Abstract
Purpose
Cancer and cardiovascular disease share risk factors, and there is some evidence that statins reduce cancer mortality. We sought to determine the accuracy of the 2013 American College of Cardiology/American Heart Association statin eligibility criteria to identify individuals at a higher risk of developing cancer or of dying as a result of cancer or other noncardiovascular causes.
Methods
We included 2,196 participants (50.5 ± 8.1 years of age; 55% female) who were statin naïve and free of cancer at baseline from the offspring and third-generation cohorts of the community-based longitudinal Framingham Heart Study. Statin eligibility was determined per American College of Cardiology/American Heart Association guidelines, and subclinical coronary atherosclerosis was assessed by computed tomography. The primary outcome was incident cancer at a median of 10.0 years (interquartile range, 9.1-10.6 years) of follow-up, and secondary outcomes were cancer mortality and noncardiovascular mortality.
Results
The incident cancer rate was 11.2% (247 of 2,196), with 58 noncardiovascular deaths, including 39 cancer deaths (1.8%). Overall, 37% (812 of 2,196) were statin eligible. Incident cancer occurred in 125 (15%) of the 812 statin-eligible participants versus 122 (8.8%) of the 1,384 of noneligible participants (subdistribution hazard ratio [SDHR], 1.8 [1.4 to 2.3]; P < .001). Cancer mortality occurred in 34 (4.2%) of the 812 statin-eligible participants versus five (0.4%) of the 1,384 noneligible participants (SDHR, 12.1 [4.7 to 31]; P < .001). Noncardiovascular mortality occurred in 49 (6.0%) of the 812 statin-eligible participants versus nine (0.7%) of the 1,384 noneligible participants (SDHR, 10.1 [5.0 to 21]; P < .001). In stratified analyses, these findings were independent of any individual causative risk factor such as body mass index, age, or smoking status.
Conclusion
In this community-based primary prevention cohort, guideline-based statin eligibility accurately identified patients at a higher risk of developing cancer and cancer-related mortality. Shared risk profiles and potential benefits of statins between cancer and cardiovascular outcomes may provide a unique opportunity to improve population health.
INTRODUCTION
Epidemiologic studies have identified common risk factors for both cancer-related morbidity and mortality and cardiovascular disease (CVD),1,2 but the extent to which cancer risk is influenced by extrinsic factors including environmental and lifestyle risk factors is not clear.3,4 Further insights are integral to strategizing cancer prevention and public health.
We have shown previously that the 2013 American College of Cardiology/American Heart Association (ACC/AHA) guidelines on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults are accurate and efficient in identifying patients at an increased risk of incident CVD and subclinical atherosclerosis.5 These guidelines for statin eligibility are based primarily on a composite cardiovascular risk profile, as opposed to cholesterol level thresholds. Because of the growing use of statins for the primary and secondary prevention of CVD, several studies have assessed the effect of statins administered for cardiovascular prevention on incident cancer events, morbidity, and mortality. Observational data suggest that statin use initiated before a diagnosis of cancer reduces cancer-related mortality and improves survival by 12% to 46%6-9 and is associated with cancer diagnosis at an earlier stage of disease (eg, lower breast cancer stage as shown in the Women’s Health Initiative).10 A large case-control study showed a 47% relative risk reduction in developing cancer (colorectal) in statin users compared with nonusers.11 One of the postulated mechanisms of the beneficial effects of statins beyond cardiovascular protection includes a limitation of the cellular proliferation required for cancer growth and metastasis via reduced cholesterol availability.12,13 Given the potential benefits of statins in cancer, it is important to know whether statin eligibility, as defined by the 2013 ACC/AHA guidelines, may also be effective in identifying those at the highest risk of developing, or dying as a result of, cancer or any other noncardiovascular causes.
Hence, we determined the accuracy of the ACC/AHA guideline eligibility criteria for statin therapy to identify participants at a higher risk of developing cancer or of dying as a result of cancer or other noncardiovascular causes in a large, prospective community-based asymptomatic cohort in the Framingham Heart Study (FHS).
METHODS
Study Population
Details regarding the FHS population, selection criteria, and design of the Framingham multidetector computed tomography (MDCT) imaging study have been published and described elsewhere.14-17 Participants in this study were drawn from the offspring and the third-generation cohorts of the FHS, who underwent MDCT between 2002 and 2005. Participants in the analysis attended the offspring seventh examination cycle (1998 to 2001) or the third-generation first examination cycle (2002 to 2005). We included men 35 years old or older and women 40 years old or older who were not pregnant. All participants weighed 350 pounds (157.5 kg) or less. For the primary analysis, we excluded participants with prevalent cancer and those who were taking lipid-lowering therapy at baseline. The institutional review boards of Boston University Medical Center and Massachusetts General Hospital approved the study. All participants provided written informed consent.
Determination of Statin Eligibility
Following the 2013 ACC/AHA guidelines on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults, we identified candidates for statins on the basis of four delineated benefit groups outlined in the document9: (1) those with clinical atherosclerotic cardiovascular disease (ASCVD), defined as coronary death or nonfatal myocardial infarction, or fatal or nonfatal stroke; (2) those with LDL ≥ 190 mg/dL; (3) those who had had diabetes for 40 to 75 years and LDL of 70 to 189 mg/dL; and (4) those with no clinical ASCVD or diabetes, LDL of 70 to 189 mg/dL, and estimated ASCVD risk ≥ 7.5%. ASCVD risk was determined using the pooled cohort calculator.18,19
Imaging for Coronary Artery Calcium
Participants underwent ECG-triggered non–contrast-enhanced cardiac computed tomography on an eight-slice MDCT scanner (LightSpeed Ultra; General Electric, Milwaukee, WI) during a breath hold.20 The effective radiation exposure was 1.0 to 1.25 mSv. The amount of coronary artery calcium (CAC) was quantified independently by experienced readers using a dedicated offline workstation (Aquarius; Terarecon, San Mateo, CA) and was expressed as the typical Agatston score.21
Outcome Definitions
All participants in the FHS undergo continuous surveillance for incident cardiovascular events, cancer diagnoses, and death. Outcome events are adjudicated by a panel of three physicians after review of all available information, hospitalization records, and physician records. Cancer cases were identified at routine examinations and health updates, through surveillance of admissions at local Framingham hospitals, or from death records. The cases were confirmed by pathology reports, and two independent investigators reviewed the medical records. Cause of death was obtained from death certificates, hospital admission records, medical records, or family members. Cancer mortality included all individuals identified as having cancer and in whom cancer was identified as the primary cause of death. Noncardiovascular mortality included all mortality, with the exception of death as a result of coronary heart disease. The final date of follow-up was December 31, 2013, for both cohorts.
Statistical Analysis
Descriptive statistics presented are mean and standard deviation or percent of participants; median and quartiles are also presented for CAC. The primary outcome was incident cancer. Secondary end points were cancer mortality and noncardiovascular mortality. Univariate Fine and Gray22 semiparametric models for subdistribution hazards, accounting for competing risk of mortality, were used to relate statin eligibility to time-to-event. For the outcomes of cancer mortality and noncardiovascular mortality, the competing risks were noncancer mortality and CVD mortality, respectively, as presented in the Appendix (online only). Plots of the cumulative incidence function over time for cancer, cancer mortality, and noncardiovascular mortality, which also accounted for competing risk of mortality, are presented by 2013 ACC/AHA guideline–based statin eligibility status; the curves were compared between statin-eligible and statin-noneligible participants by using Gray’s test.23 The plots were generated using SAS PROC LIFETEST with the event code option (SAS Institute, Cary, NC).
Stratified Fine and Gray subdistribution hazard regressions22 were repeated on the basis of specific cut points for1 body mass index (BMI; > 25 v < 25),2 smoking status (ever smoked v never smoked), and3 age (older than 50 years v younger than 50 years). The analysis was repeated for subgroups of participants according to sex and presence of coronary calcification. In a sensitivity analysis, nonmalignant neoplasms and nonmelanoma skin cancers were not included among cancer cases.
Analyses were performed with the use of SAS software, version 9.4 (SAS Institute). P values were considered significant using a two-sided .05 level of significance, and are two sided.
RESULTS
Study Population
Of the 7,634 participants in the offspring and third-generation cohorts, 4,105 were not included in the MDCT study. Of the 3,529 participants undergoing MDCT, 3,505 attended the offspring seventh examination or the third-generation first examination, and 3,496 of these had evaluable results for CAC. Of these, 3,016 were between 40 and 75 years of age inclusive, of whom 2,565 were not on lipid-lowering therapy. Of these, 2,450 had nonmissing risk factors that allowed categorization of statin eligibility and, of these, 2,196 did not have a history of cancer.
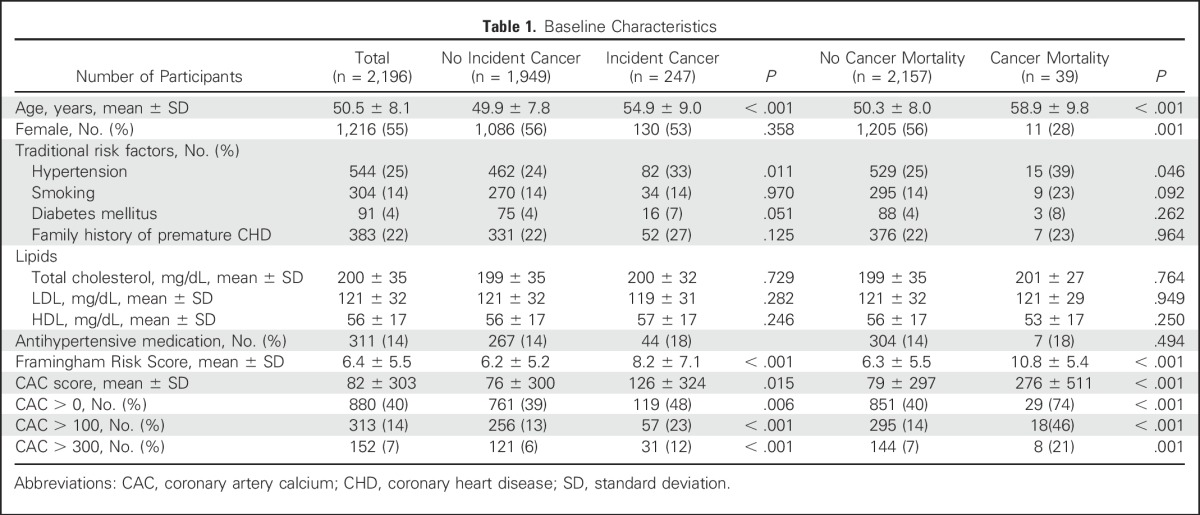
Participants were 50.5 ± 8.1 years of age, 55% were women, and the mean Framingham Risk Score (FRS) was 6.4% (Table 1). The mean LDL level was 121 mg/dL, the mean CAC score was 82 (median of 0 with quartiles of 0 to 25), and 40% of participants had a CAC score > 0. Total cholesterol and LDL levels were similar between participants who developed cancer and those who did not, and also between those who died as a result of cancer and those who did not. Although the FRS and CAC were significantly higher in participants who died as a result of cancer, after adjusting for age and sex, the FRS, as well as the presence of coronary calcification, were not significantly associated with cancer-related mortality (P = .98 for FRS and P = .44 for coronary calcification presence).
Table 1.
Baseline Characteristics
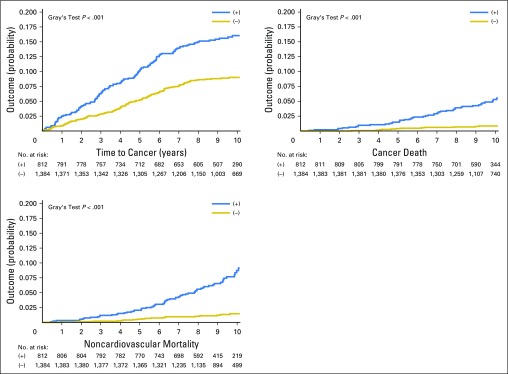
Outcomes
The median follow-up was 10.0 years (quartiles of 9.1-10.6 years). Among the 2,196 participants (50.5 ± 8.1 years of age; 55% female), the incident cancer rate was 11.2% (247 of 2,196), the cancer mortality rate was 1.8% (39 of 2,196), and the overall noncardiovascular mortality rate was 3.1% (68 deaths; Table 2). Two hundred forty-seven participants had incident cancer during follow-up. Incident cancer types included the following: breast: 30 of 247 (12.1%); prostate: 29 of 247 (11.7%); urinary bladder: 10 of 247 (4.0%); hematopoietic and reticuloendothelial systems: 10 of 247 (4.0%); lung: eight of 247 (3.2%); corpus uteri or endometrium: eight of 247 (3.2%); colon: five of 247 (2.0%); skin: 111 of 247 (44.9%); and other: 36 of 247 (14.6%).
Table 2.
Identification of Incident Cancer, Cancer Mortality, and Noncardiovascular Mortality: Observed Event Rates and SDHRs Stratified by Statin Allocation Recommendation According to 2013 ACC/AHA Guidelines
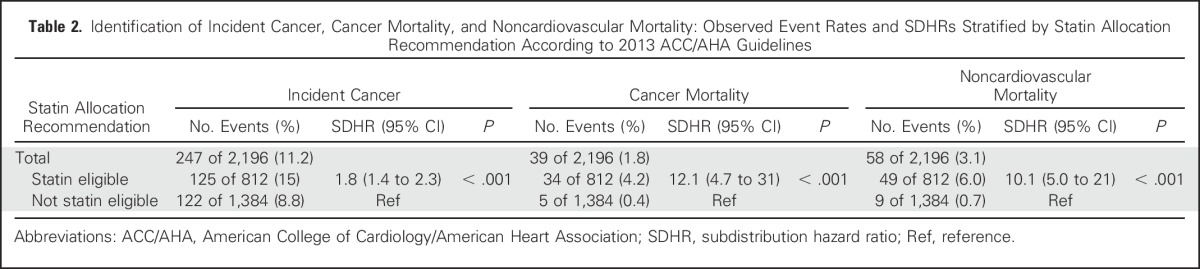
Guideline-Based Statin Eligibility and Outcomes
Overall, 37% of participants (812 of 2,196) were eligible for statins by applying the ACC/AHA guidelines. For participants older than 50 years of age, 59% (608 of 1,022) were statin eligible, whereas for participants younger than 50 years of age, 17% (204 of 1,174) were statin eligible. For smokers, 45% (525 of 1,177) were statin eligible, whereas for nonsmokers, 28% (287 of 1,019) were statin eligible. For participants with BMI > 25, 47% (668 of 1,416) were statin eligible, whereas for participants with BMI < 25, 18% (144 of 780) were statin eligible.
Incident cancer.
Among those eligible for statins according to ACC/AHA guidelines, 15% (125 of 812) developed cancer during follow-up, compared with 8.8% (122 of 1,384) among those who were not eligible (subdistribution hazard ratio [SDHR], 1.8 (1.4 to 2.3); P < .001; Fig 1). Results were similar after excluding nonmelanoma skin cancers (n = 70): 10.7% of statin-eligible participants (92 of 859) developed cancer, whereas 6.0% of noneligible participants (85 of 1,428) developed cancer (SDHR, 1.8 [1.4 to 2.5]; P < .001; Appendix Table A1, online only).
Fig 1.
Cumulative incidence function for statin-eligible versus statin-noneligible participants for (A) incident cancer, (B) cancer mortality, and (C) noncardiovascular mortality.
Cancer mortality.
Among those eligible for statins, 4.2% of participants (34 of 812) had cancer mortality, compared with only 0.4% (five of 1,384) among those who were not eligible (SDHR, 12.1 [4.7 to 31]; P < .001). Again, results were similar after excluding nonmelanoma skin cancers (n = 70): 4.4% of statin-eligible participants (38 of 859) died as a result of cancer compared with only 0.6% of noneligible participants (eight of 1,428; SDHR, 8.3 [3.8 to 18]; P < .001) died as a result of cancer.
Noncardiovascular mortality.
Among those eligible for statins, 6.0% (49 of 812) died as a result of noncardiovascular causes, compared with only 0.7% (nine of 1,384) of those noneligible for statins (SDHR, 10.1 [5.0 to 21]; P < .001).
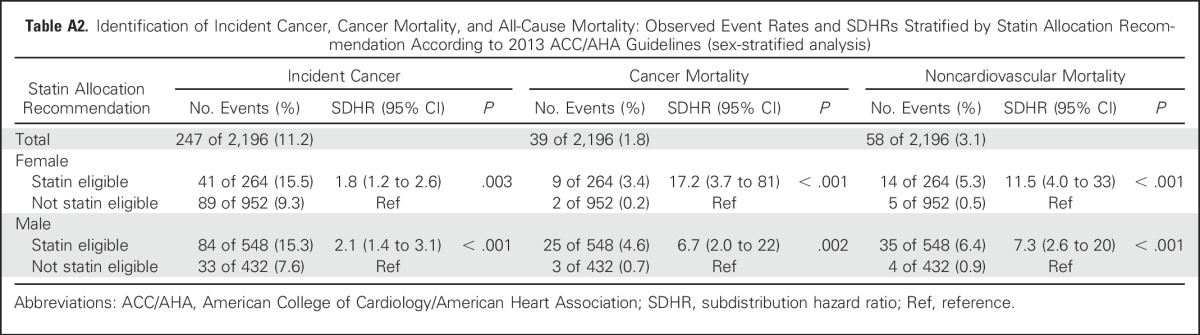
Findings for all outcomes including incident cancer, cancer mortality, and noncardiovascular mortality were similar when stratified by sex (Appendix Table A2, online only). There were no significant interactions of eligibility status with sex (P > .15 for all three outcomes). Adjusting for whether a patient had received lipid-lowering therapy during follow-up did not attenuate the SDHRs of statin-eligible versus statin-noneligible participants for each of the outcomes of incident cancer, cancer mortality, and noncardiovascular mortality (SDHR, 1.9; SDHR, 29.3; and SDHR, 22.9, respectively).
Guideline-Based Statin Eligibility and Outcomes Stratified by Risk Factors
The associations between guideline-based statin eligibility and each outcome were examined as stratified by age, smoking status, and BMI (Table 3).
Table 3.
Identification of Incident Cancer, Cancer Mortality, and All-Cause Mortality Stratified by Age, Smoking Status, and BMI: Observed Event Rates and SDHRs Stratified by Statin Allocation Recommendation According to 2013 ACC/AHA Guidelines
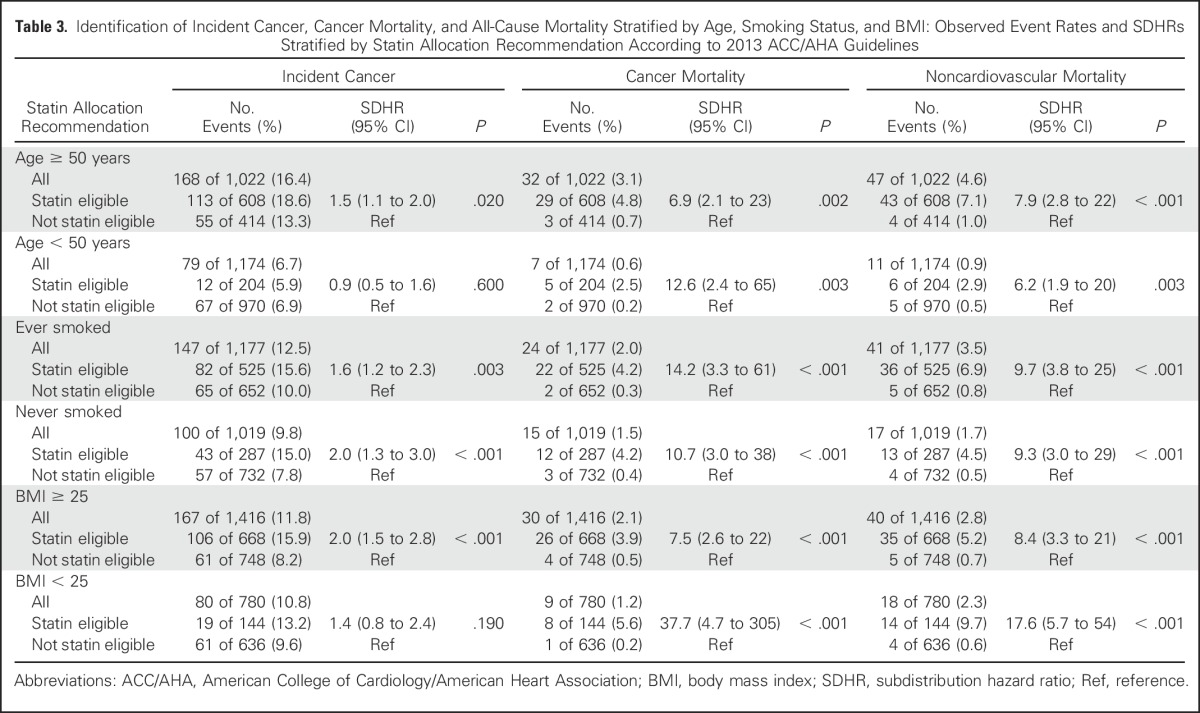
Age (older than 50 years v younger than 50 years).
Statin-eligible participants older than 50 years of age had a significantly increased likelihood of incident cancer (SDHR, 1.5 [1.1 to 2.0]; P = .020), although this was not significant among participants younger than 50 years of age. The risk of cancer mortality was elevated for statin-eligible versus statin-noneligible participants (SDHR, 6.9 [2.1 to 23] for those older than 50 years of age; SDHR, 12.6 [2.4 to 65] for those younger than 50 years of age; P = .002 and P = .003, respectively). Similarly, for both those older than 50 years of age and those younger than 50 years of age, statin-eligible participants had a significantly increased likelihood of noncardiovascular mortality compared with those not eligible (SDHR, 7.9 [2.8 to 22] for those older than 50 years of age; SDHR, 6.2 [1.9 to 20] for those younger than 50 years of age; P < .001 and P = .003, respectively). Overall, there was no significant interaction effect of eligibility status and age on any outcome (P > .15), indicating a relatively consistent effect of statin eligibility over statin ineligibility across ages.
Smoking (smokers v nonsmokers).
Statin-eligible participants had an increased likelihood of incident cancer (SDHR, 1.6 [1.2 to 2.3] for smokers; SDHR, 2.0 [1.3 to 3.0] for nonsmokers; P = .003 and P < .001, respectively). For both smokers and nonsmokers, cancer mortality was elevated for statin-eligible versus statin-noneligible participants (SDHR, 14.2 [3.3 to 61] for smokers; SDHR, 10.7 [3.0 to 38] for nonsmokers; P < .001 for both). Similarly, statin eligibility was associated with a significantly increased likelihood of noncardiovascular mortality compared with those not eligible (SDHR, 9.7 [3.8 to 25] for smokers; SDHR, 9.3 [3.0 to 29] for nonsmokers; P < .001 for both). Overall, there was no significant interaction effect of eligibility status and smoking status on any outcome (P > .15), indicating a relatively consistent effect of statin eligibility over statin ineligibility across smoking status.
BMI (> 25 v < 25).
Statin-eligible participants with a BMI > 25 had a significantly increased likelihood of incident cancer (SDHR, 2.0 [1.5 to 2.8]; P < .001), whereas the increased likelihood in participants with a BMI < 25 (SDHR, 1.4) was not significant. For participants with a BMI > 25 and those with a BMI < 25, statin eligibility was associated with a significantly increased likelihood of cancer mortality (SDHR, 7.5 [2.6 to 22] for a BMI > 25; SDHR, 37.7 [4.7 to 305] for a BMI < 25; P < .001 for both). Similar results for noncardiovascular mortality were noted among statin-eligible versus statin-noneligible participants (SDHR, 8.4 [3.3 to 21] for a BMI > 25; SDHR, 17.9 [5.7 to 54] for a BMI < 25; P < .001 for both). The interaction between BMI and statin eligibility status on cancer mortality was significant (P = .049), but the direction of the statin-eligible effect was large and in the same direction for both BMI categories. For cancer and noncardiovascular mortality, there were no significant differences between the eligibility categories across BMI categories (interaction P value > .15).
Presence of Subclinical Atherosclerosis and Outcomes
Among participants with a CAC score = 0 (n = 1,316), the incident cancer rate was 9.7% (128 of 1,316) and the noncardiovascular mortality rate was 1.2% (16 deaths), including 10 cancer deaths. Among participants with a CAC score > 0 (n = 880), the incident cancer rate was 13.5% (119 of 880) and the noncardiovascular mortality rate was 4.8% (42 deaths), including 29 cancer deaths.
For both participants with a CAC score = 0 and those with a CAC score > 0, statin eligibility was associated with a significantly increased likelihood of incident cancer (SDHR, 1.6 [1.1 to 2.3]; SDHR, 1.9 [1.3 to 2.9], respectively), cancer mortality (SDHR, 9.0 [2.3 to 34]; SDHR, 8.9 [2.1 to 38], respectively), and noncardiovascular mortality (SDHR, 5.1 [1.9 to 14]; SDHR, 13.6 [3.3 to 56], respectively), compared with participants not eligible for statin therapy.
DISCUSSION
In this community-based primary prevention cohort, we demonstrate that statin-eligible participants as defined by the 2013 ACC/AHA guidelines have a significantly increased risk of developing or dying as a result of cancer as compared with non–statin-eligible participants. These findings were maintained in analyses stratified for obesity, smoking, and age. The presence or absence of coronary calcification did not significantly modify differences in outcomes between statin-eligible and statin-noneligible subjects. Similar findings were seen for noncardiovascular mortality.
Our analysis adds to a small evidence base suggesting that a composite cardiovascular risk profile as deduced from the ASCVD risk score should be used to promote optimal cancer outcomes for any therapy by treating important cardiovascular risk factors.1 An analysis by the ARIC (The Atherosclerosis Risk In Communities) study investigators showed that adherence to several cardiovascular health metrics such as the American Heart Association 2020 goals is associated not only with CVD reduction but also with cancer incidence.2 A recent epidemiologic analysis using a metabolic risk score found that this score was linearly and positively associated with several incident cancer types.24 Our analysis extends the findings of these studies by placing them in the framework of the current ACC/AHA statin eligibility guidelines, because several observational studies have suggested a benefit of statins for cancer incidence and mortality. Surprisingly, our findings suggest that the accuracy of the ACC/AHA guideline–based statin eligibility criteria for identifying individuals at a higher 10-year cancer mortality risk is at least as high as the accuracy of this tool in identifying those at a 10-year ASCVD risk (SDHR, 12 v 7, respectively). These results also provide some context for the ongoing discussions about the importance of extrinsic versus intrinsic factors associated with the development of cancer,3,4 suggesting that extrinsic (modifiable) factors that determine ACC/AHA statin eligibility (with the exceptions of age and sex) play a highly significant role in identifying participants at risk of incident cancer and cancer mortality. However, prospective studies are needed to validate our hypotheses.
The fact that the observed risk of noncardiovascular events was consistently higher, even in stratified analyses by age, BMI, and smoking status, suggests that the association of cardiovascular risk with noncardiovascular outcomes is not driven by any one common causative risk factor in both cancer and CVD, but rather by the complex interplay of the several risk components that form the basis for determining statin eligibility, including age, blood pressure, cholesterol, smoking status, and diabetes. For example, the presence of metabolic syndrome, which involves disturbances in several of these risk factors, is associated with an increased risk of several cancer types.25 In addition, the presence of obesity, which is related to many of these factors, is thought to account for nearly 20% of cancer deaths.26 Furthermore, a recent meta-analysis showed a 41% increased risk of all-cause mortality in patients with cancer with pre-existing diabetes versus those without pre-existing diabetes.27 Some of the proposed mechanistic links between insulin resistance and obesity states and cancer include (1) insulin stimulation of cell proliferation via effects on insulin-like growth factor-1, (2) increased estrogen bio-availability by way of aromatization of androgens in adipose tissue leading to higher risk of breast and endometrial cancer, and (3) leptin secretion from adipocytes that increases cellular proliferation.28
The most important conclusion of our data is that there is an opportunity to improve public health awareness of shared risk factors for cardiovascular and cancer outcomes. If statin eligibility is, as our data suggest, not only an indicator of a higher risk of ASCVD but also of cancer incidence and mortality, the ACC/AHA guidelines present a unique and simple platform for identifying a cohort that is at a higher risk of cancer and cancer-related death.
Another interesting finding is that the association of statin eligibility and a higher risk of incident cancer and cancer mortality was not affected by the presence of CAC, which has been shown to be an effective modifier of ASCVD event risk (ie, SDHR for statin eligible to statin noneligible for CAC = 0 v CAC > 0 was 9.0 v 9.1 for cancer mortality and 1.6 v 2.0 for incident cancer, compared with 1.1. v 6.1 for ASCVD events). Intuitively, this seems to be plausible because CAC is a local manifestation of the effect of multiple risk factors over time, with a specific effect on cardiovascular health, whereas the systemic exposure to these multiple risk factors is an intermediary substrate to cancer events and noncardiovascular mortality.
Our study has limitations, including the relatively small number of events. In addition, results in white Americans may not be generalizable to other ethnic groups, and it should be noted that the reported significant differences in associations among risk factors, CAC, and outcomes in whites compared with other ethnic groups suggest that ethnic group–specific prediction rules may be required.29,30
In this community-based primary prevention cohort, ACC/AHA guideline–based statin eligibility criteria accurately identified patients at a higher risk of developing cancer and cancer-related mortality. Shared risk factor profiles between cancer and cardiovascular events, together with the potential benefits of statin therapy for both diseases, may provide a unique opportunity to improve population health.
Appendix
The SAS PHREG procedure with the event code option in the model statement was used to perform these models and to calculate the hazard ratios, referred to as subdistribution hazard ratios when a competing risk was present. The proportional hazards assumption for each end point in the presence of the competing risk was assessed by calculating the significance of the correlation of the Schoenfeld-type residuals with log of time using the methodology and SAS programming statements developed by Kohl et al (Kohl M, et al: Comput Methods Programs Biomed 118:218-233, 2015); all correlations were not significant, with P values > .2 for all end points, indicating that the proportional hazards assumption was met in all cases.
Table A1.
Identification of Incident Cancer, Cancer Mortality, and All-Cause Mortality: Observed Event Rates and SDHRs Stratified by Statin Allocation Recommendation According to 2013 ACC/AHA Guidelines (excluding nonmelanoma skin cancer)
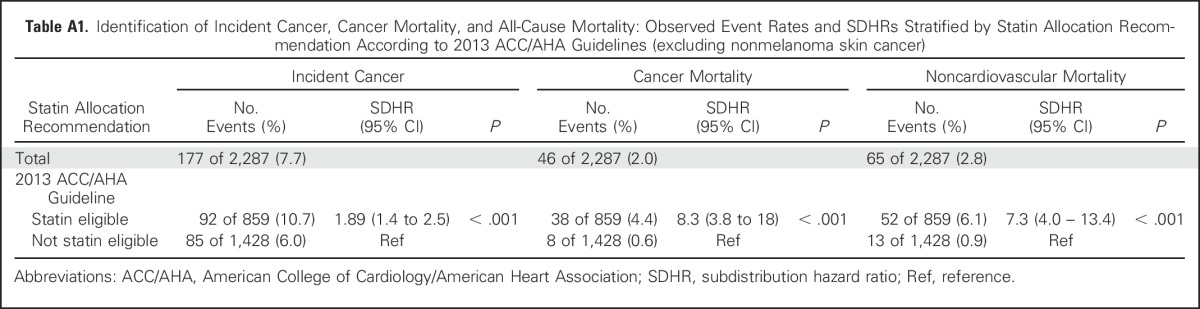
Table A2.
Identification of Incident Cancer, Cancer Mortality, and All-Cause Mortality: Observed Event Rates and SDHRs Stratified by Statin Allocation Recommendation According to 2013 ACC/AHA Guidelines (sex-stratified analysis)
Footnotes
Supported by Contract Nos. K24: 2K24HL113128, T32: 5T32HL076136, N01-HC-25195, HL076784, AG028321, HL070100, HL060040, HL080124, HL071039, HL077447, and HL107385 from the National Institutes of Health Heart, Lung, and Blood Institute’s Framingham Heart Study.
The National Institutes of Health had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Listen to the podcast by Dr Dent at ascopubs.org/jco/podcasts
AUTHOR CONTRIBUTIONS
Conception and design: Amit Pursnani, Christopher J. O'Donnell, Udo Hoffmann
Financial support: Udo Hoffmann
Administrative support: Christopher J. O'Donnell, Udo Hoffmann
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Guideline-Based Statin Eligibility, Cancer Events, and Noncardiovascular Mortality in the Framingham Heart Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Amit Pursnani
No relationship to disclose
Joseph M. Massaro
No relationship to disclose
Ralph B. D'Agostino Sr
No relationship to disclose
Christopher J. O'Donnell
No relationship to disclose
Udo Hoffmann
Research Funding: KOWA, ACRIN, HEARTFLOW
REFERENCES
- 1.Eyre H, Kahn R, Robertson RM, et al. : Preventing cancer, cardiovascular disease, and diabetes: A common agenda for the American Cancer Society, the American Diabetes Association, and the American Heart Association. Circulation 109:3244-3255, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Rasmussen-Torvik LJ, Shay CM, Abramson JG, et al. : Ideal cardiovascular health is inversely associated with incident cancer: The Atherosclerosis Risk In Communities study. Circulation 127:1270-1275, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu S, Powers S, Zhu W, et al. : Substantial contribution of extrinsic risk factors to cancer development. Nature 529:43-47, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tomasetti C, Vogelstein B: Cancer etiology. Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science 347:78-81, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pursnani A, Massaro JM, D’Agostino RB, Sr, et al. : Guideline-based statin eligibility, coronary artery calcification, and cardiovascular events. JAMA 314:134-141, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nielsen SF, Nordestgaard BG, Bojesen SE: Statin use and reduced cancer-related mortality. N Engl J Med 368:576-577, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Cardwell CR, Mc Menamin Ú, Hughes CM, et al. : Statin use and survival from lung cancer: A population-based cohort study. Cancer Epidemiol Biomarkers Prev 24:833-841, 2015 [DOI] [PubMed] [Google Scholar]
- 8.Nevadunsky NS, Van Arsdale A, Strickler HD, et al. : Association between statin use and endometrial cancer survival. Obstet Gynecol 126:144-150, 2015 [DOI] [PubMed] [Google Scholar]
- 9.Murtola TJ, Visvanathan K, Artama M, et al. : Statin use and breast cancer survival: A nationwide cohort study from Finland. PLoS One 9:e110231, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desai P, Lehman A, Chlebowski RT, et al. : Statins and breast cancer stage and mortality in the Women’s Health Initiative. Cancer Causes Control 26:529-539, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poynter JN, Gruber SB, Higgins PD, et al. : Statins and the risk of colorectal cancer. N Engl J Med 352:2184-2192, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Jakobisiak M, Golab J: Potential antitumor effects of statins (Review). Int J Oncol 23:1055-1069, 2003 [PubMed] [Google Scholar]
- 13.Chan KK, Oza AM, Siu LL: The statins as anticancer agents. Clin Cancer Res 9:10-19, 2003 [PubMed] [Google Scholar]
- 14. doi: 10.1016/j.amjcard.2008.06.038. Hoffmann U, Massaro JM, Fox CS, et al: Defining normal distributions of coronary artery calcium in women and men (from the Framingham Heart Study). Am J Cardiol 102:1136-1141, 1141.e1, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lehman SJ, Massaro JM, Schlett CL, et al. : Peri-aortic fat, cardiovascular disease risk factors, and aortic calcification: The Framingham Heart Study. Atherosclerosis 210:656-661, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Preis SR, Hwang SJ, Fox CS, et al. : Eligibility of individuals with subclinical coronary artery calcium and intermediate coronary heart disease risk for reclassification (from the Framingham Heart Study). Am J Cardiol 103:1710-1715, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thanassoulis G, Massaro JM, Cury R, et al. : Associations of long-term and early adult atherosclerosis risk factors with aortic and mitral valve calcium. J Am Coll Cardiol 55:2491-2498, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stone NJ, Robinson NJ, Lichtenstein AH, et al. : 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 63:2889-2934, 2014 [DOI] [PubMed] [Google Scholar]
- 19. doi: 10.1016/j.jacc.2013.11.005. Goff DC Jr, Lloyd-Jones DM, Bennett G, et al: 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 63:2935-2959, 2014 [Erratum J Am Coll Cardiol 63:3026, 2014] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hong C, Bae KT, Pilgram TK: Coronary artery calcium: Accuracy and reproducibility of measurements with multi-detector row CT--assessment of effects of different thresholds and quantification methods. Radiology 227:795-801, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Hoffmann U, Siebert U, Bull-Stewart A, et al. : Evidence for lower variability of coronary artery calcium mineral mass measurements by multi-detector computed tomography in a community-based cohort--consequences for progression studies. Eur J Radiol 57:396-402, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Fine JP, Gray RJ: A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94:496-509, 1999. [Google Scholar]
- 23.Gray R: A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 16:1141-1154, 1988 [Google Scholar]
- 24.Stocks T, Bjørge T, Ulmer H, et al. : Metabolic risk score and cancer risk: Pooled analysis of seven cohorts. Int J Epidemiol 44:1353-1363, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Esposito K, Chiodini P, Colao A, et al. : Metabolic syndrome and risk of cancer: A systematic review and meta-analysis. Diabetes Care 35:2402-2411, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calle EE, Rodriguez C, Walker-Thurmond K, et al. : Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 348:1625-1638, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Barone BB, Yeh HC, Snyder CF, et al. : Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: A systematic review and meta-analysis. JAMA 300:2754-2764, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calle EE, Kaaks R: Overweight, obesity and cancer: Epidemiological evidence and proposed mechanisms. Nat Rev Cancer 4:579-591, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Polonsky TS, McClelland RL, Jorgensen NW, et al. : Coronary artery calcium score and risk classification for coronary heart disease prediction. JAMA 303:1610-1616, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Safford MM, Brown TM, Muntner PM, et al. : Association of race and sex with risk of incident acute coronary heart disease events. JAMA 308:1768-1774, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]