Abstract
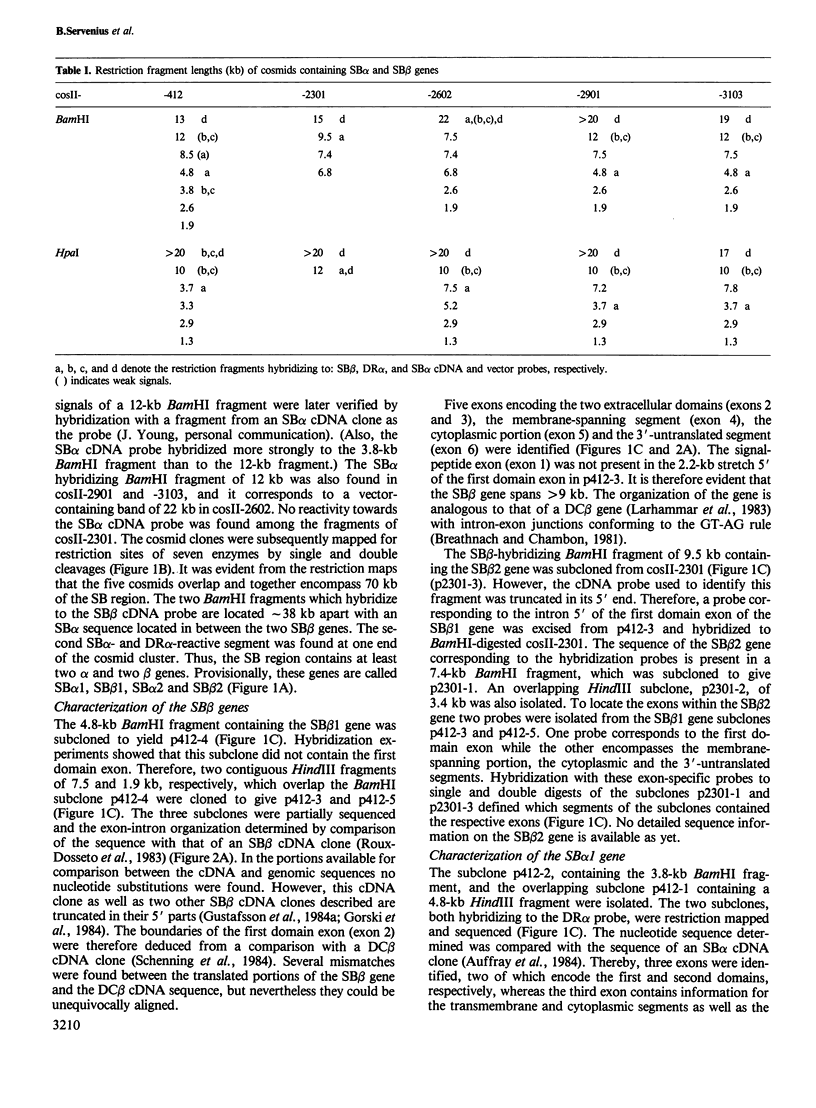
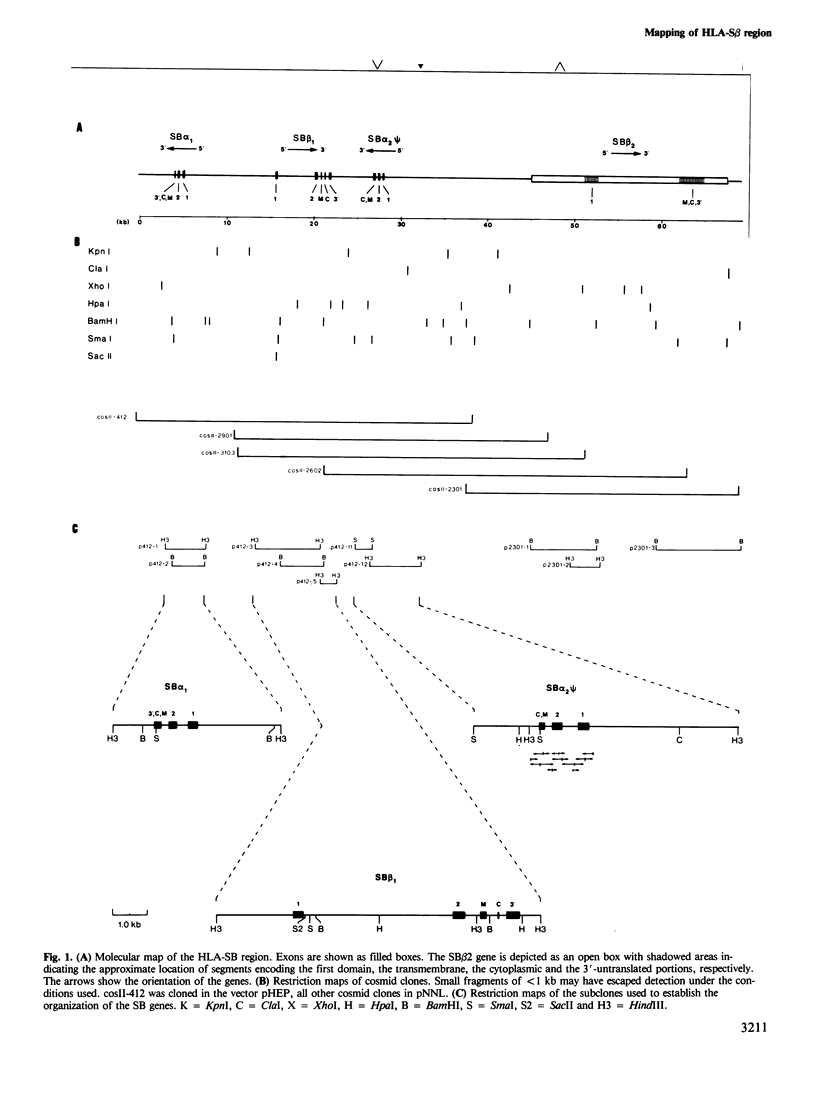
The human major histocompatibility complex contains the genes for at least three different types of class II antigens, DR, DC and SB (DR, DQ and DP). They are all composed of an alpha and a beta chain. We have cloned a chromosomal region of 70 kb containing the SB (DP) gene family in overlapping cosmid clones. This segment contains two alpha genes and two beta genes, located in the order SB alpha 1, SB beta 1, SB alpha 2 and SB beta 2. The orientation of the alpha genes is reversed compared with that of the beta genes. This organisation suggests that the SB region has arisen by duplication of a chromosomal segment encompassing one alpha and one beta gene. Partial nucleotide sequences of the SB alpha 1 and SB beta 1 exons demonstrate that the genes correspond to SB alpha and beta cDNA clones. Consequently these genes are expressed. In contrast nucleotide sequence determination of the SB alpha 2 gene shows that it is a pseudogene.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auffray C., Korman A. J., Roux-Dosseto M., Bono R., Strominger J. L. cDNA clone for the heavy chain of the human B cell alloantigen DC1: strong sequence homology to the HLA-DR heavy chain. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6337–6341. doi: 10.1073/pnas.79.20.6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auffray C., Lillie J. W., Arnot D., Grossberger D., Kappes D., Strominger J. L. Isotypic and allotypic variation of human class II histocompatibility antigen alpha-chain genes. Nature. 1984 Mar 22;308(5957):327–333. doi: 10.1038/308327a0. [DOI] [PubMed] [Google Scholar]
- Benacerraf B. Role of MHC gene products in immune regulation. Science. 1981 Jun 12;212(4500):1229–1238. doi: 10.1126/science.6165083. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Cresswell P., Geler S. S. Antisera to human B-lymphocyte membrane glycoproteins block stimulation in mixed lymphocyte culture. Nature. 1975 Sep 11;257(5522):147–149. doi: 10.1038/257147a0. [DOI] [PubMed] [Google Scholar]
- Cullen S. E., David C. S., Shreffler D. C., Nathenson S. G. Membrane molecules determined by the H-2 associated immune response region: isolation and some properties. Proc Natl Acad Sci U S A. 1974 Mar;71(3):648–652. doi: 10.1073/pnas.71.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski J., Rollini P., Long E., Mach B. Molecular organization of the HLA-SB region of the human major histocompatibility complex and evidence for two SB beta-chain genes. Proc Natl Acad Sci U S A. 1984 Jul;81(13):3934–3938. doi: 10.1073/pnas.81.13.3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosveld F. G., Lund T., Murray E. J., Mellor A. L., Dahl H. H., Flavell R. A. The construction of cosmid libraries which can be used to transform eukaryotic cells. Nucleic Acids Res. 1982 Nov 11;10(21):6715–6732. doi: 10.1093/nar/10.21.6715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson K., Emmoth E., Widmark E., Böhme J., Peterson P. A., Rask L. Isolation of a cDNA clone coding for an SB beta-chain. Nature. 1984 May 3;309(5963):76–78. doi: 10.1038/309076a0. [DOI] [PubMed] [Google Scholar]
- Gustafsson K., Wiman K., Emmoth E., Larhammar D., Böhme J., Hyldig-Nielsen J. J., Ronne H., Peterson P. A., Rask L. Mutations and selection in the generation of class II histocompatibility antigen polymorphism. EMBO J. 1984 Jul;3(7):1655–1661. doi: 10.1002/j.1460-2075.1984.tb02026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley C. K., Shaw S., Nadler L., Schlossman S., Capra J. D. Alpha and beta chains of SB and DR antigens are structurally distinct. J Exp Med. 1982 Nov 1;156(5):1557–1562. doi: 10.1084/jem.156.5.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klareskog L., Sandgerg-Trägårdh L., Rask L., Lindblom J. B., Curman B., Peterson P. A. Chemical properties of human Ia antigens. Nature. 1977 Jan 20;265(5591):248–251. doi: 10.1038/265248a0. [DOI] [PubMed] [Google Scholar]
- Korman A. J., Auffray C., Schamboeck A., Strominger J. L. The amino acid sequence and gene organization of the heavy chain of the HLA-DR antigen: homology to immunoglobulins. Proc Natl Acad Sci U S A. 1982 Oct;79(19):6013–6017. doi: 10.1073/pnas.79.19.6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larhammar D., Gustafsson K., Claesson L., Bill P., Wiman K., Schenning L., Sundelin J., Widmark E., Peterson P. A., Rask L. Alpha chain of HLA-DR transplantation antigens is a member of the same protein superfamily as the immunoglobulins. Cell. 1982 Aug;30(1):153–161. doi: 10.1016/0092-8674(82)90021-6. [DOI] [PubMed] [Google Scholar]
- Larhammar D., Hyldig-Nielsen J. J., Servenius B., Andersson G., Rask L., Peterson P. A. Exon-intron organization and complete nucleotide sequence of a human major histocompatibility antigen DC beta gene. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7313–7317. doi: 10.1073/pnas.80.23.7313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larhammar D., Schenning L., Gustafsson K., Wiman K., Claesson L., Rask L., Peterson P. A. Complete amino acid sequence of an HLA-DR antigen-like beta chain as predicted from the nucleotide sequence: similarities with immunoglobulins and HLA-A, -B, and -C antigens. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3687–3691. doi: 10.1073/pnas.79.12.3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. S., Trowsdale J., Travers P. J., Carey J., Grosveld F., Jenkins J., Bodmer W. F. Sequence of an HLA-DR alpha-chain cDNA clone and intron-exon organization of the corresponding gene. Nature. 1982 Oct 21;299(5885):750–752. doi: 10.1038/299750a0. [DOI] [PubMed] [Google Scholar]
- Long E. O., Wake C. T., Gorski J., Mach B. Complete sequence of an HLA-dR beta chain deduced from a cDNA clone and identification of multiple non-allelic DR beta chain genes. EMBO J. 1983;2(3):389–394. doi: 10.1002/j.1460-2075.1983.tb01435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Roux-Dosseto M., Auffray C., Lillie J. W., Boss J. M., Cohen D., DeMars R., Mawas C., Seidman J. G., Strominger J. L. Genetic mapping of a human class II antigen beta-chain cDNA clone to the SB region of the HLA complex. Proc Natl Acad Sci U S A. 1983 Oct;80(19):6036–6040. doi: 10.1073/pnas.80.19.6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenning L., Larhammar D., Bill P., Wiman K., Jonsson A. K., Rask L., Peterson P. A. Both alpha and beta chains of HLA-DC class II histocompatibility antigens display extensive polymorphism in their amino-terminal domains. EMBO J. 1984 Feb;3(2):447–452. doi: 10.1002/j.1460-2075.1984.tb01826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw S., Johnson A. H., Shearer G. M. Evidence for a new segregant series of B cell antigens that are encoded in the HLA-D region and that stimulate secondary allogenic proliferative and cytotoxic responses. J Exp Med. 1980 Sep 1;152(3):565–580. doi: 10.1084/jem.152.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz M., Hood L. Genes of the major histocompatibility complex in mouse and man. Science. 1983 Nov 18;222(4625):727–733. doi: 10.1126/science.6356354. [DOI] [PubMed] [Google Scholar]
- Steinmetz M., Minard K., Horvath S., McNicholas J., Srelinger J., Wake C., Long E., Mach B., Hood L. A molecular map of the immune response region from the major histocompatibility complex of the mouse. Nature. 1982 Nov 4;300(5887):35–42. doi: 10.1038/300035a0. [DOI] [PubMed] [Google Scholar]
- Trowsdale J., Kelly A., Lee J., Carson S., Austin P., Travers P. Linkage map of two HLA-SB beta and two HLA-SB alpha-related genes: an intron in one of the SB beta genes contains a processed pseudogene. Cell. 1984 Aug;38(1):241–249. doi: 10.1016/0092-8674(84)90546-4. [DOI] [PubMed] [Google Scholar]


