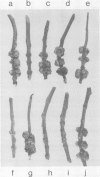
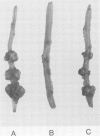
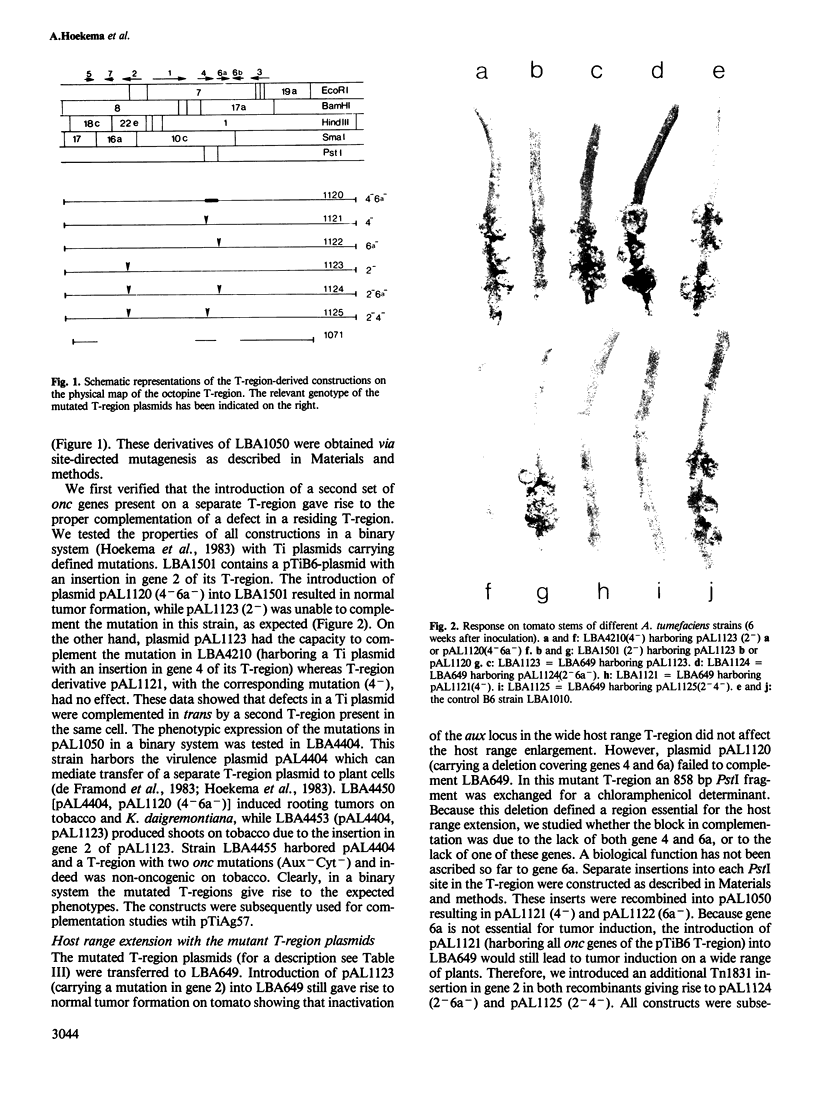
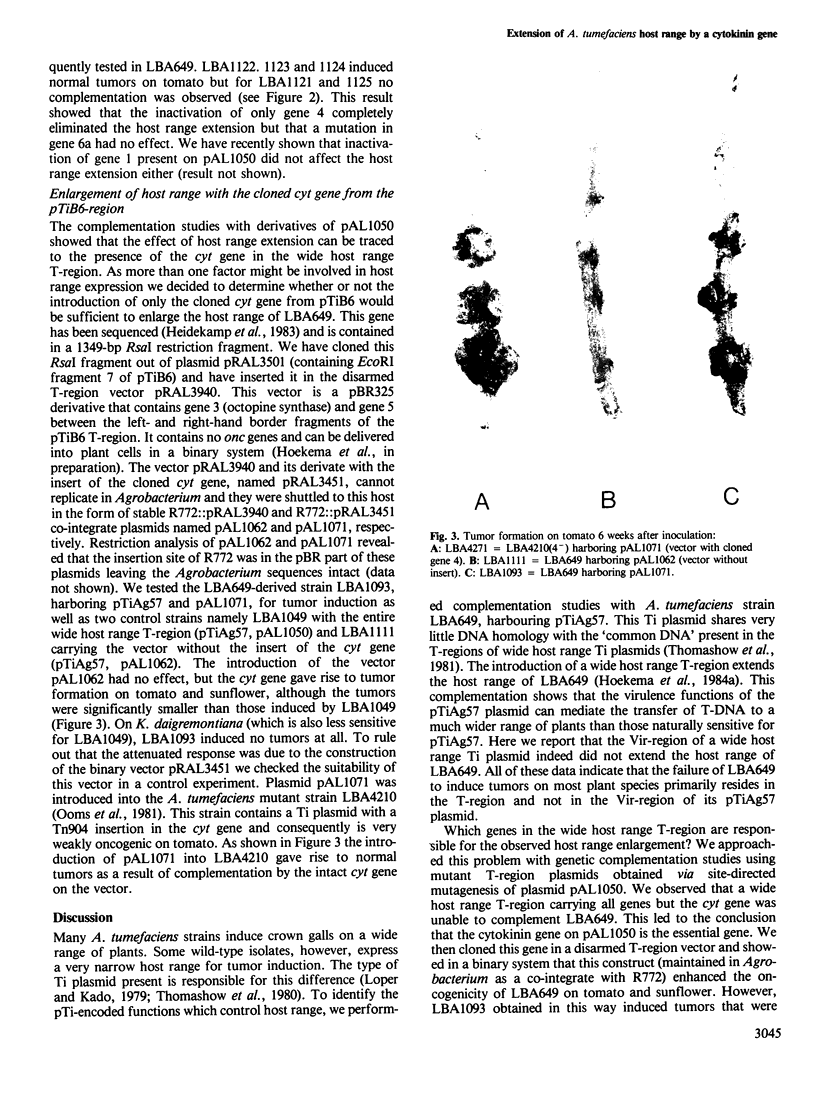
Abstract
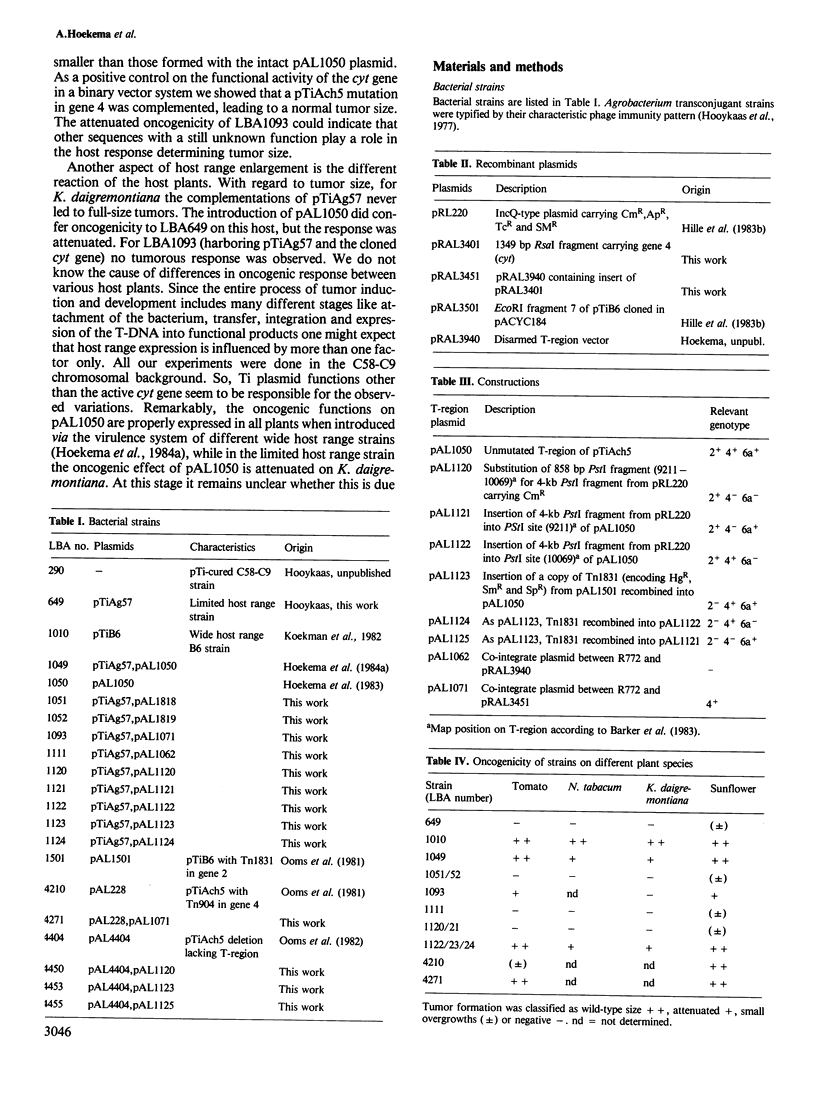
The host range of Agrobacterium tumefaciens strain LBA649 (pTiAg57) is limited to grapevine and a few other plant species. Its host range was extended through the introduction of the T-region from the wide host range octopine plasmid pTiAch5. In contrast, R prime plasmids harboring the entire wide host range virulence region were unable to achieve this effect. Via site-directed mutagenesis a search was performed to identify the T-DNA genes which were responsible for the observed host range extension. Inactivation of one of the onc-genes (the cyt gene) was found to abolish the capacity of the T-region to extend the host range of LBA649. Therefore, we cloned the cyt gene into a disarmed T-region plant vector and used it in complementation studies with pTiAg57 via the binary vector strategy. We show that the mere presence of the cyt gene from a wide host range Ti plasmid is sufficient to extend the host range of LBA649 to certain plants. We conclude that the limited host range of LBA649 is not caused by a lack of recognition of plants but is mainly due to the absence or inactivity of a cyt gene in the T-region of pTiAg57.
Keywords: Agrobacterium tumefaciens, host range, crown gall, cytokinin-gene, binary vector system
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyoshi D. E., Morris R. O., Hinz R., Mischke B. S., Kosuge T., Garfinkel D. J., Gordon M. P., Nester E. W. Cytokinin/auxin balance in crown gall tumors is regulated by specific loci in the T-DNA. Proc Natl Acad Sci U S A. 1983 Jan;80(2):407–411. doi: 10.1073/pnas.80.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry G. F., Rogers S. G., Fraley R. T., Brand L. Identification of a cloned cytokinin biosynthetic gene. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4776–4780. doi: 10.1073/pnas.81.15.4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilton M. D., Drummond M. H., Merio D. J., Sciaky D., Montoya A. L., Gordon M. P., Nester E. W. Stable incorporation of plasmid DNA into higher plant cells: the molecular basis of crown gall tumorigenesis. Cell. 1977 Jun;11(2):263–271. doi: 10.1016/0092-8674(77)90043-5. [DOI] [PubMed] [Google Scholar]
- Heidekamp F., Dirkse W. G., Hille J., van Ormondt H. Nucleotide sequence of the Agrobacterium tumefaciens octopine Ti plasmid-encoded tmr gene. Nucleic Acids Res. 1983 Sep 24;11(18):6211–6223. doi: 10.1093/nar/11.18.6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille J., Klasen I., Schilperoort R. Construction and application of R prime plasmids, carrying different segments of an octopine Ti plasmid from Agrobacterium tumefaciens, for complementation of vir genes. Plasmid. 1982 Mar;7(2):107–118. doi: 10.1016/0147-619x(82)90071-3. [DOI] [PubMed] [Google Scholar]
- Hille J., van Kan J., Klasen I., Schilperoort R. Site-directed mutagenesis in Escherichia coli of a stable R772::Ti cointegrate plasmid from Agrobacterium tumefaciens. J Bacteriol. 1983 May;154(2):693–701. doi: 10.1128/jb.154.2.693-701.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekema A., Hooykaas P. J., Schilperoort R. A. Transfer of the octopine T-DNA segment to plant cells mediated by different types of Agrobacterium tumor- or root-inducing plasmids: generality of virulence systems. J Bacteriol. 1984 Apr;158(1):383–385. doi: 10.1128/jb.158.1.383-385.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekema A., Roelvink P. W., Hooykaas P. J., Schilperoort R. A. Delivery of T-DNA from the Agrobacterium tumefaciens chromosome into plant cells. EMBO J. 1984 Nov;3(11):2485–2490. doi: 10.1002/j.1460-2075.1984.tb02160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koekman B. P., Hooykaas P. J., Schilperoort R. A. A functional map of the replicator region of the octopine Ti plasmid. Plasmid. 1982 Mar;7(2):119–132. doi: 10.1016/0147-619x(82)90072-5. [DOI] [PubMed] [Google Scholar]
- Leemans J., Deblaere R., Willmitzer L., De Greve H., Hernalsteens J. P., Van Montagu M., Schell J. Genetic Identification of functions of TL-DNA transcripts in octopine crown galls. EMBO J. 1982;1(1):147–152. doi: 10.1002/j.1460-2075.1982.tb01138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loper J. E., Kado C. I. Host range conferred by the virulence-specifying plasmid of Agrobacterium tumefaciens. J Bacteriol. 1979 Aug;139(2):591–596. doi: 10.1128/jb.139.2.591-596.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooms G., Hooykaas P. J., Moolenaar G., Schilperoort R. A. Grown gall plant tumors of abnormal morphology, induced by Agrobacterium tumefaciens carrying mutated octopine Ti plasmids; analysis of T-DNA functions. Gene. 1981 Jun-Jul;14(1-2):33–50. doi: 10.1016/0378-1119(81)90146-3. [DOI] [PubMed] [Google Scholar]
- Ooms G., Hooykaas P. J., Van Veen R. J., Van Beelen P., Regensburg-Tuïnk T. J., Schilperoort R. A. Octopine Ti-plasmid deletion mutants of agrobacterium tumefaciens with emphasis on the right side of the T-region. Plasmid. 1982 Jan;7(1):15–29. doi: 10.1016/0147-619x(82)90023-3. [DOI] [PubMed] [Google Scholar]
- Panagopoulos C. G., Psallidas P. G. Characteristics of Greek Isolates of Agrobacterium tumefaciens (E. F. Smith & Townsend) Conn. J Appl Bacteriol. 1973 Jun;36(2):233–240. doi: 10.1111/j.1365-2672.1973.tb04096.x. [DOI] [PubMed] [Google Scholar]
- Prakash R. K., Schilperoort R. A., Nuti M. P. Large plasmids of fast-growing rhizobia: homology studies and location of structural nitrogen fixation (nif) genes. J Bacteriol. 1981 Mar;145(3):1129–1136. doi: 10.1128/jb.145.3.1129-1136.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder G., Waffenschmidt S., Weiler E. W., Schröder J. The T-region of Ti plasmids codes for an enzyme synthesizing indole-3-acetic acid. Eur J Biochem. 1984 Jan 16;138(2):387–391. doi: 10.1111/j.1432-1033.1984.tb07927.x. [DOI] [PubMed] [Google Scholar]
- Thomashow M. F., Knauf V. C., Nester E. W. Relationship between the limited and wide host range octopine-type Ti plasmids of Agrobacterium tumefaciens. J Bacteriol. 1981 May;146(2):484–493. doi: 10.1128/jb.146.2.484-493.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmitzer L., Simons G., Schell J. The TL-DNA in octopine crown-gall tumours codes for seven well-defined polyadenylated transcripts. EMBO J. 1982;1(1):139–146. doi: 10.1002/j.1460-2075.1982.tb01137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]