Abstract
Purpose
Concurrent chemoradiotherapy is standard treatment for patients with stage III non–small-cell lung cancer. Elderly patients may experience increased rates of adverse events (AEs) or less benefit from concurrent chemoradiotherapy.
Patients and Methods
Individual patient data were collected from 16 phase II or III trials conducted by US National Cancer Institute–supported cooperative groups of concurrent chemoradiotherapy alone or with consolidation or induction chemotherapy for stage III non–small-cell lung cancer from 1990 to 2012. Overall survival (OS), progression-free survival, and AEs were compared between patients age ≥ 70 (elderly) and those younger than 70 years (younger). Unadjusted and adjusted hazard ratios (HRs) for survival time and CIs were estimated by single-predictor and multivariable frailty Cox models. Unadjusted and adjusted odds ratio (ORs) for AEs and CIs were obtained from single-predictor and multivariable generalized linear mixed-effect models.
Results
A total of 2,768 patients were classified as younger and 832 as elderly. In unadjusted and multivariable models, elderly patients had worse OS (HR, 1.20; 95% CI, 1.09 to 1.31 and HR, 1.17; 95% CI, 1.07 to 1.29, respectively). In unadjusted and multivariable models, elderly and younger patients had similar progression-free survival (HR, 1.01; 95% CI, 0.93 to 1.10 and HR, 1.00; 95% CI, 0.91 to 1.09, respectively). Elderly patients had a higher rate of grade ≥ 3 AEs in unadjusted and multivariable models (OR, 1.35; 95% CI, 1.07 to 1.70 and OR, 1.38; 95% CI, 1.10 to 1.74, respectively). Grade 5 AEs were significantly higher in elderly compared with younger patients (9% v 4%; P < .01). Fewer elderly compared with younger patients completed treatment (47% v 57%; P < .01), and more discontinued treatment because of AEs (20% v 13%; P < .01), died during treatment (7.8% v 2.9%; P < .01), and refused further treatment (5.8% v 3.9%; P = .02).
Conclusion
Elderly patients in concurrent chemoradiotherapy trials experienced worse OS, more toxicity, and had a higher rate of death during treatment than younger patients.
INTRODUCTION
Lung cancer is the leading cause of cancer-related mortality in the United States, and a majority of patients have the non–small-cell lung cancer (NSCLC) subtype.1,2 Approximately 20% to 25% of patients with lung cancer present with locally advanced disease, and for patients with unresectable stage IIIA or IIIB NSCLC and good performance status, concurrent chemoradiotherapy is the standard therapy. With concurrent chemoradiotherapy, the 3- and 5-year overall survival rates are 24% and 15%, respectively.3 However, this treatment paradigm is associated with a significant rate of severe toxicity. A variety of concurrent chemotherapy and radiation therapy combinations and schedules are currently used, and the outcomes with the different treatment paradigms are similar.
The median age of patients with lung cancer is 70 years, and many patients have comorbidities associated with advanced age or tobacco use.1 Cancer clinical trials select for a younger patient population than the general cancer population, and elderly patients are frequently underrepresented in clinical trials.4-6 The eligibility criteria of concurrent chemoradiotherapy clinical trials select for the subset of patients most likely to tolerate and benefit from concurrent chemoradiotherapy. A significant proportion of patients seen in clinical practice do not meet the standard trial eligibility criteria, and clinicians extrapolate the benefit and toxicity of concurrent chemoradiotherapy to frail patients with significant comorbidities. Clinicians are frequently faced with the difficult decision about whether to treat an elderly patient with a potentially curative therapy that is associated with significant toxicity or with an inferior treatment paradigm, such as radiation therapy alone or sequential chemotherapy and radiation therapy.3,7,8
Retrospective subset analyses of elderly patients treated in concurrent chemoradiotherapy trials have been discrepant. Some analyses have revealed similar outcomes and toxicity in elderly patients, whereas others have revealed age as a poor prognostic factor or a factor associated with a higher rate of toxicity.9-14 In previous analyses, approximately 20% to 25% of patients enrolled in the trials analyzed were age ≥ 70 years, and the elderly subsets in these analyses were small (24 to 130 patients), which limits the interpretation. The US National Cancer Institute (NCI) –supported cooperative groups (now known as the National Clinical Trials Network) have performed phase II or III clinical trials investigating concurrent chemoradiotherapy. We investigated the outcomes and adverse events (AEs) of elderly and younger patients enrolled in cooperative group trials to estimate the benefit and toxicity of concurrent chemoradiotherapy in elderly patients. We used the age cutoff of 70 years because that is the age that has been used to define elderly patients in previous prospective trials and retrospective analyses and for ease of comparison of our analysis with other studies.15-18
PATIENTS AND METHODS
Data-sharing agreements with the relevant cooperative groups were developed, and individual patient data (IPD) were obtained for patients with NSCLC or small-cell lung cancer treated in National Clinical Trials Network trials from 1990 to 2012. A centralized database was developed, and for this analysis, IPD were restricted to trials of concurrent chemoradiotherapy for unresectable stage IIIA or IIIB NSCLC. The study protocols and final publications were reviewed for inclusion, and only trials that included concurrent chemoradiotherapy alone or with induction or consolidation chemotherapy were included. IPD for patient populations with poor performance status or defined as poor risk were excluded. IPD from trials of a targeted therapy without chemotherapy were excluded, but IPD from trials of a targeted therapy in combination with chemotherapy were included. Patients age younger than 70 years were defined as younger, and patients age ≥ 70 years were defined as elderly, because this age cutoff has been used in previous prospective and retrospective analyses for patients with lung cancer.15-18 The primary end point investigated was overall survival (OS), and secondary end points were progression-free survival (PFS), rate of severe AEs, and reasons for treatment discontinuation. OS was defined as the time between random assignment or registration to death resulting from any cause. PFS was defined as the time between random assignment or registration to disease progression or death (whichever occurred first). Severe AEs were defined as NCI Common Terminology Criteria for Adverse Events grade ≥ 3. Individual grade ≥ 3 AEs occurring at a rate of ≥ 2.5% in elderly or younger patients (excluding leukopenia and lymphopenia) were compared. The AEs were reported for the entire study treatment. The reasons for treatment discontinuation were assessed by the study team and reported as part of the protocol. Reasons reported for treatment discontinuation included treatment completed, AE, disease progression, patient refused further treatment, patient died during treatment, treatment never started, patient developed other disease, and no response to treatment; reasons not included in this list were categorized as other. Treatments were divided into the following treatment paradigms: induction chemotherapy followed by concurrent chemoradiotherapy, concurrent chemoradiotherapy alone, and concurrent chemoradiotherapy followed by consolidation chemotherapy. We then performed supplementary analyses of age as a continuous variable and using the age cutoff of 65 years and trial enrollment completed in 2000 or later or before 2000.
Statistical Methods
The association between age group with other patient characteristics was tested using the χ2 test for categorical variables and Wilcoxon rank sum test for continuous variables. Kaplan-Meier curves were used to characterize PFS and OS for younger and older patients.19 The distribution difference of OS and PFS for younger and older patients was tested by log-rank test, and the age-group effect was further quantified by unadjusted and adjusted hazard ratios (HRs) for PFS and OS, estimated with CIs using univariable and multivariable frailty Cox models.19-22 Unadjusted and adjusted odds ratios (ORs) for AEs and their CIs were calculated using univariable and multivariable generalized linear mixed-effect models for binary outcome.23-25 Frailty Cox models and generalized linear mixed-effect models adopted here were one-stage approaches to analyze pooled IPD, which included study trial because random effects accounted for the clustering effect of patients with studies and the between-trial variance that could not be captured by covariates. Candidate covariates used for stepwise selection were: treatment group, age group, performance status, sex, race, weight loss, histology, number of chemotherapy agents, and recent trial or not (accrual closed after or before 2000). Treatment paradigm and age group were included in all adjusted models because they corresponded to the effects of primary interest. Variables included in the final model for the OS analysis were: treatment group, age group, performance status, sex, weight loss, and number of chemotherapy agents. Variables included in the final model for the PFS analysis were: treatment paradigm, age group, performance status, stage IIIA or IIIB, sex, weight loss, histology, number of chemotherapy agents, and recent trial. Variables included in the final model for the AE analysis were: treatment paradigm, age group, performance status, sex, histology, and number of chemotherapy agents. The adjusted HRs or ORs from the pooled analysis were compared with the adjusted HRs or ORs of each trial. Trial-level adjusted HRs or ORs and their CIs were estimated using multivariable Cox models or logistic regression models, adjusted for selected covariates. The P values testing specific effects for these regression models were based on the Wald test. When comparing the rates of specific AEs, the χ2 test was used to calculate P values, and for reasons for treatment discontinuation, the χ2 test was used to calculate P values, except when the association was tested on binary variables (treatment never started, developed other disease, and no response to treatment), where Fisher’s exact test was used. All P values reported are two sided and were not adjusted for multiple comparisons. The study was approved by the Duke University Institutional Review Board. Data management and statistical analyses were performed by statisticians at the Duke Department of Biostatistics and Bioinformatics using SAS (version 9.3; SAS Institute, Cary, NC) and R (version 3.2; R Foundation, Vienna, Austria) statistical software.
RESULTS
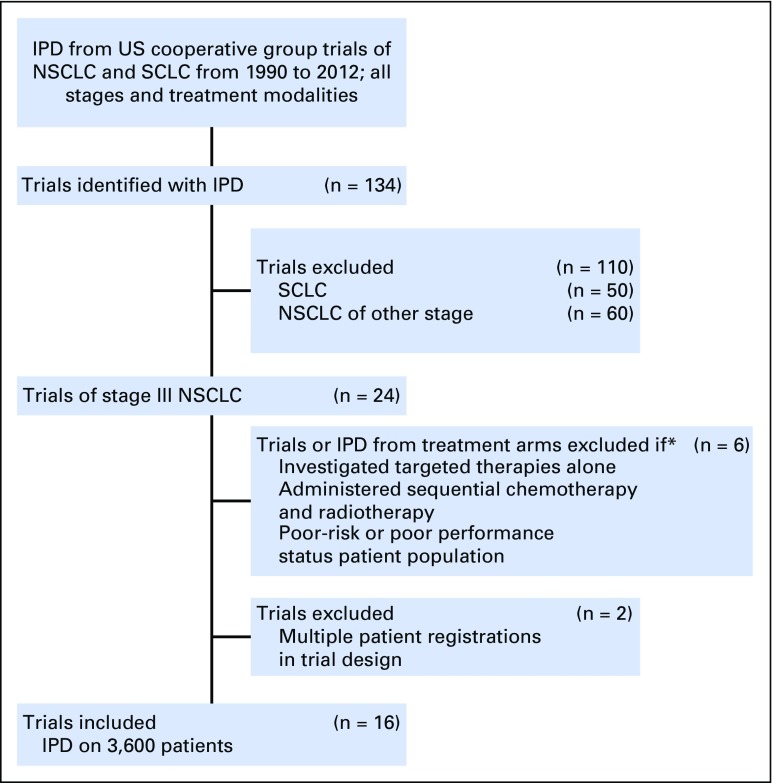
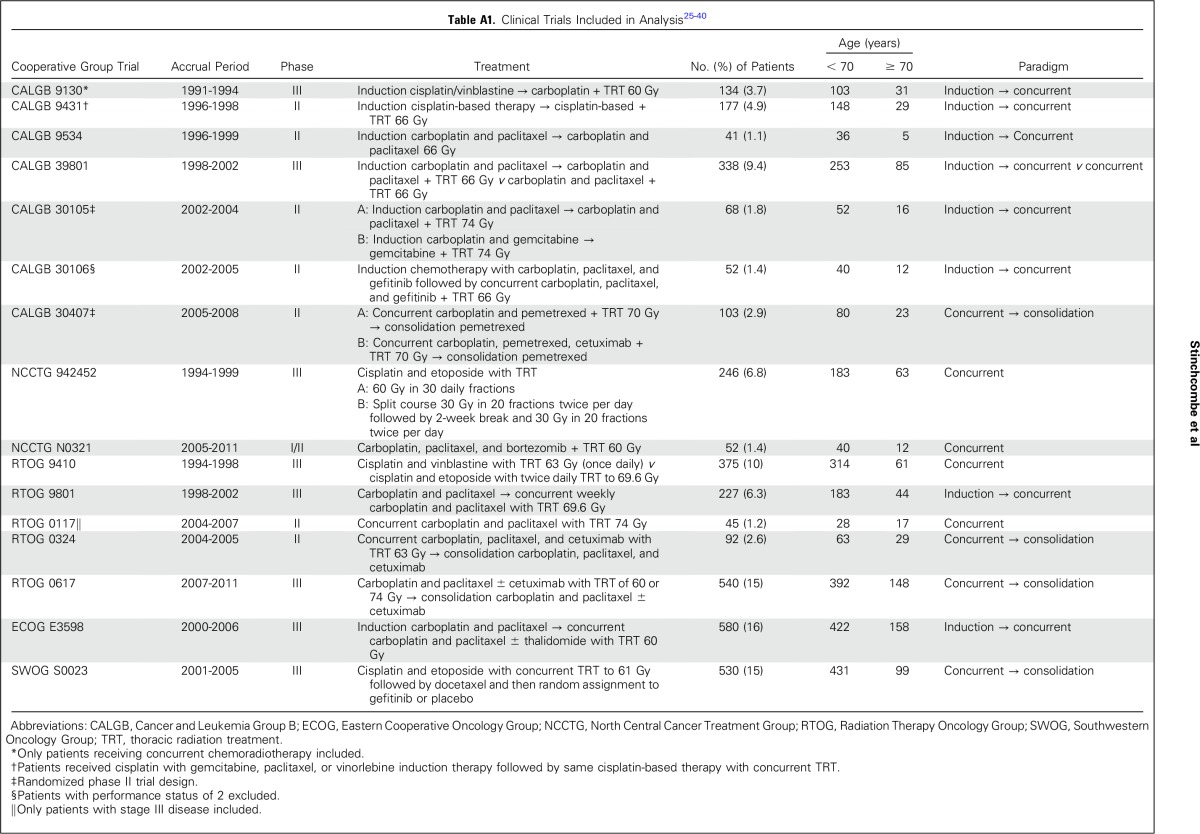
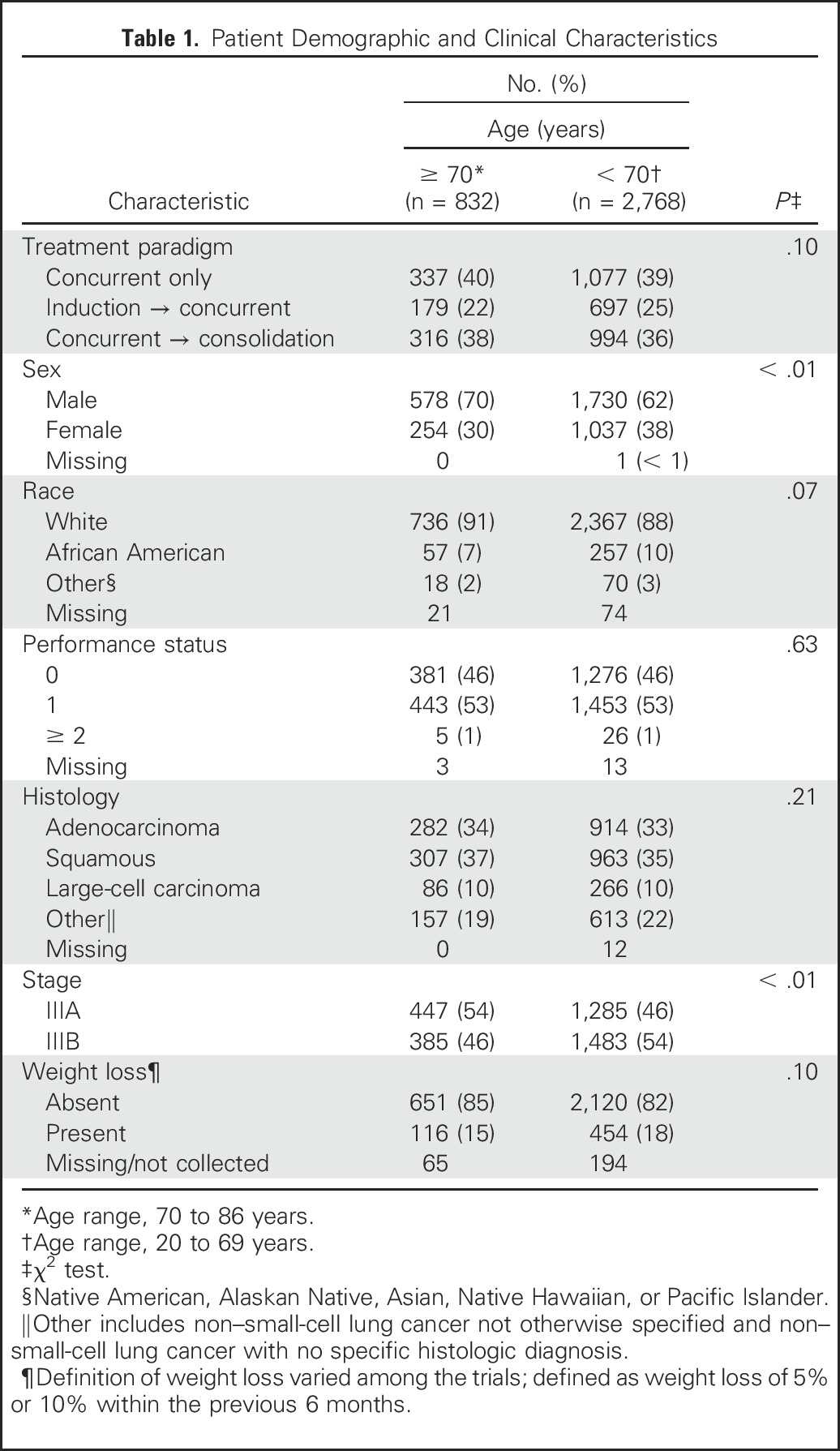
IPD from 3,600 patients from 16 trials were included in this analysis (Fig 1; Appendix Table A1, online only); 2,768 patients were categorized as younger, and 832 were categorized as elderly.26-41 Seven trials used induction chemotherapy followed by concurrent chemoradiotherapy, four trials used concurrent chemoradiotherapy followed by consolidation chemotherapy, four trials used concurrent chemoradiotherapy alone, and one trial randomly assigned patients between induction chemotherapy followed by concurrent chemoradiotherapy and concurrent chemoradiotherapy. Nine trials used carboplatin and paclitaxel, two trials used cisplatin and etoposide, one trial used cisplatin and a third-generation agent, and one trial used carboplatin and pemetrexed. Four trials used a cisplatin-based therapy, and 11 trials used a carboplatin-based therapy, and one trial used cisplatin induction chemotherapy and concurrent therapy with carboplatin. The patient characteristics are listed in Table 1. A statistically significantly higher percentage of elderly patients were male and had stage IIIA disease. These trials were conducted before routine collection of smoking history.
Fig 1.
CONSORT diagram of clinical trials. IPD, individual patient data; NSCLC, non–small-cell lung cancer; SCLC, small-cell lung cancer.
*IPD from treatment arms within that investigated targeted therapy alone, administered sequential chemotherapy and radiotherapy, or poor risk or poor performance status patient population.
Table 1.
Patient Demographic and Clinical Characteristics
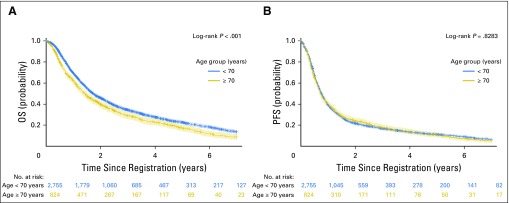
In the unadjusted and multivariable models, elderly patients had a statistically significantly worse OS (HR, 1.20; 95% CI, 1.09 to 1.31 and HR, 1.17; 95% CI, 1.07 to 1.29, respectively). The median OS in the elderly and younger patients were 17.0 and 20.7 months, respectively (P < .01). In elderly patients, the 3- and 5-year OS rates were 29% and 17%, respectively, and in younger patients, the 3- and 5-year OS rates were 34% and 23%, respectively (Fig 2A). When the treatment paradigms were analyzed, the test for treatment paradigm and age interaction was not statistically significant in the unadjusted (P = .81) or adjusted model (P = .97). In unadjusted and multivariable models, elderly and younger patients had a similar PFS (HR, 1.01; 95% CI, 0.93 to 1.10 and HR, 1.00; 95% CI, 0.91 to 1.09, respectively). The median PFS in elderly and younger patients were 8.7 and 9.1 months, respectively (P = .68; Fig 2B). The treatment paradigm and age interaction test was not statistically significant in the unadjusted (P = .26) or adjusted model (P = .46). The outcomes observed with each of the different treatment paradigms are listed in Appendix Table A2 (online only).
Fig 2.
Kaplan-Meier curves for (A) overall survival (OS) and (B) progression-free survival (PFS); product-limit survival estimates based on LIFETEST procedure in SAS software. Crosses indicate censored patients; shaded areas indicate 95% CIs.
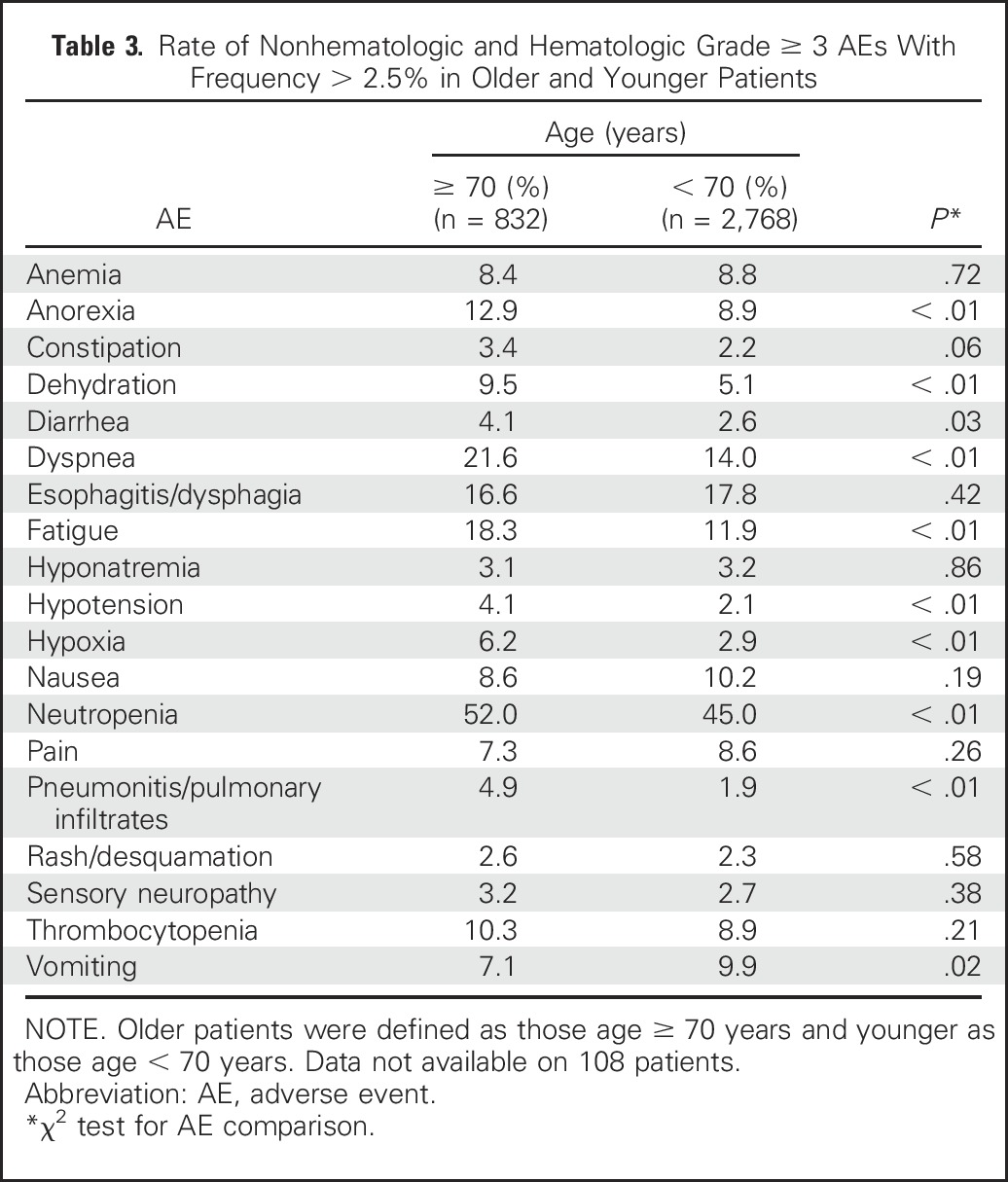
Elderly patients had a higher rate of grade ≥ 3 AEs in the unadjusted and multivariable models (OR, 1.35; 95% CI, 1.07 to 1.70 and OR, 1.38; 95% CI, 1.10 to 1.74, respectively). When the rates of all grade ≥ 3, hematologic grade ≥ 3, and nonhematologic grade ≥ 3 AEs in elderly patients were compared with those in younger patients, we found that elderly patients experienced a statistically significantly higher rate of grade ≥ 3 AEs in each category (Table 2). The treatment paradigm and age interactions in the unadjusted and adjusted models were not statistically significant (grade ≥ 3 AEs: unadjusted model, P = .81 and adjusted model, P = .77; grade ≥ 3 hematologic AEs: unadjusted model, P = 0.36 and adjusted model, P = .35; grade ≥ 3 nonhematologic AEs: unadjusted model, P = .79 and adjusted model, P = .71). The rate of grade 5 AEs was higher in elderly patients compared with younger patients (9.0% v 4.4%; P < .01). The rate of deaths attributed to treatment by the investigator was similar in elderly compared with younger patients (3.2% v 2%; P = .12); data were available for 2,091 patients for this analysis. The specific grade ≥ 3 AEs that occurred at a rate of ≥ 2.5% in elderly or younger patients are listed in Table 3.
Table 2.
Rate of AEs Among Older and Younger Patients
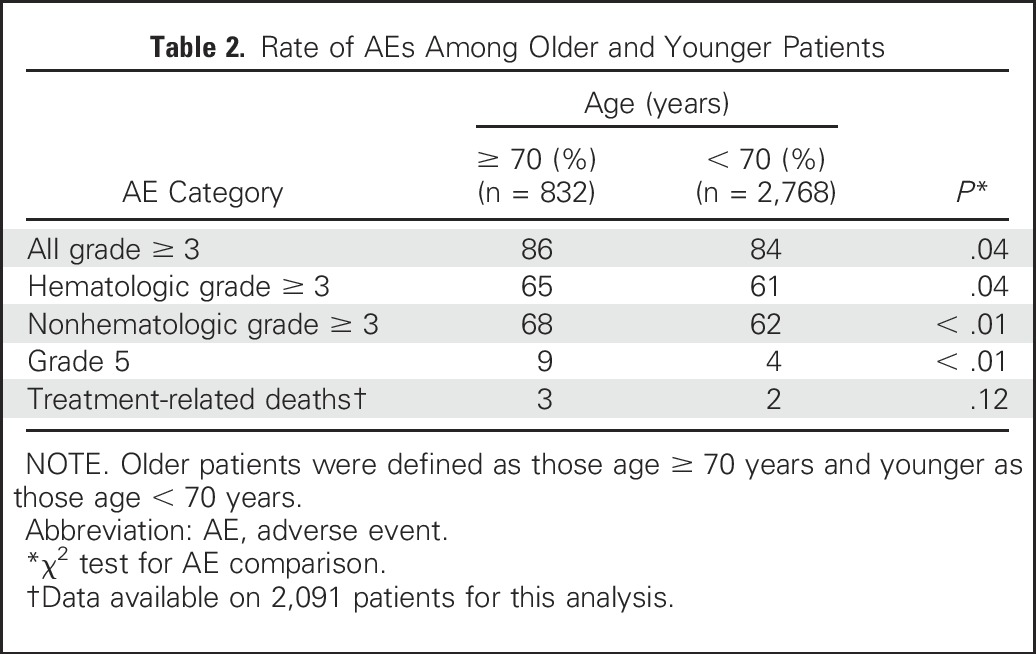
Table 3.
Rate of Nonhematologic and Hematologic Grade ≥ 3 AEs With Frequency > 2.5% in Older and Younger Patients
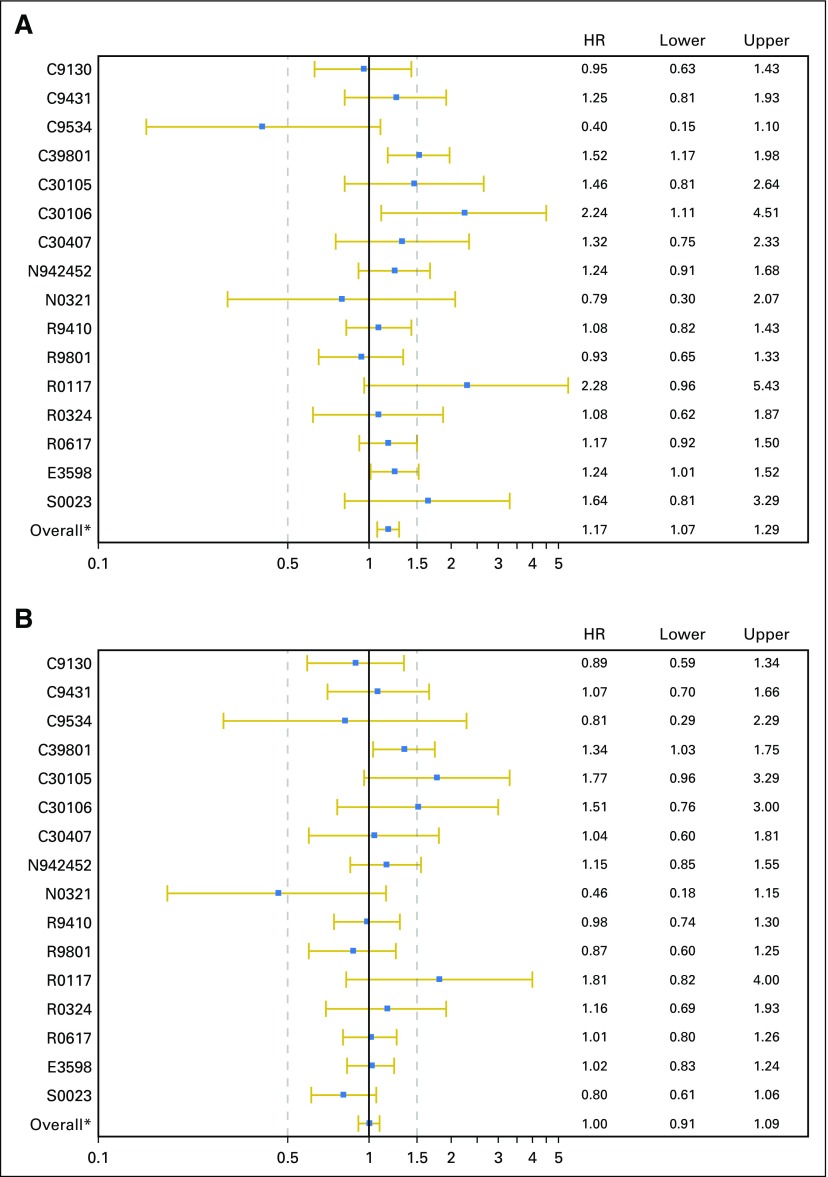
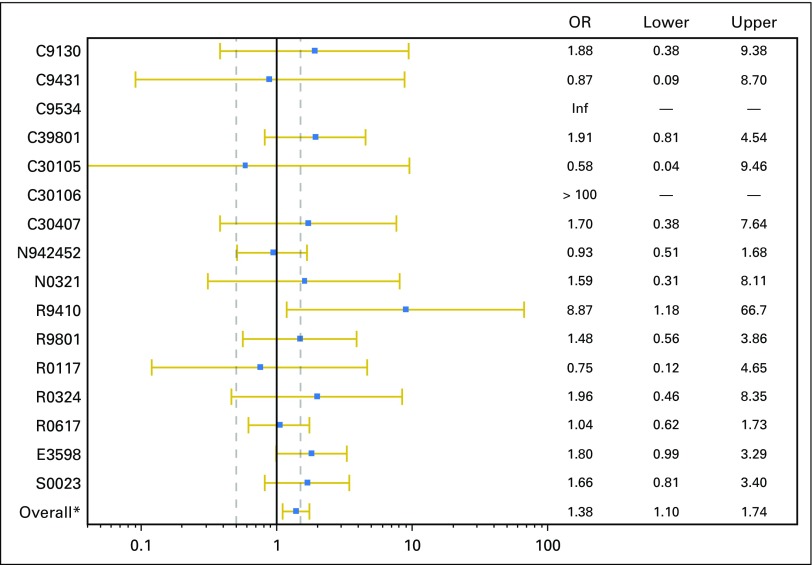
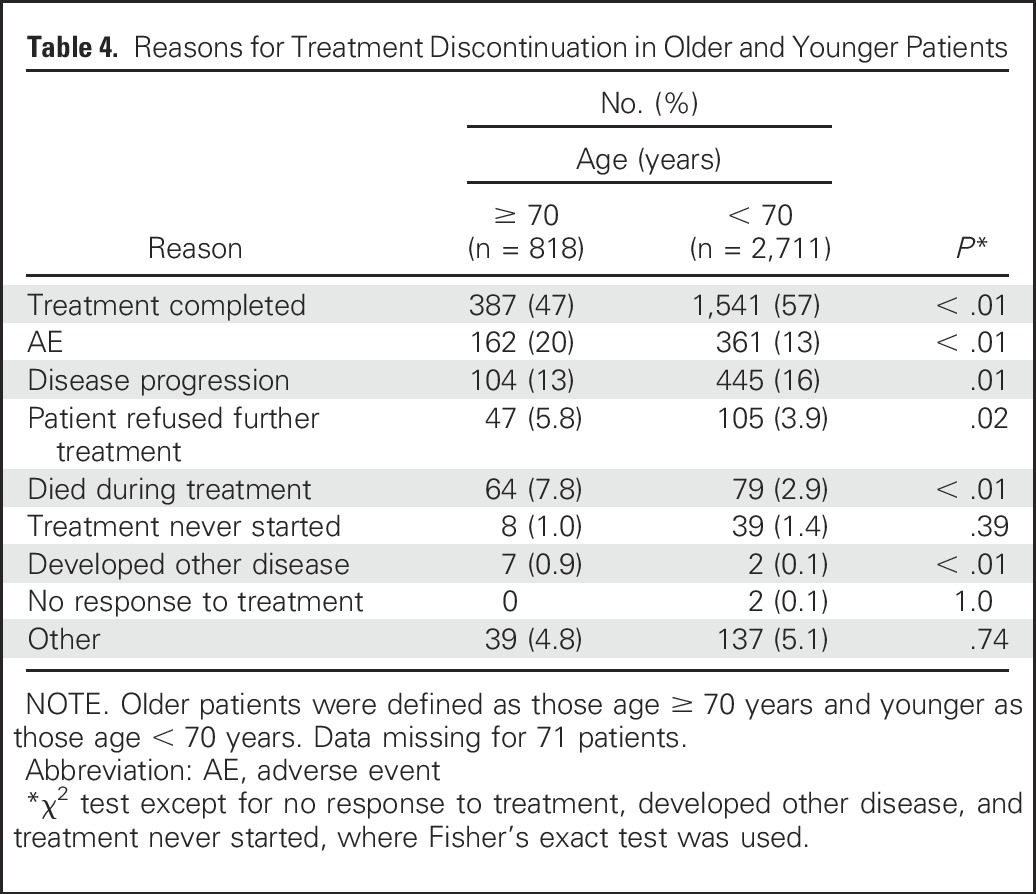
The reasons for treatment discontinuation were analyzed, and significant differences in the reasons for treatment discontinuation were observed (Table 4). Elderly patients completed treatment at a lower rate (47% v 57%; P < .01), discontinued treatment secondary to an AE at a higher rate (20% v 13%; P < .01), refused treatment at a higher rate (5.8% v 3.9%; P = .02), and died during treatment at a higher rate (7.8% v 2.9%; P < .01). This analysis was performed using an age cutoff of 65 years and using age as a continuous variable, and the conclusions remained the same with these additional analyses (data not shown). The individual trial–level HRs for OS and PFS are shown in Appendix Figures A1A and A1B (online only), and the individual trial–level ORs for grade ≥ 3 AEs are shown in Appendix Figure A2 (online only). We performed a supplemental analysis of trials where accrual was closed in 2000 or later compared before 2000, and the conclusions remained the same.
Table 4.
Reasons for Treatment Discontinuation in Older and Younger Patients
DISCUSSION
The main finding from this analysis is that elderly patients experienced more toxicity and poorer survival after concurrent chemoradiotherapy. The similar PFS and higher rate of grade 5 AEs suggest early deaths were a factor in the worse OS. The higher rate of toxicity was predictable because elderly patients can be frailer, and many clinicians already know elderly patients have difficulty tolerating concurrent chemoradiotherapy. The elderly patients enrolled in these trials met the trial eligibility criteria and represent a subset of elderly patients often described as the fit elderly. It seems that even fit elderly patients had comorbidities exacerbated by concurrent chemoradiotherapy, and the vulnerability of these patients was seen. It is also possible physicians were more conservative in the enrollment of elderly patients and more lenient in the enrollment of younger patients with comorbidities, and our analysis may have underestimated the impact of age. A difference in treatment selection according to age was observed in a previous analysis of chemotherapy for advanced NSCLC.42
These findings suggest the current methods of assessing elderly patients would benefit from further refinement. In the treatment of metastatic lung cancer, there has been interest in comprehensive geriatric assessment to assess patients before chemotherapy and better define who will tolerate or benefit from chemotherapy.43,44 Because concurrent chemoradiotherapy for stage III NSCLC carries significant toxicity, and over- or undertreatment can have significant clinical consequences, patients with stage III disease may be better candidates for investigating comprehensive geriatric assessment. The preliminary results of a prospective study of comprehensive geriatric assessment and the Vulnerable Elders Survey-13 (VES-13; a vulnerability screening tool that classifies patients as fit, medium fit, or unfit based on a numeric score) in elderly patients with stage III NSCLC treated with concurrent chemoradiotherapy revealed that in multivariable analysis, comprehensive geriatric assessment and the VES-13 had independent prognostic value. A higher VES-13 score was associated with statistically significantly shorter OS and higher rate of grade 3 or 4 toxicity.45 The hope is that geriatric assessment will be able to identify which patients will tolerate and benefit from concurrent chemoradiotherapy, as well as identify previously undetected comorbidities that can be addressed before initiation of therapy.
Three trials included in this pooled analysis were previously evaluated, comparing the results of concurrent chemoradiotherapy in elderly patients with those in younger patients. Previous analyses of cooperative group trials have revealed a numerically higher rate of esophagitis and a higher rate of grade 4 pneumonitis, hematologic toxicities, and renal toxicities during cisplatin induction chemotherapy in elderly patients.12-14,26,33,35 With much greater patient numbers, our analysis provided the power to detect that elderly patients experienced a higher rate of other AEs and provided an estimate of the occurrence rates of specific AEs.
One of the advantages of using clinical trial data is the prospective collection of data on AEs and reasons for treatment discontinuation. Despite the standardized AE reporting using the NCI Common Terminology Criteria for Adverse Events, there can be ambiguity when interpreting clinical events because multiple interrelated processes contribute to the clinical situation. For instance, the attribution of death resulting from treatment can be challenging when the patient has treatment-related AEs, underlying comorbidities, and potentially intercurrent illness. In our analysis, a statistically significant difference in treatment-related deaths between elderly and younger patients was not observed (P = .12), but there were fewer IPD available for this analysis. Elderly patients had a statistically significantly higher rate of grade 5 AEs and a statistically significant higher number of elderly patients died during treatment as a reason for treatment discontinuation. This indicates treatment with concurrent chemoradiotherapy carries greater risk in elderly patients.
It is possible that by modifying the chemotherapy, the AEs associated with concurrent chemoradiotherapy could be reduced. In our analysis, a majority of the trials used carboplatin and paclitaxel (Appendix Table A1). A recent retrospective analysis and systemic analysis revealed similar efficacy between treatment with carboplatin and paclitaxel and treatment with cisplatin and etoposide with concurrent thoracic radiotherapy, with a higher rate of toxicity with cisplatin and etoposide. Neither analysis investigated whether there was an interaction between age and rate of toxicity with the two treatments.46,47 A phase III trial compared cisplatin with pemetrexed or etoposide, and during the overall study period, the rate of grade 3 or 4 neutropenia was lower with cisplatin and pemetrexed.48 In our study, when the different treatment paradigms were analyzed for interaction between age and treatment paradigm, a statistically significant interaction was not observed, suggesting that it is unlikely that a change in treatment sequence will significantly improve the tolerability of the therapy.
Our analysis has limitations. We were not able to identify if the AEs or treatment discontinuations occurred during the concurrent chemoradiotherapy or the induction or consolidation chemotherapy segments of the treatment regimens. This issue is particularly relevant because a recent phase III trial raised questions about the benefit of consolidation chemotherapy after concurrent chemoradiotherapy.49 We also do not know the rate of chemotherapy dose adjustments, delays, and omissions or the rate of radiation treatment interruptions or dose adjustments. These trials also enrolled patients over an extended period of time, and there is the potential for variation in the characteristics of the elderly patient population enrolled in these trials compared with those of the current populations of elderly patients with stage III disease.
Treating a patient with concurrent chemoradiotherapy is always an individual decision based on each patient’s comorbidities, symptoms, and specific clinical situation. There should be a discussion with the patient and his or her family about the risks and benefits and his or her preferences before initiating therapy. Fit elderly patients with stage III NSCLC should be encouraged to receive concurrent chemoradiotherapy, preferably in clinical trials, but physicians should be cautious when monitoring the effects of therapy in elderly patients because of an increased incidence of severe toxicity. Future research should seek ways to decrease toxicity, especially in the elderly. Trials specifically designed for elderly patients with stage III disease may improve outcomes as well. Toxicity may be lessened with the use of modified radiation therapy treatments and changes in systemic therapy, such as the use of a single chemotherapy agent during concurrent chemoradiotherapy. Additionally, subgroups of elderly patients may benefit from targeted therapy and immunotherapy. Assessment of frailty or comorbidities could potentially be used to individualize therapy by better matching patient subgroups to optimal therapies. Comprehensive geriatric assessment could be used to better characterize elderly patients and assess the potential for toxicity.43,50
Appendix
Table A1.
Table A2.
AEs and Outcomes Observed in Treatment Paradigms
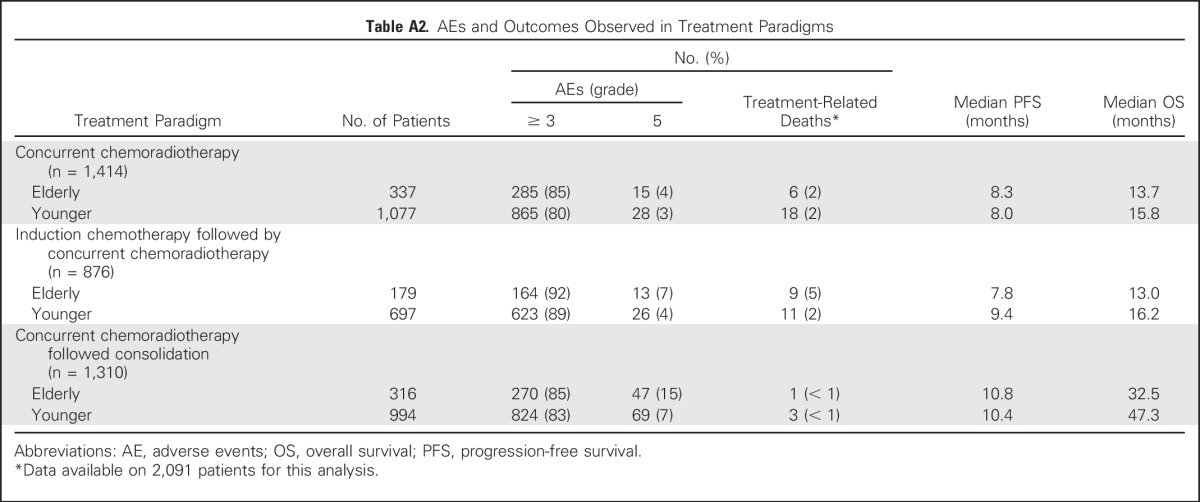
Fig A1.
Individual trial–level hazard ratios (HRs) for (A) overall survival and (B) progression-free survival. (*) Overall represents the polled estimate form the 16 trials.
Fig A2.
Individual trial–level odds ratios (ORs) for grade ≥ 3 adverse events. Inf, infinity. (*) Overall indicates the pooled O estimate from all 16 trials.
Footnotes
Supported by National Institutes of Health Grant No. R21-AG042894 (T.E.S., E.E.V., H.H.P., X.W.), Health and Medical Research Fund No. 12133251 (H.H.P.), and National Cancer Institute Grant No. P01-CA142538 (X.W.).
See accompanying Editorial on page 2860
AUTHOR CONTRIBUTIONS
Conception and design: Thomas E. Stinchcombe, Ying Zhang, Everett E. Vokes, Mark A. Socinski, Harvey J. Cohen, Herbert H. Pang, Xiaofei Wang
Provision of study materials or patients: Joan H. Schiller, Jeffrey D. Bradley, Walter J. Curran Jr, Mark A. Socinski
Collection and assembly of data: Thomas E. Stinchcombe, Ying Zhang, Jeffrey D. Bradley, Steven E. Schild, Benjamin Movsas, Mark A. Socinski, Neal E. Ready, Herbert H. Pang, Xiaofei Wang
Data analysis and interpretation: Thomas E. Stinchcombe, Ying Zhang, Everett E. Vokes, Joan H. Schiller, Jeffrey D. Bradley, Karen Kelly, Walter J. Curran Jr, Steven E. Schild, Gerald Clamon, Ramaswamy Govindan, George R. Blumenschein, Mark A. Socinski, Wallace L. Akerley, Harvey J. Cohen, Herbert H. Pang, Xiaofei Wang
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Pooled Analysis of Individual Patient Data on Concurrent Chemoradiotherapy for Stage III Non–Small-Cell Lung Cancer in Elderly Patients Compared With Younger Patients Who Participated in US National Cancer Institute Cooperative Group Studies
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Thomas E. Stinchcombe
Consulting or Advisory Role: ARIAD Pharmaceuticals, Boehringer Ingelheim, Celgene, AbbVie
Research Funding: Genentech (Inst), EMD Serono (Inst), Bristol-Myers Squibb (Inst)
Ying Zhang
No relationship to disclose
Everett E. Vokes
Stock or Other Ownership: McKesson
Consulting or Advisory Role: AbbVie, Amgen, AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Eli Lilly, Genentech, Leidos Biomedical Research, Merck, Regeneron, Merck Serono, Takeda Pharmaceuticals,VentiRx
Joan H. Schiller
Consulting or Advisory Role: Roche, Synta, Clovis Oncology, Genentech, Eli Lilly, Vertex, Oncogenex, AstraZeneca, Halozyme, Merck, EMD Serono
Research Funding: Synta (Inst), Astex Pharmaceuticals (Inst), Genentech (Inst), Clovis Oncology (Inst), AbbVie (Inst), Xcovery (Inst)
Other Relationship: Free to Breathe
Jeffrey D. Bradley
Honoraria: ViewRay, Varian Proton
Consulting or Advisory Role: ViewRay, Varian Proton
Research Funding: Mevion Medical Systems (Inst), ViewRay (Inst)
Travel, Accommodations, Expenses: Mevion Medical Systems
Karen Kelly
Honoraria: Roche, Bristol-Myers Squibb
Consulting or Advisory Role: Clovis Oncology, Transgene, Celgene, Synta, AstraZeneca, Eli Lilly, ARIAD Pharmaceuticals, Boehringer Ingelheim, Bristol-Myers Squibb, Genentech, Eli Lilly
Research Funding: Millennium Pharmaceuticals (Inst), Novartis (Inst), Synta (Inst), EMD Serono (Inst), Eli Lilly (Inst), Genentech (Inst), AbbVie (Inst), Gilead Sciences (Inst), Celgene (Inst), Five Prime Therapeutics (Inst)
Patents, Royalties, Other Intellectual Property: Author royalties from UpToDate
Travel, Accommodations, Expenses: AstraZeneca, Roche, Bristol-Myers Squibb, Celgene, ARIAD Pharmaceuticals, Genentech
Walter J. Curran Jr
No relationship to disclose
Steven E. Schild
No relationship to disclose
Benjamin Movsas
Research Funding: Philips Healthcare (Inst), Varian Medical Systems (Inst)
Patents, Royalties, Other Intellectual Property: Patent on lung phantom of computed tomography–magnetic resonance imaging fusion (Inst)
Gerald Clamon
Stock or Other Ownership: Pfizer
Research Funding: Merck (Inst)
Ramaswamy Govindan
Honoraria: Boehringer Ingelheim
Consulting or Advisory Role: GlaxoSmithKline, Boehringer Ingelheim, Clovis Oncology, Helsinn Therapeutics, Genentech, AbbVie, Celgene, Bayer HealthCare Pharmaceuticals, Novartis
Research Funding: Bayer HealthCare Pharmaceuticals (Inst), GlaxoSmithKline (Inst), MethylGene (Inst), AbbVie (Inst)
Travel, Accommodations, Expenses: Boehringer Ingelheim, Celgene, Merck, Amgen, Genentech, GlaxoSmithKline
George R. Blumenschein
Consulting or Advisory Role: Bristol-Myers Squibb, Clovis Oncology, AbbVie, Bayer HealthCare Pharmaceuticals, AstraZeneca, Merck, Celgene, ARIAD Pharmaceuticals
Research Funding: Bayer HealthCare Pharmaceuticals (Inst), Bristol-Myers Squibb (Inst), Celgene (Inst), Merck (Inst), Novartis (Inst), Xcovery (Inst), Adaptimmune (Inst), Immatics (Inst), AstraZeneca (Inst), GlaxoSmithKline (Inst)
Mark A. Socinski
No relationship to disclose
Neal E. Ready
Honoraria: Bristol-Myers Squibb, Celgene, Merck, AstraZeneca, ARIAD Pharmaceuticals
Consulting or Advisory Role: Bristol-Myers Squibb, Celgene, Merck, AstraZeneca, ARIAD Pharmaceuticals
Wallace L. Akerley
No relationship to disclose
Harvey J. Cohen
No relationship to disclose
Herbert H. Pang
No relationship to disclose
Xiaofei Wang
No relationship to disclose
REFERENCES
- 1. National Cancer Institute: SEER stat fact sheets: Lung and bronchus cancer. http://seer.cancer.gov/statfacts/html/lungb.html-accessed8.30.2016.
- 2.Govindan R, Page N, Morgensztern D, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: Analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol. 2006;24:4539–4544. doi: 10.1200/JCO.2005.04.4859. [DOI] [PubMed] [Google Scholar]
- 3.Aupérin A, Le Péchoux C, Rolland E, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non–small-cell lung cancer. J Clin Oncol. 2010;28:2181–2190. doi: 10.1200/JCO.2009.26.2543. [DOI] [PubMed] [Google Scholar]
- 4.Hutchins LF, Unger JM, Crowley JJ, et al. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341:2061–2067. doi: 10.1056/NEJM199912303412706. [DOI] [PubMed] [Google Scholar]
- 5.Lewis JH, Kilgore ML, Goldman DP, et al. Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol. 2003;21:1383–1389. doi: 10.1200/JCO.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 6.Pang HH, Wang X, Stinchcombe TE, et al. Enrollment trends and disparity among patients with lung cancer in national clinical trials, 1990 to 2012. J Clin Oncol. 2016;34:3992–3999. doi: 10.1200/JCO.2016.67.7088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dawe DE, Christiansen D, Swaminath A, et al. Chemoradiotherapy versus radiotherapy alone in elderly patients with stage III non-small cell lung cancer: A systematic review and meta-analysis. Lung Cancer. 2016;99:180–185. doi: 10.1016/j.lungcan.2016.07.016. [DOI] [PubMed] [Google Scholar]
- 8.Schild SE, Mandrekar SJ, Jatoi A, et al. The value of combined-modality therapy in elderly patients with stage III nonsmall cell lung cancer. Cancer. 2007;110:363–368. doi: 10.1002/cncr.22780. [DOI] [PubMed] [Google Scholar]
- 9.Ademuyiwa FO, Johnson CS, White AS, et al. Prognostic factors in stage III non-small-cell lung cancer. Clin Lung Cancer. 2007;8:478–482. doi: 10.3816/CLC.2007.n.031. [DOI] [PubMed] [Google Scholar]
- 10.Stinchcombe TE, Hodgson L, Herndon JE, II, et al. Treatment outcomes of different prognostic groups of patients on cancer and leukemia group B trial 39801: Induction chemotherapy followed by chemoradiotherapy compared with chemoradiotherapy alone for unresectable stage III non-small cell lung cancer. J Thorac Oncol. 2009;4:1117–1125. doi: 10.1097/JTO.0b013e3181b27b33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Socinski MA, Zhang C, Herndon JE, II, et al. Combined modality trials of the Cancer and Leukemia Group B in stage III non-small-cell lung cancer: Analysis of factors influencing survival and toxicity. Ann Oncol. 2004;15:1033–1041. doi: 10.1093/annonc/mdh282. [DOI] [PubMed] [Google Scholar]
- 12.Schild SE, Stella PJ, Geyer SM, et al. The outcome of combined-modality therapy for stage III non–small-cell lung cancer in the elderly. J Clin Oncol. 2003;21:3201–3206. doi: 10.1200/JCO.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 13. Langer CJ, Hsu C, Curran W, et al: Do elderly patients with locally advanced Non-Small Cell Lung Cancer benefit from combined modality therapy? A secondary analysis of RTOG 94-10. Int J Radiat Oncol Biol Phys 51:20-21, 2001. [Google Scholar]
- 14.Rocha Lima CM, Herndon JE, II, Kosty M, et al. Therapy choices among older patients with lung carcinoma: An evaluation of two trials of the Cancer and Leukemia Group B. Cancer. 2002;94:181–187. doi: 10.1002/cncr.10174. [DOI] [PubMed] [Google Scholar]
- 15.Quoix E, Zalcman G, Oster JP, et al. Carboplatin and weekly paclitaxel doublet chemotherapy compared with monotherapy in elderly patients with advanced non-small-cell lung cancer: IFCT-0501 randomised, phase 3 trial. Lancet. 2011;378:1079–1088. doi: 10.1016/S0140-6736(11)60780-0. [DOI] [PubMed] [Google Scholar]
- 16.Atagi S, Kawahara M, Yokoyama A, et al. Thoracic radiotherapy with or without daily low-dose carboplatin in elderly patients with non-small-cell lung cancer: A randomised, controlled, phase 3 trial by the Japan Clinical Oncology Group (JCOG0301) Lancet Oncol. 2012;13:671–678. doi: 10.1016/S1470-2045(12)70139-0. [DOI] [PubMed] [Google Scholar]
- 17.Ramalingam SS, Dahlberg SE, Langer CJ, et al. Outcomes for elderly, advanced-stage non–small-cell lung cancer patients treated with bevacizumab in combination with carboplatin and paclitaxel: Analysis of Eastern Cooperative Oncology Group Trial 4599. J Clin Oncol. 2008;26:60–65. doi: 10.1200/JCO.2007.13.1144. [DOI] [PubMed] [Google Scholar]
- 18.Effects of vinorelbine on quality of life and survival of elderly patients with advanced non-small-cell lung cancer: The Elderly Lung Cancer Vinorelbine Italian Study GroupJ Natl Cancer Inst 9166–72.1999 [DOI] [PubMed] [Google Scholar]
- 19.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 20.Cox DR. Regression models and life-tables. J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 21. Hougaard P: Analysis of multivariate survival data, in Dietz K, Gail M, Krickeberg K, et al (eds): Statistics for Biology and Health. New York, NY, Springer Science+Business Media, 2000, pp 345-384. [Google Scholar]
- 22.Hougaard P. Frailty models for survival data. Lifetime Data Anal. 1995;1:255–273. doi: 10.1007/BF00985760. [DOI] [PubMed] [Google Scholar]
- 23.Cox DR. The regression analysis of binary sequences (with discussion) J R Stat Soc B. 1958;20:215–242. [Google Scholar]
- 24.McCullagh P, Nelder JA: Generalized Linear Models (ed 2) New York, NY, Chapman and Hall/CRC, 1989 [Google Scholar]
- 25.Agresti A: Categorical Data Analysis (ed 3) Hoboken, NJ, Wiley, 2012 [Google Scholar]
- 26.Clamon G, Herndon J, Cooper R, et al. Radiosensitization with carboplatin for patients with unresectable stage III non–small-cell lung cancer: A phase III trial of the Cancer and Leukemia Group B and the Eastern Cooperative Oncology Group. J Clin Oncol. 1999;17:4–11. doi: 10.1200/JCO.1999.17.1.4. [DOI] [PubMed] [Google Scholar]
- 27.Vokes EE, Herndon JE, II, Crawford J, et al. Randomized phase II study of cisplatin with gemcitabine or paclitaxel or vinorelbine as induction chemotherapy followed by concomitant chemoradiotherapy for stage IIIB non-small-cell lung cancer: Cancer and Leukemia Group B study 9431. J Clin Oncol. 2002;20:4191–4198. doi: 10.1200/JCO.2002.03.054. [DOI] [PubMed] [Google Scholar]
- 28.Akerley W, Herndon JE, Jr, Lyss AP, et al. Induction paclitaxel/carboplatin followed by concurrent chemoradiation therapy for unresectable stage III non-small-cell lung cancer: A limited-access study—CALGB 9534. Clin Lung Cancer. 2005;7:47–53. doi: 10.3816/CLC.2005.n.021. [DOI] [PubMed] [Google Scholar]
- 29.Vokes EE, Herndon JE, II, Kelley MJ, et al. Induction chemotherapy followed by chemoradiotherapy compared with chemoradiotherapy alone for regionally advanced unresectable stage III non–small-cell lung cancer: Cancer and Leukemia Group B. J Clin Oncol. 2007;25:1698–1704. doi: 10.1200/JCO.2006.07.3569. [DOI] [PubMed] [Google Scholar]
- 30.Socinski MA, Blackstock AW, Bogart JA, et al. Randomized phase II trial of induction chemotherapy followed by concurrent chemotherapy and dose-escalated thoracic conformal radiotherapy (74 Gy) in stage III non–small-cell lung cancer: CALGB 30105. J Clin Oncol. 2008;26:2457–2463. doi: 10.1200/JCO.2007.14.7371. [DOI] [PubMed] [Google Scholar]
- 31.Ready N, Jänne PA, Bogart J, et al. Chemoradiotherapy and gefitinib in stage III non-small cell lung cancer with epidermal growth factor receptor and KRAS mutation analysis: Cancer and leukemia group B (CALEB) 30106, a CALGB-stratified phase II trial. J Thorac Oncol. 2010;5:1382–1390. doi: 10.1097/JTO.0b013e3181eba657. [DOI] [PubMed] [Google Scholar]
- 32.Govindan R, Bogart J, Stinchcombe T, et al. Randomized phase II study of pemetrexed, carboplatin, and thoracic radiation with or without cetuximab in patients with locally advanced unresectable non–small-cell lung cancer: Cancer and Leukemia Group B trial 30407. J Clin Oncol. 2011;29:3120–3125. doi: 10.1200/JCO.2010.33.4979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schild SE, Stella PJ, Geyer SM, et al. Phase III trial comparing chemotherapy plus once-daily or twice-daily radiotherapy in stage III non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2002;54:370–378. doi: 10.1016/s0360-3016(02)02930-9. [DOI] [PubMed] [Google Scholar]
- 34.Zhao Y, Foster NR, Meyers JP, et al. A phase I/II study of bortezomib in combination with paclitaxel, carboplatin, and concurrent thoracic radiation therapy for non-small-cell lung cancer: North Central Cancer Treatment Group (NCCTG)-N0321. J Thorac Oncol. 2015;10:172–180. doi: 10.1097/JTO.0000000000000383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Curran WJ, Jr, Paulus R, Langer CJ, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: Randomized phase III trial RTOG 9410. J Natl Cancer Inst. 2011;103:1452–1460. doi: 10.1093/jnci/djr325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Movsas B, Scott C, Langer C, et al. Randomized trial of amifostine in locally advanced non–small-cell lung cancer patients receiving chemotherapy and hyperfractionated radiation: Radiation Therapy Oncology Group trial 98-01. J Clin Oncol. 2005;23:2145–2154. doi: 10.1200/JCO.2005.07.167. [DOI] [PubMed] [Google Scholar]
- 37.Bradley JD, Bae K, Graham MV, et al. Primary analysis of the phase II component of a phase I/II dose intensification study using three-dimensional conformal radiation therapy and concurrent chemotherapy for patients with inoperable non–small-cell lung cancer: RTOG 0117. J Clin Oncol. 2010;28:2475–2480. doi: 10.1200/JCO.2009.27.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blumenschein GR, Jr, Paulus R, Curran WJ, et al. Phase II study of cetuximab in combination with chemoradiation in patients with stage IIIA/B non–small-cell lung cancer: RTOG 0324. J Clin Oncol. 2011;29:2312–2318. doi: 10.1200/JCO.2010.31.7875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): A randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16:187–199. doi: 10.1016/S1470-2045(14)71207-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoang T, Dahlberg SE, Schiller JH, et al. Randomized phase III study of thoracic radiation in combination with paclitaxel and carboplatin with or without thalidomide in patients with stage III non–small-cell lung cancer: The ECOG 3598 study. J Clin Oncol. 2012;30:616–622. doi: 10.1200/JCO.2011.36.9116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kelly K, Chansky K, Gaspar LE, et al. Phase III trial of maintenance gefitinib or placebo after concurrent chemoradiotherapy and docetaxel consolidation in inoperable stage III non–small-cell lung cancer: SWOG S0023. J Clin Oncol. 2008;26:2450–2456. doi: 10.1200/JCO.2007.14.4824. [DOI] [PubMed] [Google Scholar]
- 42.Chrischilles EA, Pendergast JF, Kahn KL, et al. Adverse events among the elderly receiving chemotherapy for advanced non–small-cell lung cancer. J Clin Oncol. 2010;28:620–627. doi: 10.1200/JCO.2009.23.8485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: A prospective multicenter study. J Clin Oncol. 2011;29:3457–3465. doi: 10.1200/JCO.2011.34.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Corre R, Greillier L, Le Caër H, et al. Use of a comprehensive geriatric assessment for the management of elderly patients with advanced non–small-cell lung cancer: The phase III randomized ESOGIA-GFPC-GECP 08-02 study. J Clin Oncol. 2016;34:1476–1483. doi: 10.1200/JCO.2015.63.5839. [DOI] [PubMed] [Google Scholar]
- 45. Rebollo MA, Aceituno JL, Saldana J, et al: A prospective study assessing the value of geriatric assessment in elderly patients with stage III NSCLC for concurrent chemoradiation. J Clin Oncol 34, 2016 (suppl; abstr 8509) [Google Scholar]
- 46. doi: 10.1001/jamaoncol.2016.4280. Steuer CE, Behera M, Ernani V, et al: Comparison of concurrent use of thoracic radiation with either carboplatin-paclitaxel or cisplatin-etoposide for patients with stage iii non–small-cell lung cancer: A systematic review. JAMA Oncol [epub ahead of print on December 15, 2016] [DOI] [PubMed] [Google Scholar]
- 47.Santana-Davila R, Devisetty K, Szabo A, et al. Cisplatin and etoposide versus carboplatin and paclitaxel with concurrent radiotherapy for stage III non–small-cell lung cancer: An analysis of Veterans Health Administration data. J Clin Oncol. 2015;33:567–574. doi: 10.1200/JCO.2014.56.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Senan S, Brade A, Wang LH, et al. PROCLAIM: Randomized phase III trial of pemetrexed-cisplatin or etoposide-cisplatin plus thoracic radiation therapy followed by consolidation chemotherapy in locally advanced nonsquamous non–small-cell lung cancer. J Clin Oncol. 2016;34:953–962. doi: 10.1200/JCO.2015.64.8824. [DOI] [PubMed] [Google Scholar]
- 49.Ahn JS, Ahn YC, Kim JH, et al. Multinational randomized phase III trial with or without consolidation chemotherapy using docetaxel and cisplatin after concurrent chemoradiation in inoperable stage III non–small-cell lung cancer: KCSG-LU05-04. J Clin Oncol. 2015;33:2660–2666. doi: 10.1200/JCO.2014.60.0130. [DOI] [PubMed] [Google Scholar]
- 50.Hurria A, Mohile S, Gajra A, et al. Validation of a prediction tool for chemotherapy toxicity in older adults with cancer. J Clin Oncol. 2016;34:2366–2371. doi: 10.1200/JCO.2015.65.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]