Abstract
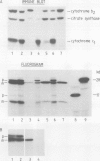
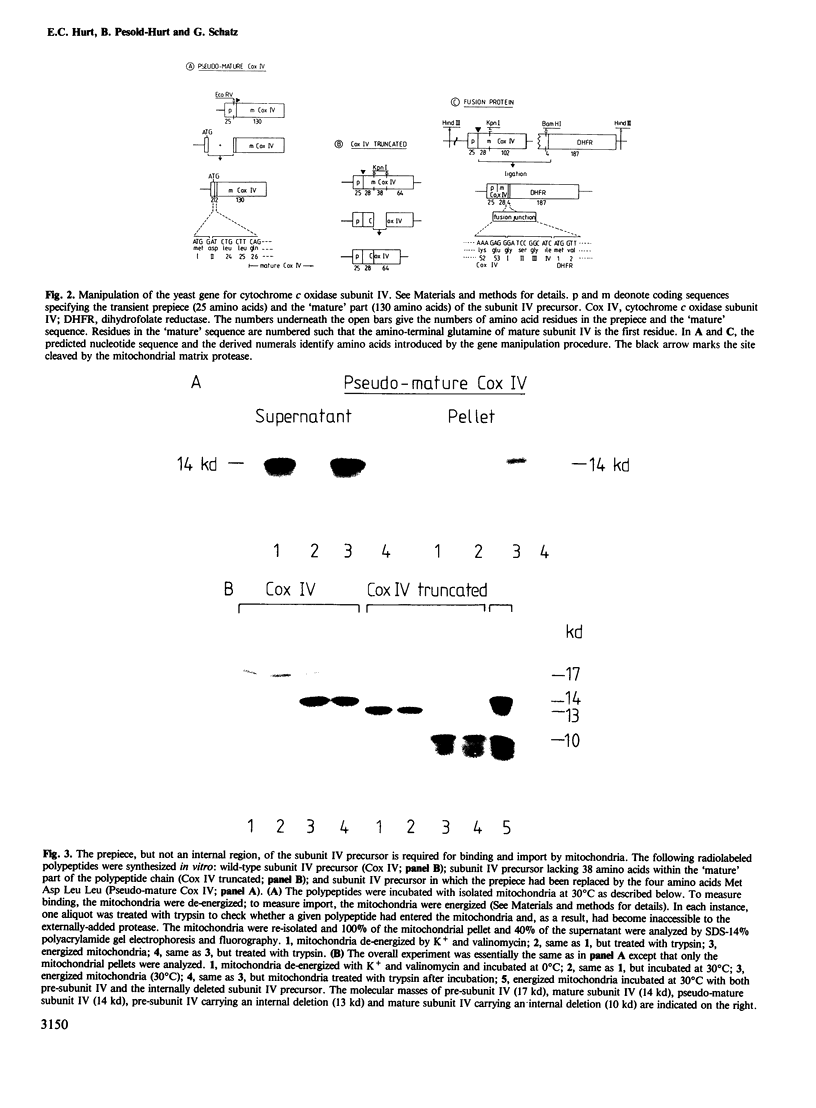
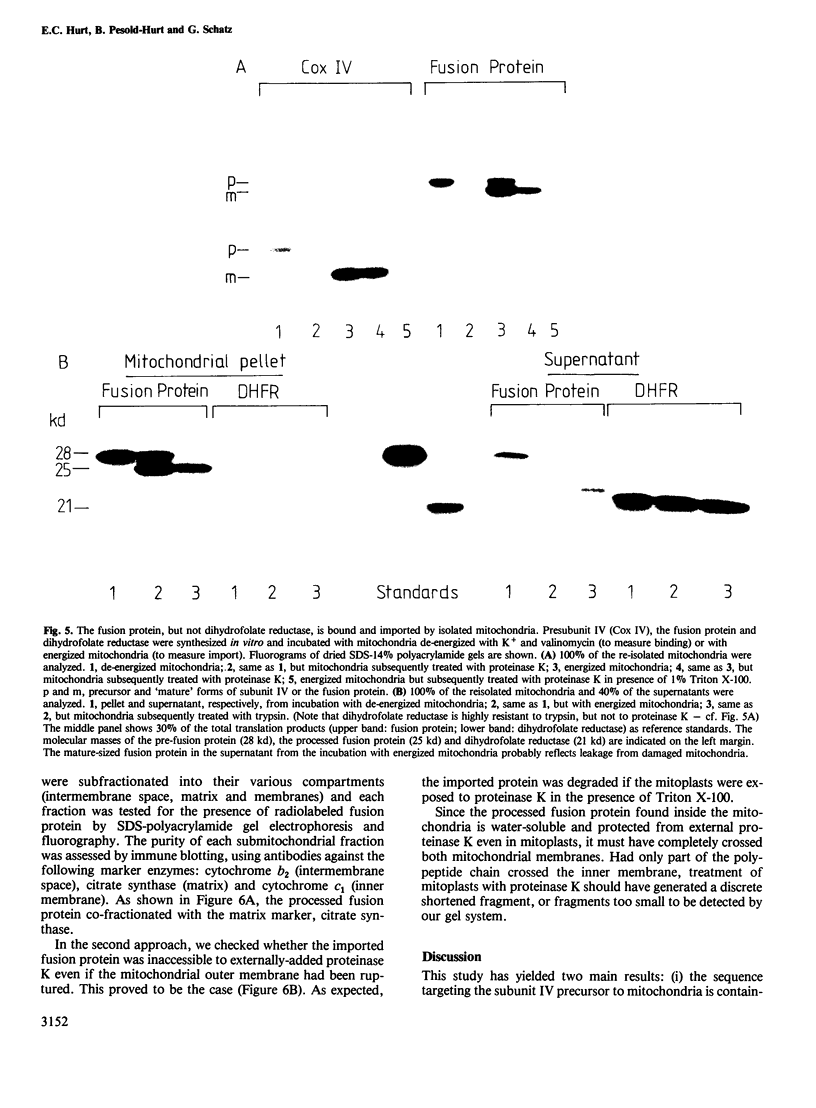
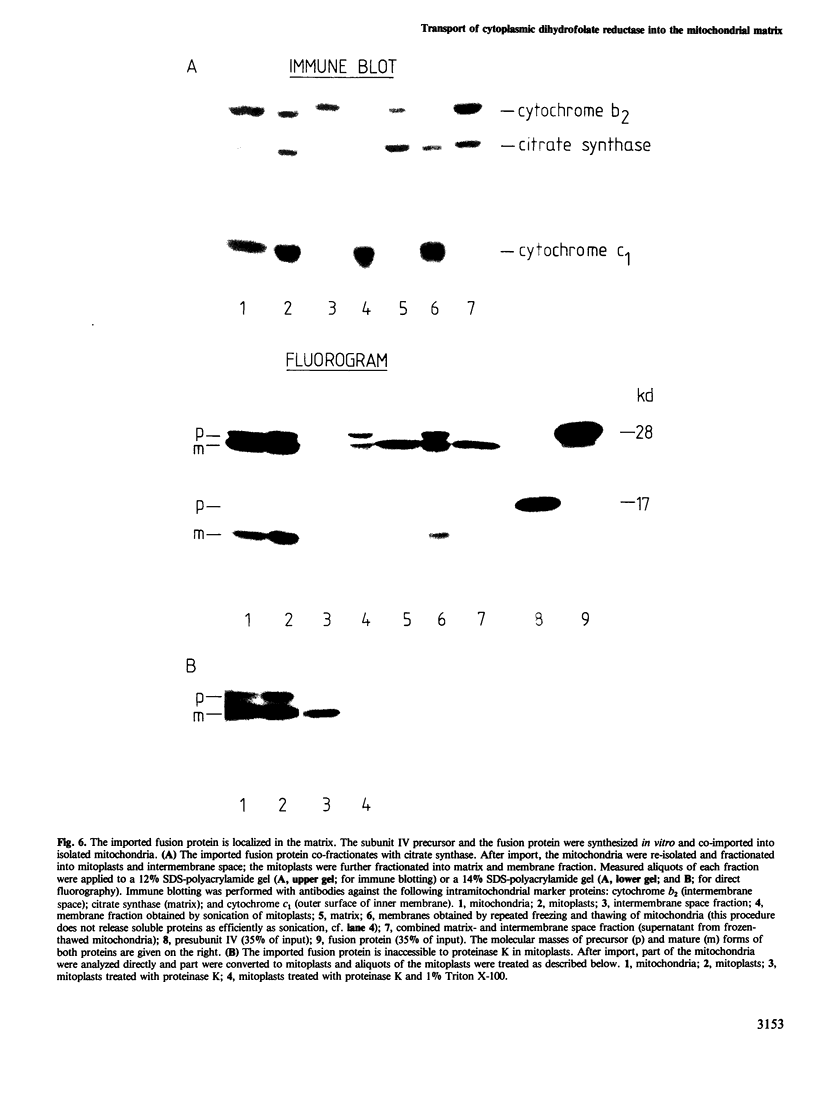
Subunit IV of yeast cytochrome c oxidase is encoded by a nuclear gene, synthesized in the cytosol as a precursor with a transient amino-terminal extension of 25 amino acids, and imported into the mitochondria. By gene fusion, we have attached the amino-terminal 53 amino acids of the subunit IV precursor to the amino terminus of the mouse cytosolic enzyme dihydrofolate reductase. When the resulting fusion protein was synthesized in a transcription-translation system and then incubated with energized yeast mitochondria, it was imported into the mitochondrial matrix space and processed to a shorter form by the chelator-sensitive matrix protease. No evidence was obtained that the fusion protein became stuck across one of the two mitochondrial membranes. Thus, a non-mitochondrial protein can be transported into the mitochondrial matrix if it is fitted with a mitochondrial targeting sequence.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhni P. C., Daum G., Schatz G. Import of proteins into mitochondria. Partial purification of a matrix-located protease involved in cleavage of mitochondrial precursor polypeptides. J Biol Chem. 1983 Apr 25;258(8):4937–4943. [PubMed] [Google Scholar]
- Douglas M. G., Geller B. L., Emr S. D. Intracellular targeting and import of an F1-ATPase beta-subunit-beta-galactosidase hybrid protein into yeast mitochondria. Proc Natl Acad Sci U S A. 1984 Jul;81(13):3983–3987. doi: 10.1073/pnas.81.13.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dretzen G., Bellard M., Sassone-Corsi P., Chambon P. A reliable method for the recovery of DNA fragments from agarose and acrylamide gels. Anal Biochem. 1981 Apr;112(2):295–298. doi: 10.1016/0003-2697(81)90296-7. [DOI] [PubMed] [Google Scholar]
- Gasser S. M., Daum G., Schatz G. Import of proteins into mitochondria. Energy-dependent uptake of precursors by isolated mitochondria. J Biol Chem. 1982 Nov 10;257(21):13034–13041. [PubMed] [Google Scholar]
- Haid A., Suissa M. Immunochemical identification of membrane proteins after sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Methods Enzymol. 1983;96:192–205. doi: 10.1016/s0076-6879(83)96017-2. [DOI] [PubMed] [Google Scholar]
- Hay R., Böhni P., Gasser S. How mitochondria import proteins. Biochim Biophys Acta. 1984 Jan 27;779(1):65–87. doi: 10.1016/0304-4157(84)90004-2. [DOI] [PubMed] [Google Scholar]
- Kedes L. H., Chang A. C., Houseman D., Cohen S. N. Isolation of histone genes from unfractionated sea urchin DNA by subculture cloning in E. coli. Nature. 1975 Jun 12;255(5509):533–538. doi: 10.1038/255533a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lewin A. S., Gregor I., Mason T. L., Nelson N., Schatz G. Cytoplasmically made subunits of yeast mitochondrial F1-ATPase and cytochrome c oxidase are synthesized as individual precursors, not as polyproteins. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3998–4002. doi: 10.1073/pnas.77.7.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- McAda P. C., Douglas M. G. A neutral metallo endoprotease involved in the processing of an F1-ATPase subunit precursor in mitochondria. J Biol Chem. 1982 Mar 25;257(6):3177–3182. [PubMed] [Google Scholar]
- Mihara K., Blobel G. The four cytoplasmically made subunits of yeast mitochondrial cytochrome c oxidase are synthesized individually and not as a polyprotein. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4160–4164. doi: 10.1073/pnas.77.7.4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura S., Mori M., Amaya Y., Tatibana M. A mitochondrial protease that cleaves the precursor of ornithine carbamoyltransferase. Purification and properties. Eur J Biochem. 1982 Mar 1;122(3):641–647. [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Power S. D., Lochrie M. A., Sevarino K. A., Patterson T. E., Poyton R. O. The nuclear-coded subunits of yeast cytochrome c oxidase. I. Fractionation of the holoenzyme into chemically pure polypeptides and the identification of two new subunits using solvent extraction and reversed phase high performance liquid chromatography. J Biol Chem. 1984 May 25;259(10):6564–6570. [PubMed] [Google Scholar]
- Riezman H., Hase T., van Loon A. P., Grivell L. A., Suda K., Schatz G. Import of proteins into mitochondria: a 70 kilodalton outer membrane protein with a large carboxy-terminal deletion is still transported to the outer membrane. EMBO J. 1983;2(12):2161–2168. doi: 10.1002/j.1460-2075.1983.tb01717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz G. How mitochondria import proteins from the cytoplasm. FEBS Lett. 1979 Jul 15;103(2):203–211. doi: 10.1016/0014-5793(79)81328-9. [DOI] [PubMed] [Google Scholar]
- Stone D., Phillips A. W. The amino acid sequence of dihydrofolate reductase from L1210 cells. FEBS Lett. 1977 Feb 15;74(1):85–88. doi: 10.1016/0014-5793(77)80758-8. [DOI] [PubMed] [Google Scholar]
- Stueber D., Bujard H. Transcription from efficient promoters can interfere with plasmid replication and diminish expression of plasmid specified genes. EMBO J. 1982;1(11):1399–1404. doi: 10.1002/j.1460-2075.1982.tb01329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stueber D., Ibrahimi I., Cutler D., Dobberstein B., Bujard H. A novel in vitro transcription-translation system: accurate and efficient synthesis of single proteins from cloned DNA sequences. EMBO J. 1984 Dec 20;3(13):3143–3148. doi: 10.1002/j.1460-2075.1984.tb02271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viebrock A., Perz A., Sebald W. The imported preprotein of the proteolipid subunit of the mitochondrial ATP synthase from Neurospora crassa. Molecular cloning and sequencing of the mRNA. EMBO J. 1982;1(5):565–571. doi: 10.1002/j.1460-2075.1982.tb01209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F. K., Koch J., Stokstad E. L. Folate coenzyme pattern, folate linked enzymes and methionine biosynthesis in rat liver mitochondria. Biochem Z. 1967 Jan 27;346(5):458–466. [PubMed] [Google Scholar]
- Wiedmann M., Huth A., Rapoport T. A. Xenopus oocytes can secrete bacterial beta-lactamase. Nature. 1984 Jun 14;309(5969):637–639. doi: 10.1038/309637a0. [DOI] [PubMed] [Google Scholar]