbon nanofibers. Acc Chem Res. 2013;46:642–649. doi: 10.1021/ar300004a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallo D, Fanizza C, Ursini CL, Casciardi S, Paba E, Ciervo A, Fresegna AM, Maiello R, Marcelloni AM, Buresti G, Tombolini F, Bellucci S, Iavicoli S. Multi-walled carbon nanotubes induce cytotoxicity and genotoxicity in human lung epithelial cells. J Appl Toxicol. 2012;32:454–464. doi: 10.1002/jat.2711. [DOI] [PubMed] [Google Scholar]
- Eastman A, Barry MA. The origins of DNA breaks: a consequence of DNA damage, DNA repair, or apoptosis? Cancer Investig. 1992;10:229–240. doi: 10.3109/07357909209032765. [DOI] [PubMed] [Google Scholar]
- Elgrabli D, Abella-Gallart S, Aguerre-Chariol O, Robidel F, Rogerieux F, Boczkowski J, Lacroix G. Effect of BSA on carbon nanotube dispersion for in vivo and in vitro studies. Nanotoxicology. 2007;1:266–278. [Google Scholar]
- Hasan W, Chu K, Gullapalli A, Dunn SS, Enlow EM, Luft JC, Tian S, Napier ME, Pohlhaus PD, Rolland JP, Desimone JM. Delivery of multiple siRNAs using lipid-coated PLGA nanoparticles for treatment of prostate cancer. Nano Lett. 2012;12:287–292. doi: 10.1021/nl2035354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Young SH, Schwegler-Berry D, Chisholm WP, Fernback JE, Ma Q. Multiwalled carbon nanotubes induce a fibrogenic response by stimulating reactive oxygen species production, activating NF-κB signaling, and promoting fibroblast-to-myofibroblast transformation. Chem Res Toxicol. 2011;24:2237–2248. doi: 10.1021/tx200351d. [DOI] [PubMed] [Google Scholar]
- Johnston HJ, Hutchison GR, Christensen FM, Peters S, Hankin S, Aschberger K, Stone V. A critical review of the biological mechanisms underlying the in vivo and in vitro toxicity of carbon nanotubes: the contribution of physico-chemical characteristics. Nanotoxicology. 2010;4:207–246. doi: 10.3109/17435390903569639. [DOI] [PubMed] [Google Scholar]
- Kim JS, Song KS, Lee JH, Yu IJ. Evaluation of biocompatible dispersants for carbon nanotube toxicity tests. Arch Toxicol. 2011;85:1499–1508. doi: 10.1007/s00204-011-0723-0. [DOI] [PubMed] [Google Scholar]
- Li R, Wang X, Ji Z, Sun B, Zhang H, Chang CH, Lin S, Meng H, Liao YP, Wang M, Li Z, Hwang AA, Song TB, Xu R, Yang Y, Zink JI, Nel AE, Xia T. Surface charge and cellular processing of covalently functionalized multiwall carbon nanotubes determine pulmonary toxicity. ACS Nano. 2013;7:2352–2368. doi: 10.1021/nn305567s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg HK, Falck GCM, Singh R, Suhonen S, Järventaus H, Vanhala E, Catalán J, Farmer PB, Savolainen KM, Norppa H. Genotoxicity of short single-wall and multi-wall carbon nanotubes in human bronchial epithelial and mesothelial cells in vitro. Toxilogy. 2013;313:24–37. doi: 10.1016/j.tox.2012.12.008. [DOI] [PubMed] [Google Scholar]
- Magrez A, Kasas S, Salicio V, Pasquier N, Seo JW, Celio M, Catsicas S, Schwaller B, Forró L. Cellular toxicity of carbon-based nanomaterials. Nano Lett. 2006;6:1121–1125. doi: 10.1021/nl060162e. [DOI] [PubMed] [Google Scholar]
- Mali N, Jadhav S, Karpe M, Kadam V. Carbon nanotubes as carriers for delivery of bioactive and therapeutic agents: an overview. Int J Pharm Pharm Sci. 2011;3:45–52. [Google Scholar]
- Martín O, Gutierrez HR, Maroto-Valiente A, Terrones M, Blanco T, Baselga J. An efficient method for the carboxylation of few-wall carbon nanotubes with little damage to their sidewalls. Mater Chem Phys. 2013;140:499–507. [Google Scholar]
- Mauter MS, Elimelech M. Environmental applications of carbon-based nanomaterials. Environ Sci Technol. 2008;42:5843–5859. doi: 10.1021/es8006904. [DOI] [PubMed] [Google Scholar]
- Mu Q, Broughton DL, Yan B. Endosomal leakage and nuclear translocation of multiwalled carbon nanotubes: developing a model for cell uptake. Nano Lett. 2009;9:4370–4375. doi: 10.1021/nl902647x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai H, Okazaki Y, Chew SH, Misawa N, Yamashita Y, Akatsuka S, Ishihara T, Yamashita K, Yoshikawa Y, Yasui H, Jiang L, Ohara H, Takahashi T, Ichihara G, Kostarelos K, Miyata Y, Shinohara H, Toyokuni S. Diameter and rigidity of multiwalled carbon nanotubes are critical factors in mesothelial injury and carcinogenesis. Proc Natl Acad Sci. 2011;108:E1330–E1338. doi: 10.1073/pnas.1110013108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogasawara Y, Umezu N, Ishii K. DNA damage in human pleural mesothelial cells induced by exposure to carbon nanotubes. Nihon eiseigaku zasshi. 2012;67:76–83. doi: 10.1265/jjh.67.76. Japanese journal of hygiene. [DOI] [PubMed] [Google Scholar]
- Palomäki J, Välimäki E, Sund J, Vippola M, Clausen PA, Jensen KA, Savolainen K, Matikainen S, Alenius H. Long, needle-like carbon nanotubes and asbestos activate the NLRP3 inflammasome through a similar mechanism. ACS Nano. 2011;5:6861–6870. doi: 10.1021/nn200595c. [DOI] [PubMed] [Google Scholar]
- Patlolla AK, Hussain SM, Schlager JJ, Patlolla S, Tchounwou PB. Comparative study of the clastogenicity of functionalized and nonfunctionalized multiwalled carbon nanotubes in bone marrow cells of Swiss-Webster mice. Environ Toxicol. 2010;25:608–621. doi: 10.1002/tox.20621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichardo S, Gutiérrez-Praena D, Puerto M, Sánchez E, Grilo A, Cameán AM, Jos Á. Oxidative stress responses to carboxylic acid functionalized single wall carbon nanotubes on the human intestinal cell line Caco-2. Toxicol in Vitro. 2012;26:672–677. doi: 10.1016/j.tiv.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Smart SK, Cassady AI, Lu GQ, Martin DJ. The biocompatibility of carbon nanotubes. Carbon. 2006;44:1034–1047. [Google Scholar]
- Srivastava RK, Pant AB, Kashyap MP, Kumar V, Lohani M, Jonas L, Rahman Q. Multi-walled carbon nanotubes induce oxidative stress and apoptosis in human lung cancer cell line-A549. Nanotoxicology. 2011;5:195–207. doi: 10.3109/17435390.2010.503944. [DOI] [PubMed] [Google Scholar]
- Tagmatarchis N, Prato M. Functionalization of carbon nanotubes via 1,3-dipolar cycloadditions. J Mater Chem. 2004;14:437–439. [Google Scholar]
- Ursini CL, Cavallo D, Fresegna AM, Ciervo A, Maiello R, Buresti G, Casciardi S, Tombolini F, Bellucci S, Iavicoli S. Comparative cyto-genotoxicity assessment of functionalized and pristine multiwalled carbon nanotubes on human lung epithelial cells. Toxicol in Vitro. 2012;26:831–840. doi: 10.1016/j.tiv.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Van Berlo D, Clift M, Albrecht C, Schins R. Carbon nanotubes: an insight into the mechanisms of their potential genotoxicity. Swiss Med Wkly. 2012:142. doi: 10.4414/smw.2012.13698. [DOI] [PubMed] [Google Scholar]
- Vashist SK, Zheng D, Pastorin G, Al-Rubeaan K, Luong JHT, Sheu FS. Delivery of drugs and biomolecules using carbon nanotubes. Carbon. 2011;49:4077–4097. [Google Scholar]
- Wick P, Manser P, Limbach LK, Dettlaff-Weglikowska U, Krumeich F, Roth S, Stark WJ, Bruinink A. The degree and kind of agglomeration affect carbon nanotube cytotoxicity. Toxicol Lett. 2007;168:121–131. doi: 10.1016/j.toxlet.2006.08.019. [DOI] [PubMed] [Google Scholar]


